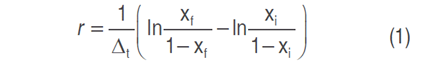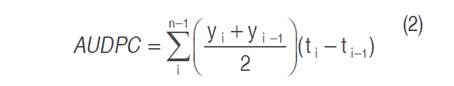Enterolobium cyclocarpum (Jacq.) Griseb. and Platymiscium pinnatum (Jacq.) Dugand (Fabaceae family) are common trees of the Colombian Caribbean region, with a high presence in the city of Santa Marta (department of Magdalena). E. cyclocarpum (subfamily: Mimosoideae) is distributed throughout the dry forests of Central America and northern South America (Rocha et al., 2018). This fabaceous is a species widely known for its uses in different human activities and, in forestry systems, its wood is used for construction, production of household utensils, furniture, musical instruments, among others (Pacheco et al., 2012). Its wide canopy provides shade appreciated in silvopastoral systems, as well as in urban centers, where it is planted as an ornamental tree along with parks and roads (Cordero and Boshier, 2003). Additionally, its fruits and seeds are suitable for human and animal consumption, making it possible to produce flours (Cordero and Boshier, 2003). On the other hand, extracts from different parts of the tree have been used to alleviate various ailments in humans and animals (Pacheco et al., 2012).
Platymiscium pinnatum (subfamily: Faboideae) is a neotropical tree distributed from Mexico to Brazil, preferably occupying dry habitats; however, its growth is common in humid ecosystems (Saslis-Lagoudakis et al., 2008). P. pinnatum is highly valued in forestry for its wood, which is used for multiple purposes, such as construction, fine wood carvings, furniture making, and musical instruments (Cordero and Boshier, 2003). This species has been described as a nitrogen fixer, being ideal for agroforestry systems and enrichment of degraded forests (Cordero and Boshier, 2003). In Central America, for example, P. pinnatum has been associated with organic and conventional agroforestry systems of shade coffee (Haggar et al., 2015). On the other hand, the use of leaf infusions has been reported for the treatment of infectious diseases in the skin and eyes (Pabón et al., 2017).
In a survey of pathogens in the urban trees of Santa Marta, Colombia, Colletotrichum spp. were associated with foliar diseases in E. cyclocarpum and P. pinnatum (Cantillo, 2014). The symptoms associated with Colletotrichum in E. cyclocarpum consist of whitish rounded spots at the leaflet base on the leaf underside, while in P. pinnatum, the symptom corresponds to a light or dark brown anthracnose surrounded by a chlorotic halo (Cantillo, 2014; Restrepo-Leal and Rada-González, 2017). Furthermore, in previous tests, the fungal pathogenicity was verified in both developing and mature foliar tissues of these forest species with the expression of characteristic symptoms in each plant (Restrepo-Leal and Rada-González, 2017). Due to these symptoms, the name "foliar Anthracnose" was defined to refer to this disorder or pathology.
Colletotrichum is one of the most important phytopathogenic fungi in the world because it affects a large number of plant species, causing huge economical losses (Dean et al., 2012). Moreover, this pathogen has a regular occurrence in different forest species, where it mainly has a causal relationship with foliar diseases that are expressed as spots, blights or anthracnose (Arguedas-Gamboa and Cots-Ibiza, 2012). In general, Colletotrichum spp. cause the disease called Anthracnose and, in aerial organs, these fungi cause sunken necrotic lesions, subcircular or angular, where the acervuli are formed (Dean et al., 2012; Arguedas-Gamboa and Cots-Ibiza, 2012).
To develop efficient control strategies, it is necessary: i) to carry out epidemiological studies of the disease that allow understanding its behavior, including aspects related to the infective period (Miles et al., 2013), and ii) to know the effect of the environmental variables on the disease development (Moral et al., 2012). In this regard, some research indicate that Colletotrichum species can be inactive during dry periods and change to infective when temperatures oscillate between 25 and 30 °C, and the relative humidity is greater than 80% (Miles et al., 2013; Lima et al., 2015). The severity of the disease is conditioned by the intensity and duration of precipitation, duration of humidity on the leaf surface, luminosity, among others (Huertas-Palacios et al., 2009; Moral et al., 2012).
