Due to the climate change, it is necessary to search for crops resistant to harsh climates, pests and poor soils that can replace popular crops (Zhang et al., 2018). The search for new plants with antioxidant compounds has increased considerably during the last 5 years (Pisoschi et al., 2016), mainly due to the ability of antioxidants to neutralize free radicals that help prevent cardiovascular and cerebrovascular diseases as well as cancer (Gul et al., 2016). Antioxidants can also prevent atherosclerosis, arthritis, diabetes, and other diseases (Zhang et al., 2015). In the food industry, antioxidants are used to reduce the rate of oxidation of the products and thus, extend their shelf life (Xu et al., 2017). Peru has a high biodiversity of food and medicinal plants with nutraceutical and antioxidant potential, among which are Andean tuberous as mashua (Tropaeolum tuberosum R. & P.) (Campos et al., 2018; Pacheco et al., 2020). This tuber is a perennial herbaceous plant native to the Andean region with a high nutraceutical potential, which grows between 2,800 and 4,000 masl. Its spread and distribution includes Colombia, Ecuador, Peru, Argentina and Bolivia (Roca et al., 2007; Valle-Parra et al., 2018; Choquechambi et al., 2019; Apaza et al., 2020). In the Andean region, Peru and Bolivia represent the largest planting areas, which is generally grown in association with other tubers such as oca (Oxalis tuberosa), ulluco (Ullucus tuberosus) and potato (Solanum tuberosum) (Manrique et al., 2014). Mashua has a high diversity in morphology and color, which ranges from beige to dark purple. In Peru, more than 100 accessions have been recognized due to their variability in morphology and color, which would be correlated with their levels of phenolic compounds (Campos et al., 2018). Economically, it is the less important Andean tubers; however, it contains phenolic compounds with high antioxidant activity (Campos et al., 2006). Previous studies show that mashua contains glucosinolates (Martin and Higuera, 2016; Villacrés et al., 2016), phenolics and high antioxidant activity (Chirinos et al., 2015). Within the Andean tubers such as potatoes (Solanum sp.), oca (O. tuberosa) and olluco (U. tuberosum), mashua has the highest antioxidant activity (Campos et al., 2006). Furthermore, mashua has high resistance to pests and plant diseases, helps prevent soil erosion, adapts to cold temperatures and poor soils, has medicinal properties and can be used as a bioinsecticide. It is used in ethnomedicine to relieve kidney, liver, and prostate disorders, obtaining favorable results due to its bioactive compounds (Grau et al., 2003).
In Peru, Puno Region is the mean producer of mashua followed by the Cusco and Ayacucho Regions. It has a planting area of 4,828 ha that produces only 7,368 t year-1, compared to the potato production that is 742,924 t year-1 in the same region (Ministerio de Agricultura y Riego, 2018). This low production is due to its minimal demand since it has a bitter taste because of the presence of its glucosinolates (Martin and Higuera, 2016). Despite the fact that its planting area is less than those other Andean tubers, its cultivation is still important, since it is part of the food security of thousands of peasant families in the Andes through self-consumption or generation of income from the sale of this product (Apaza et al., 2020). Several studies recommend using it as a nutraceutical product or in the food preservation industry (Campos et al., 2006; Chirinos et al., 2007; Chirinos et al., 2008; Chirinos et al., 2015). In the Puno Region, one of the provinces with the highest production of mashua is Yunguyo. Therefore, the aim of this study was to characterize the physicochemical and antioxidant properties of six accessions of mashua (T. tuberosum R. & P.) of greatest economic importance in the province of Yunguyo (Puno Region, Peru), which vary according to the shape and color.