Plant health studies in forest species have been limited to describing the pathogens associated with the crops; similarly, the phytosanitary regulatory bodies in each country have focused on keeping the phytosanitary status updated and, with some exceptions, on designing dispersal models or indicating the distribution of pests that threat forest production (Cordero and Boshier, 2003; Arguedas-Gamboa and Cots-Ibiza, 2012). In Colombia and Latin America, there are few investigations related to the epidemiology of pathologies in forest species, and Anthracnose caused by Colletotrichum species is not an exception.
Information on the development of Colletotrichum spp. in E. cyclocarpum and P. pinnatum is non-existent. Additionally, in Santa Marta and the Caribbean region, there are few studies on forest health, which makes it difficult to develop phytosanitary management strategies for trees. The previous situation has motivated the beginning of an investigative process on the interaction Colletotrichum spp. - forest species, raising the hypothesis that the epidemiological behavior of foliar anthracnose associated with Colletotrichum spp. varies between forest tree species. This study aimed to analyze the behavior of "foliar Anthracnose" associated with Colletotrichum spp. in E. cyclocarpum and P. pinnatum under field conditions.
MATERIALS AND METHODS
Area of study
The research was carried out in trees at the Universidad del Magdalena Campus, located in the city of Santa Marta (Colombia), in an area between 11°13'43" and 11°13'22" North latitude, and 74°11'00" and 74°11'16" West longitude, at an altitude of approximately 20 m. Santa Marta city presents an average temperature of 28.3 °C, average relative humidity of 76% and an average annual rainfall of 545 mm (IDEAM, 2014).
Four individuals of E. cyclocarpum and P. pinnatum were selected from a population of 97 and 40 trees, respectively. In each tree, four monitoring sites were marked, corresponding to the four cardinal points. Every monitoring site corresponded to five leaves located in the terminal part of a branch and positioned in the under-canopy layer.
Epidemiological variables
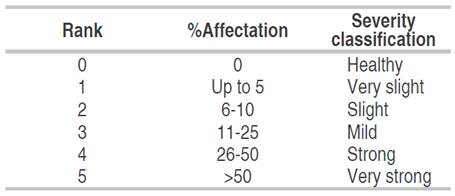
The epidemiological parameters were evaluated during 33 weeks, from March 26, 2016, to November 6, 2016, obtaining 29 measurements for each monitoring site. The number of leaflets with symptoms and the number of total leaflets were recorded; in this way, the incidence of Colletotrichum spp. at each monitored time was calculated. The severity of the disease was estimated according to scale diagrams designed for each forest species (Figure 1), following the severity scale described by Páez et al. (2003), with modifications. This scale involved six levels of affectation, as indicated in Table 1. Additionally, defoliation was determined for each monitoring site, based on the differences in the total records of leaves from one measurement with respect to the previous one. Based on weekly data, disease development curves were constructed for each forest species and Spearman correlations were made between these variables (incidence, severity and defoliation).
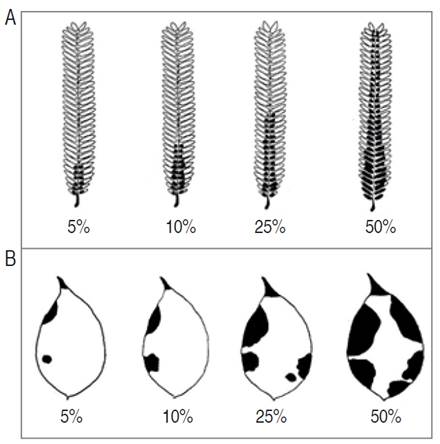
Figure 1 Scale diagrams to measure the severity of foliar Anthracnose (Colletotrichum spp.). A. Leaflets of E. cyclocarpum. B. Leaflets of P. pinnatum.
The disease development rate (r) was determined, according to Equation (1) proposed by Van der Plank (1963):
where r is the rate of disease development, ∆t is the time difference, xf is the final value of the disease and xi the initial value of the disease.
Likewise, the area under the disease progress curve (AUDPC) was calculated, according to Equation (2) indicated by López-Vásquez et al. (2013):
where AUDPC is the area under the disease progress curve, yi is the final severity, yi-1 is the initial severity, ti is the final time and ti-1 the initial time.
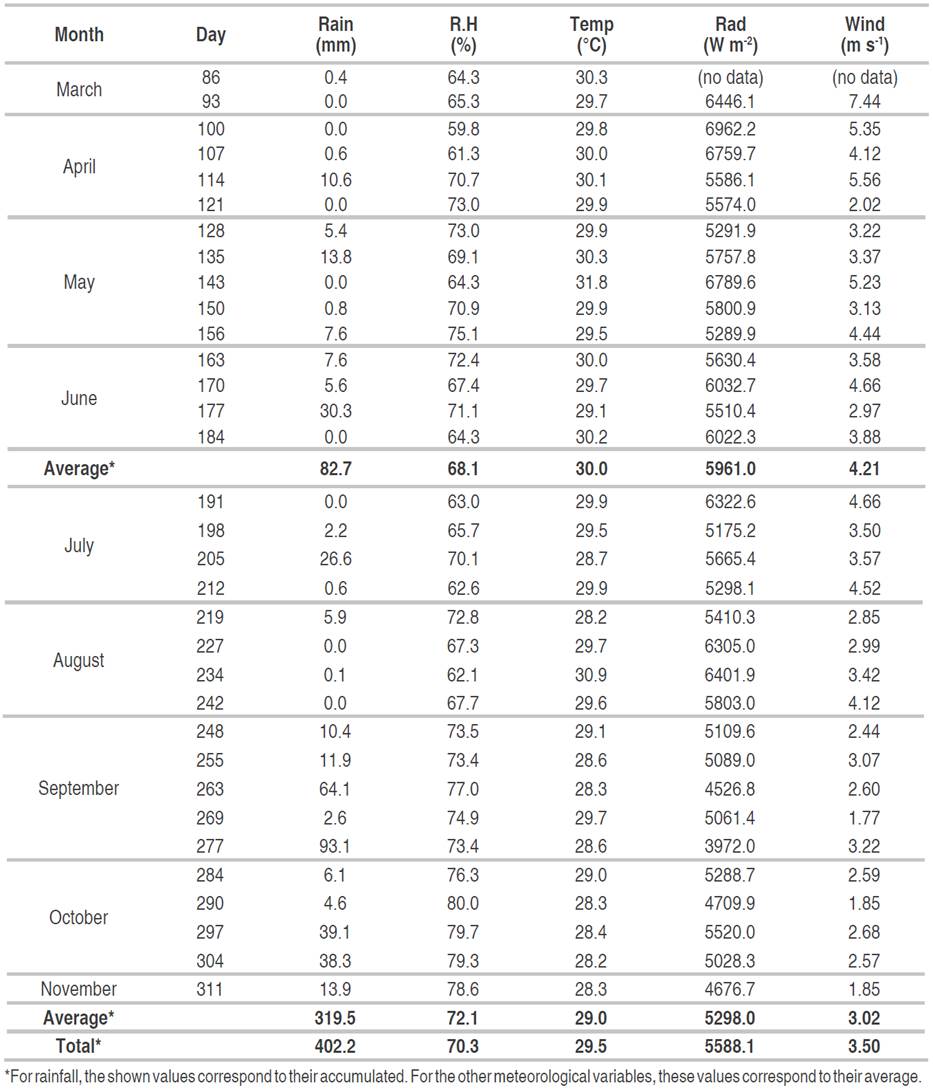
Both the development rate and the AUDPC were determined based on the severity values recorded for two follow-up periods: the first one, considered dry or with less rainfall, corresponding to March to June (first semester of the year); and the second one, considered rainy or with higher rainfall, between July and November (Table 2).