MATERIALS AND METHODS
Plant material
Six accessions of mashua (T. tuberosum R. & P.) were collected in the Yunguyo district, Yunguyo Province, Puno Region, Peru (16°14'39"S, 69°05'34"W). Yunguyo is one of the 13 provinces of the Puno Region, it has an altitude of 3,826 m, an average temperature of 8 °C, a maximum temperature of 17.3 °C and a minimum temperature of -1.3 °C. Three purple-colored mashua: Tt-03 (peel/pulp, purple/purple), Tt-23 (peel/pulp, purple/purple) and Tt-25 (peel/pulp, purple/purple); and three yellow-colored mashua Tt-02 (peel/pulp, yellow/yellow), Tt-11 (peel/pulp, yellow/yellow) and Tt-19 (peel/pulp, yellow/yellow), were provided by the National Institute of Agricultural Innovation ILLPA-Puno of Peru and numbered using the prefix Tt (T. tuberosum) (Figure 1). Approximately five units of each accession were disinfected by submersion in 5% sodium hypochlorite for 15 min, then cut to 2.5 mm in thickness and lyophilized for 7.5 h with a minimum temperature of -40 °C and 13.33 Pa by a freeze dryer device (Stellar, Millrock Technology, NY, USA). Subsequently, grinding process was performed in a rotor mill (mesh 0.08 mm) and the lyophilized powder was stored at -20 °C until the extraction and purification of phenolic compounds took place.
Sample preparation
The extraction of phenolic compounds was performed according to Chirinos et al. (2007), with minor modifications. Briefly, 5 g of the lyophilized powder from each accession was weighed using an analytical balance (Sartorius Extend Scale, ED224S) and homogenized for 20 min with methanol (CH₃OH) (Sigma-Aldrich, USA), acetone (C3H6O) (Sigma-Aldrich, USA), and destilated water (45:45:10), then acidified with 5 drops of 1% chloride acid (HCl) (Sigma-Aldrich, USA). The purification of phenolic compounds was carried out by solid phase separation in columns RP-18 (Lichrolut, Germany) of 60 mL. The column was conditioned with 60 mL of acidified methanol and 50 mL of acidified water, pH 2. Then, 60 mL of the sample was added, the column was washed with 40 mL of acidified water, and the elution was performed with 40 mL of acidified methanol, all the solvent was removed under vacuum on a rotary evaporator (Selecta, Spain) at 38 °C for 30 min. The purified solid residue was diluted in methanol (10 mg mL-1) and kept at -20 °C in a freezer (Thermo Scientific, USA) until use.
Total polyphenols
The content of total polyphenols was determined according to Herrera-Calderon et al. (2016). Briefly, 0.1 mL of sample was mixed with 1 mL of 10% Folin-Ciocalteu reagent for 5 min at 25 °C, then 1 mL of 5% sodium carbonate (Na2CO3) (Sigma-Aldrich, USA) was added and the mixture was placed in a water bath at 45 °C for 30 min. Absorbance was read by means of a spectrophotometer (Pharo 300, Spectroquant, USA) at 725 nm. The results were expressed in mg gallic acid equivalent 100 g-1 fresh weight (mg GAE 100 g-1 FW).
Total flavonoids
Total flavonoid content was performed according to Wolfe et al. (2008). To 0.250 mL of sample, 1250 mL of 5% sodium nitrite (NaNO2) (Sigma-Aldrich, USA) was added and it was left to react for 5 min, then 0.150 mL of aluminum chloride (AlCl3) was added, and the mixture could stand for 5 min. Finally, 0.5 mL of 1 M sodium hydroxide (NaOH) was added to the mixture and it was left in contact for 15 min. The reading was performed at 510 nm by a spectrophotometer (Pharo 300, Spectroquant, USA). The results were expressed in mg catechin equivalent 100 g-1 fresh weight (mg CE 100 g-1 FW).
Antioxidant capacity by ferric reducing antioxidant power (FRAP)
Antioxidant activity evaluated by the FRAP assay was performed according to the methodology proposed by Szollosi and Varga (2002). Briefly, to 20 µL of sample, 1 mL of distilled water and 1 mL of FRAP reagent (Sigma-Aldrich, USA) were added, the mixture was placed in a water bath at 37 °C and allowed to react for 15 min. The reading was made using a spectrophotometer (Pharo 300, Spectroquant, USA) at 593 nm. A standard curve was prepared using different concentrations of Fe2+ ranged from 15 to 75 mM. The results were expressed in mM Fe2+ 100 g-1 fresh weight (mM Fe2+ 100 g-1 FW).
Antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
Antioxidant activity evaluated by the DPPH radical scavenging assay was determined through the method developed by Brand-Williams et al. (1995), with minor modifications. It was performed using different concentrations of sample, which were placed in test tubes containing 1 mL of 0.1 M Acetate buffer pH 6.0, 1.5 mL methanol and 0.5 mL of 0.1 mM DPPH, then the mixture was stirred at 2500 rpm for 1 min and incubated at 37 °C for 30 min. Absorbance was read by a spectrophotometer (Pharo 300, Spectroquant, USA) at 517 nm. The results were expressed as µM Trolox equivalent antioxidant capacity 100 g-1 fresh weight (µM TEAC 100 g-1 FW).
Identification of phenolics compounds by HPLC-DAD
HPLC analyses of caffeic acid, rutin, chlorogenic acid, quercetin, apigenin and kaempferol were performed according to Chirinos et al. (2008), with minor modifications. All phenolic compound standards were obtained from Sigma-Aldrich, USA. Briefly, a VWR HITACHI Chromaster 600 HPLC with a diode array detector (HPLC-DAD 300), autosampler and a reversed phase C18 column (5 µm particle size, i.d. 4.6x250 mm) was used. The mobile phase consisted of 0.1% acetic acid solution (A) and 100% acetonitrile (B). The gradient profile was from 10 to 90% B from 0 to the desired gradient time (28, 39 and 55 min) at a flow rate of 0.5 mL min-1. Detection was performed at 272 and 414 nm using a photodiode array detector. Calibration curves were made in triplicate using five different concentrations (10, 20, 30, 40, 60 µg mL-1) for all phenolic compounds evaluated (Seal 2016). Data were processed using OpenLAB CDS software (Agilent Technologies, USA).
Statistical analysis
All the assays were carried out in triplicate. The results were expressed as mean±standard deviation (SD), and analyzed using SPSS software for Windows version 26.0 (SPSS, Inc., Chicago, IL, USA). The means were compared by one-way ANOVA followed by Tukey's multiple comparison test, at a significance level of P<0.05. Statistical correlation among different variables was performed using the Pearson coefficient (r) and results were statistically significant when P<0.05.
RESULTS AND DISCUSSION
Total polyphenols
Table 1 shows the total content of poyphenols and flavonoids, DPPH radical scavenging and FRAP activity of all samples evaluated. The content of total polyphenols ranged from 75.08 to 221.07 mg GAE 100 g-1 FW. All three purple-colored mashua showed significantly higher levels of total polyphenols compared to the three yellow-colored mashua (P<0.05). Within the group of purple-colored mashua, the Tt-23 accession presented significantly higher levels of total polyphenols compared to the Tt-03 and Tt-25 accessions (220.83±0.42, 172.62±0.76 and 173.55±0.10, respectively).
Table 1 Total content of poyphenols and flavonoids, DPPH radical scavenging and FRAP activity of six accessions of mashua (T. tuberosum R. & P.) from the Puno Region, Peru.

These results agreed with a previous study (Chirinos et al., 2006) in three purple-colored mashua, which showed a total polyphenol content that ranged from 174.9 to 374.4 mg GAE 100 g-1 FW. In another study (Campos et al., 2006), the content of total polyphenols was evaluated in four Andean tubers species. In the native potato (Solanum sp.), oca (O. tuberosa Molina) and ulluco (U. tuberosus Caldas), the content of total polyphenols was ranged from 64 to 232 mg chlorogenic acid equivalent 100 g-1 FW, 71 to 131 mg chlorogenic acid equivalent 100 g-1 FW and 41 to 77 mg chlorogenic acid equivalent 100 g-1 FW, respectively; while in mashua, in the case of the 11 genotypes evaluated, the total polyphenol levels ranged from 92 to 337 mg chlorogenic acid equivalent 100 g-1 FW. Studies carried out on different foods show that content greater than 100 mg GAE 100 g-1 is recognized as a high content of polyphenols (Ovaskainen et al., 2008). These results showed that the Tt-23 accession purple-colored had a high content of total polyphenols, even higher than other Andean tubers previously evaluated (Campos et al., 2006).