Effect of meteorological variables on the disease development
In order to know the effect of the meteorological variables on the behavior of foliar Anthracnose, a Spearman correlation analysis and a multiple regression analysis by ordinary least squares were performed between both incidence and severity with rainfall (mm), relative humidity (%), average temperature (°C), solar radiation (W m-2) and wind speed (m s-1). The meteorological data were provided by the Institute of Hydrology, Meteorology and Environmental Studies (IDEAM, 2014), according to the readings of the Meteorological Station of the Universidad del Magdalena.
The measurements were analyzed weekly, averaging the values of each meteorological variable, except for rainfall, which was calculated cumulatively. All statistical analyzes were performed in Statgraphics® Centurion XVI program.
During the 33 weeks of field follow-up, the total rainfall was 402.2 mm. In the dry period (March-June) the accumulated rainfall was 82.7 mm, while in the rainy period (July-November), it was 319.5 mm, being October the rainiest month with 17.8 mm (Table 2).
From March to June, the highest values of temperature (average of 30 °C), solar radiation (average of 5961.0 W m-2), and wind speed (average of 4.21 m s-1) were recorded, while relative humidity (average of 68.1%) presented the lowest values during this follow-up period. In the second period, average temperature, solar radiation, and wind speed were 29 °C, 5298.0 W m-2 and 3.02 m s-1, respectively. The average relative humidity was higher than the first semester with a value of 72.1% (Table 2).
This meteorological behavior is typical of the Colombian Caribbean region, where there is a unimodal rain distribution, with a short rainy period accentuated in the second semester of the year, and a long dry period that covers almost the entire first semester and some of the second. The temperature and relative humidity present fluctuating values throughout the year, with a slight increase in temperature in the first two months of the year and an increase of humidity in September to November, due to the effect of the rains. Wind speed is generally higher between December to March (IDEAM, 2014).
RESULTS AND DISCUSSION
Incidence and severity of foliar anthracnose and their relationship with tree defoliation
The mean of incidence and severity, for both follow-up periods (i.e. dry and rainy), are shown in Table 3. E. cyclocarpum presented higher values of the disease than P. pinnatum in the two periods. Both incidence and severity in E. cyclocarpum increased in the rainy season, registering a mean incidence of 83.9%, in contrast with the mean incidence during the dry period (44.3%). Mean severity in rainy months was 11.3%, while in dry months was 8.2%.
Table 3 Mean of incidence and severity of foliar Anthracnose, and defoliation in E. cyclocarpum and P. pinnatum in two follow-up periods. Universidad del Magdalena, Santa Marta, Colombia. 2016.
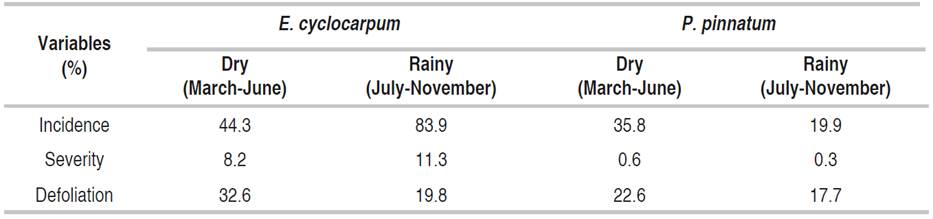
In E. cyclocarpum, from 26-03-2016 (day 86) and until 04-06-2016 (day 156), a decrease in the incidence of the disease was observed (from 68.0 to 28.8%). Starting on day 156, a linear growth was evidenced until 04-09-2016 (day 248), when the disease reached 100% and stabilized until the end of monitoring (06-11-2016; Figure 2A). The disease severity had a similar behavior; it decreased in the first six weeks, reaching a minimum percentage on 04-07-2016 (day 191) with 2.0%. Subsequently, from day 198 to the end of monitoring on 06-11-2016 (day 311), a linear increase was recorded, with a maximum value of 19.6%, considered moderate (Figure 2B).