Total flavonoids
In the mashua accessions evaluated, the total flavonoid levels were ranged from 2.54 to 79.77 mg CE 100 g-1 FW. As shown in Table 1, the purple-colored mashua (Tt-23 and Tt-03) presented significantly higher content of total flavonoids (79.66±0.19 and 77.30±0.20 mg CE 100 g-1, respectively), approximately 8 times higher compared to the other mashua accessions evaluated (P<0.05). There are no reports on total flavonoid levels in mashua, however; some flavonoids such as flavan 3-ols, anthocyanins, flavones, flavonols and flavanones have been detected by high-performance liquid chromatography (Chirinos et al., 2008; Pacheco et al., 2019).
In vitro antioxidant activity by FRAP and DPPH radical scavenging assays
In the different mashua accessions evaluated, the FRAP activity ranged 376.89 to 2327.18 mM Fe2+ 100 g-1 FW, while the DPPH radical scavenging activity varied from 376.89 to 68.25±1.80 µM TEAC 100 g-1 FW. As shown in Table 1, all the purple-colored mashua presented significantly higher FRAP and DPPH radical scavenging activity compared to all yellow-colored mashua (P<0.05), being again the Tt-23 accession purple-colored, the one that presented the highest FRAP and DPPH radical scavenging activity compared to the other accessions (P<0.05).
Mashua accesions present a high diversity in their morphology and color, ranging from beige to purple. Previous studies have shown that purple-colored mashua have 8 to 10 times more antioxidant activity than yellow-colored mashua. This increase in the in vitro antioxidant activity is due partially to its high anthocyanin content (Campos et al., 2006; Chirinos et al., 2006; Chirinos et al., 2008). Chirinos et al. (2006) reported that mashua anthocyanins contributed to the total antioxidant activity in only one of the three purple-colored mashua, which allows to hypothesize that other phenolic compounds could participate in its antioxidant activity. Some studies have evaluated the in vitro antioxidant activity in different genotypes of mashua using the 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and oxygen radical absorbance capacity (ORAC) assays (Campos et al., 2006; Chirinos et al., 2006; Chirinos et al., 2008). A study in 11 pigmented genotypes of mashua (T. tuberosum R. & P.) reported that the in vitro antioxidant activity evaluated by the ABTS method ranged between 3.82 and 39.15 μmol Trolox equivalent g-1 FW, finding that the purple-colored DP-02-24, ARB-5241 and ARV-5366 genotypes showed the highest antioxidant activity (Campos et al., 2006). Another study in three purple-colored mashua showed an in vitro antioxidant capacity evaluated by the ABTS method, which ranged from 16.2 to 45.7 μmol Trolox equivalent g-1 FW (Chirinos et al., 2006). Furthermore, when the antioxidant activity was evaluated by the ORAC method in two purple-colored mashua, the values ranged from 221 to 359 μmol Trolox equivalents g-1 dry matter. These results agree with the present study, which among the different accessions of mashua evaluated, all purple-colored mashua (peel/pulp, purple/purple) showed an increased for the for the in vitro antioxidant activity.
Identification of phenolic compounds by HPLC-DAD
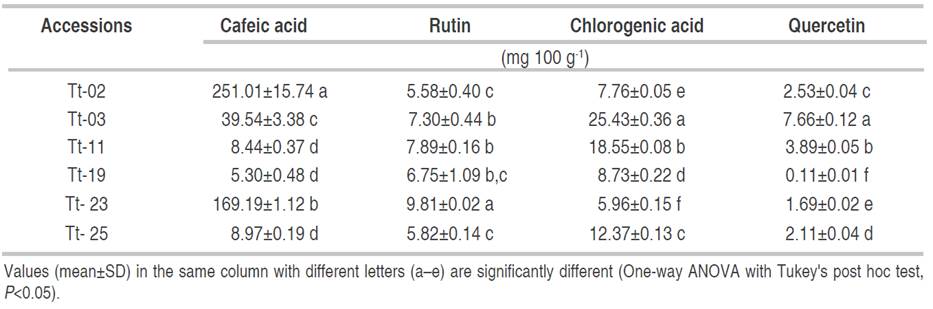
Table 2 shows the phenolic compounds analysis of the six accessions. The HPLC-DAD analysis showed detectable values for caffeic acid, rutin, chlorogenic acid and quercetin. As shown in Table 2, the highest levels of phenolic compounds were observed for caffeic acid, which ranged from 4.77 to 267.25 mg 100 g-1, lower levels were detected for the other phenolic compounds.