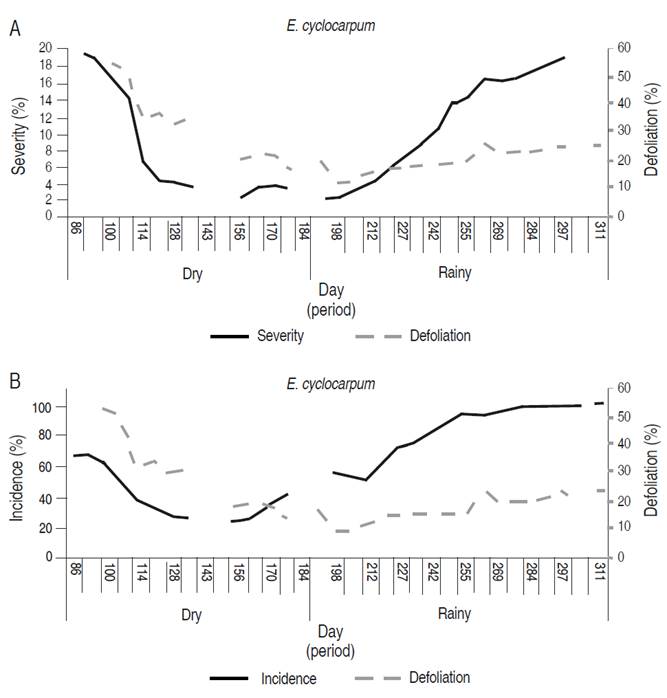
Figure 2 Development curve of foliar Anthracnose associated with Colletotrichum spp. in E. cyclocarpum. A. Severity and defoliation.
Defoliation in E. cyclocarpum was strong in the first weeks, with 54.6% in 09-04-2016 (day 100), and progressively decreased until 16-07-2016 (day 198), with values of 12.3 %. From this date, the defoliation increased until the end of the follow-up period, reaching moderate values of 25.5% (Figure 2).
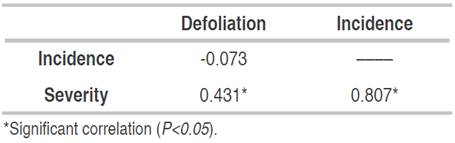
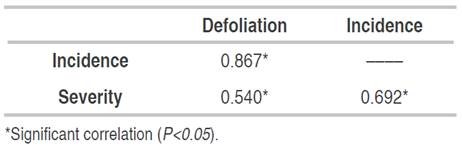
According to Spearman's correlation analysis (Table 4), the severity of the disease showed a significant relationship with defoliation (correlation=0.431; P<0.05) indicating that as the affected leaf area increases, the greater the induction of leaflet defoliations. The high correlation between incidence and severity (correlation=0.807; P<0.05) explains that in higher inoculum pressure, new leaflets are infected.
Table 4 Correlation coefficients between epidemiological variables of foliar Anthracnose and defoliation in E. cyclocarpum.
In P. pinnatum, the incidence and severity of the disease decreased from 26-03-2016 (day 86) to 14-05-2016 (day 135); the incidence was reduced from 68.9 to 15.0%, while the severity decreased from 1.3 to 0.2%. From day 135, there was a slight increase in the values of both variables, presenting 25.0 and 0.3% of incidence and severity on day 311 (11-06-2016). Defoliation presented a similar behavior to the other two variables, decreasing to 11.6% on day 135 (14-05-2016). From that date, it showed a slight increase, registering 20.7% at the end of the follow-up period (Figure 3).
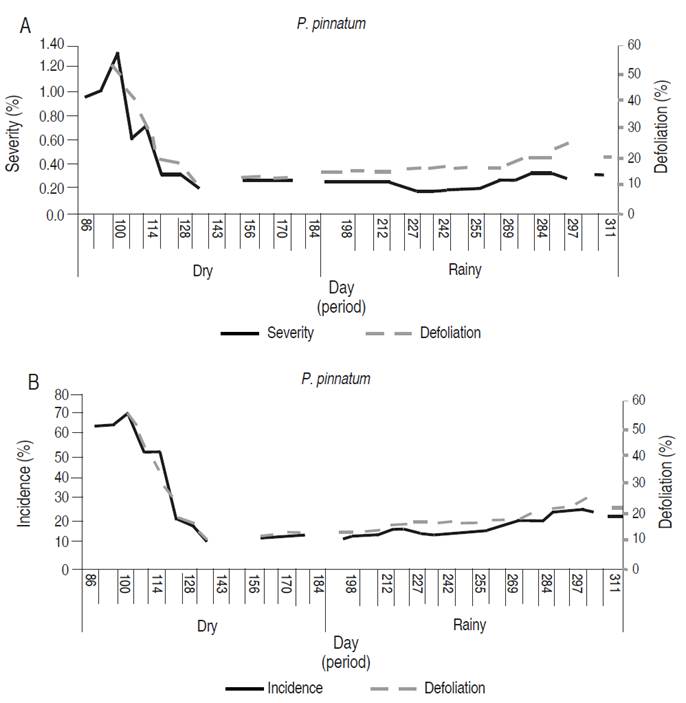
Figure 3 Development curve of the foliar Anthracnose associated with Colletotrichum spp. in P. pinnatum. A. Severity and defoliation. B. Incidence and defoliation.
According to the correlation analysis, there was a significant direct relationship between incidence and severity in P. pinnatum with defoliation, and between incidence and severity (Table 5).
Table 5 Correlation between epidemiological variables of foliar Anthracnose and defoliation in P. pinnatum.
The evaluated epidemiological variables indicated that E. cyclocarpum has a greater susceptibility to Colletotrichum spp., compared to P. pinnatum. One explanation for the differences in the amount of disease present in each forest species may be related to the production of secondary metabolites and the anatomy of their leaves. In Platymisicium species, the presence of flavonoids, isoflavonoids and other compounds with antifungal and cytotoxic properties has been reported (Cardoso-Lopes et al., 2008; Cuellar et al., 2020), although plant metabolites produced by E. cyclocarpum may also have antifungal activity (Pacheco et al., 2012; Biabiany et al., 2013). Arambarri et al. (2006) observed the presence of conspicuous epicuticular waxes in Enterolobium spp., a common feature of species adapted to dry environments, which can confer resistance to penetrating pathogens. Regarding P. pinnatum, de Enrech and Agostini (1987) indicated that the cuticle of this species presents a greater thickness, compared to other Platymiscium species. It is known that a greater thickness in the cuticle, as well as the presence of additional waxes, confers greater resistance to the penetration of fungi; however, it is worthy to mention that many pathogens can establish infections in plants with a considerable cuticle thickness (Freeman and Battie, 2008). In this research, the characteristics described for the leaves of both forest species did not prevent the development of the disease. It would be necessary to carry out studies on the physical and chemical composition of the leaves in these forest trees, as well as their relationship with resistance to Colletotrichum species.
The development of the disease depended fundamentally on the phenological development of the hosts since this defines successive sprouts and defoliation that, in turn, influence the diseases values over time. Initially, in the available leaf area, the disease values increase, being more evident with the first defoliation; however, as defoliation becomes widespread, the affected tissue is removed and the amount of initial disease decreases. In other words, the reduction in the amount of disease (incidence and severity) is mainly due to the loss of the plant organ (defoliation) and the appearance of new healthy tissue. This behavior has been used to establish management techniques for foliar Anthracnose where, through artificial defoliation, the severity of the disease is reduced (Guyot et al., 2005). However, a progressive increase in defoliation is an indicator of disease severity (Guyot et al., 2005; Huertas-Palacios et al., 2009).