Table 2 Phenolic compounds measured by HPLC-DAD in six accessions of mashua (T. tuberosum R. & P.) from the Puno Region, Peru
A previous study, in 3 genotypes of colored mashua from Peru, the presence of gallic acid, gallocatechin, epigallocatechin, procyanidin B2 and derivatives of epigallocatechin, rutin and/or derivatives of myricetin and different derivatives of hydroxycinnamic and hydroxybenzoic acid were identified in these accesions (Chirinos et al., 2008). Furthermore, Pacheco et al. (2019) in a mashua from Ecuador reported the presence of flavonols and flavan 3-ols of 68.8 and 31.2%, respectively. Among the flavanols, (+)-gallocatechin, (-)-epigallocatech and (-)-epicatechin were identified, being the latter the most abundant (9.22 μg g-1 dry matter). Isorhamnetin 3-rutinoside, quercetin 3-rutinoside, and quercertin and myricetin derivatives were found. Furthermore, in purple-colored mashua, the presence of 11 anthocyanins such as delphinidin 3-glucoside-5-acetylrhamnoside, delphinidin 3-sophoroside-5-acetylrhamnoside, delphinidin 3-glucoside-5-rhamnoside, delphinidin 3-sophoroside-5-rhamnoside, delphinidin 3-glucoside, cyanidin 3-sophoroside, cyanidin 3-sophoroside-5-rhamnoside, cyaniding 3-glucoside, cyanidin 3-rutinoside, pelargonidin 3-sophoroside and pelargonidin 3-sophoroside-5-rhamnoside were identified; of which the first 2 pigments were found in a higher concentration. This anthocyanin-rich fraction was significantly correlated with the in vitro antioxidant activity evaluated by an ABTS assay and the content of phenolic compounds (r=0.6379 and r=0.9873, respectively) (Chirinos et al., 2006).
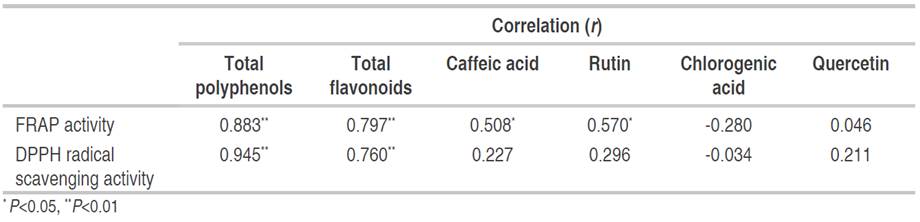
Other phenolic compounds have not been evaluated in mashua. The present study found an association between in vitro antioxidant activity and the content of phenolic compounds other than anthocyanins. Table 3 exposes th ecorrelation between in vitro antioxidant activity and levels of total polyphenols, total flavonoids and phenolic compounds identified. A significant correlation was observed between the FRAP activity and the total content of polyphenols and flavonoids (P<0.01), as well as with caffeic acid and rutin levels (P<0.05), while the DPPH activity was correlated with the total content of polyphenols and flavonoids (P<0.01) (Table 3). Furthermore, a significant correlation was observed between FRAP and DPPH radical scavenging activity (r=0.873, P<0.01, data not shown). These findings show that phenolic compounds other than anthocyanins could also contribute to the in vitro antioxidant activity presented by different accessions of mashua.
CONCLUSIONS
The present study shows that among the six mashua accessions evaluated in the Puno Region, Peru; the purple-colored mashua present a high content of total polyphenols and flavonoids, as well as a high in vitro antioxidant activity; the latter significantly correlated with the levels of total polyphenols and flavonoids, as well as phenolic compounds such as caffeic acid and rutin. These results highlight that the high antioxidant activity of this Andean tuber could be explained by bioactive compounds other than anthocyanins, which could be used as a nutraceutical in food and beverages to minimize the risk of diseases caused by oxidative stress, liver and kidney diseases, as well as urinary and prostate disorders.