The real effect of the fungus on defoliation is not understood nor if this is a plant defense mechanism against infection. In this research, the highest defoliation values, in both species, were recorded during the driest months. E. cyclocarpum is a semi-deciduous species that loses part of its foliage during the driest months, beginning defoliation at the end of the rainy season. On the other hand, the increase in foliage occurs when rainfall increases after the end of the dry season (Rocha et al., 2018). P. pinnatum is deciduous during the dry season, where trees can lose between 50 and 80% of their foliage, and as rainfall increases, the fall of the foliage decreases (Gómez, 2010). It is recommended to evaluate the effect of foliar Anthracnose in the defoliation of these forest species in future investigations.
Development rate and area under the disease progress curve (AUDPC)
In E. cyclocarpum, during the dry period (March to June), the foliar Anthracnose presented a development rate of 0.136 units week-1. Starting with the rains of July, and increasing in September and October, r reached 0.187 units week-1. The AUDPC was lower in the first period (dry), with 51 units week-1, compared to 186 units week-1 obtained during the rainy period (Table 6). These results indicated a more rapid development of the disease during the second half of the year.
Table 6 Development rate and area under the progress curve of foliar Anthracnose associated with Colletotrichum spp. in E. cyclocarpum and P. pinnatum for two follow-up periods.
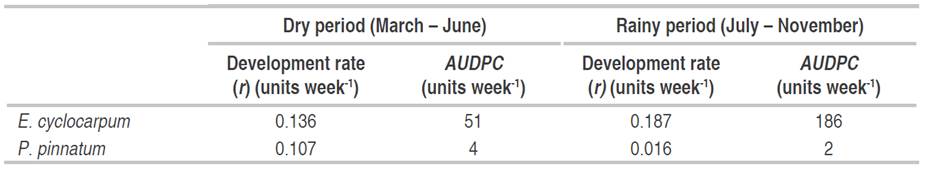
In P. pinnatum, the disease presented a development rate of 0.107 units week-1 during the first semester of the year. In the rainiest months, the development rate was lower (0.016 units week-1). The AUDPC indicated that foliar Anthracnose was higher in the months with less rainfall, registering a value of 4 units week-1, while in the second period the AUDPC was 2 units week-1 (Table 6). In this host, different behavior of the disease was registered to that obtained in E. cyclocarpum, which makes to consider other factors than the environment.
In this study, P. pinnatum presented lower development of foliar Anthracnose, especially when the climatic conditions were more favorable for the synthesis. In contrast, E. cyclocarpum was more susceptible to the action of the pathogen, observing a greater progression of the disease during periods where conditions of high humidity prevail. This greater disease progress during the rainy season agrees with studies in other pathosystems, including Colletotrichum species (Huertas-Palacios et al., 2009; Moral et al., 2012; Miles et al., 2013; Lima et al., 2015).
Effect of meteorological variables on the development of the disease
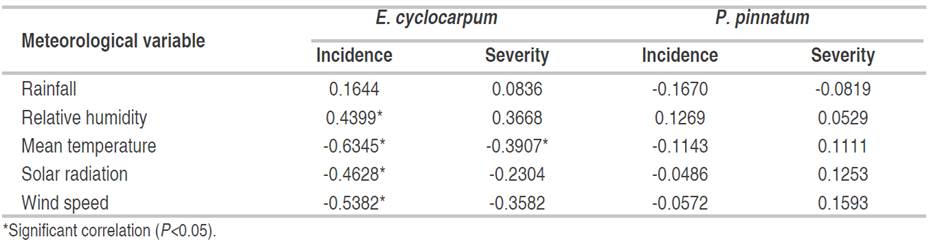
In E. cyclocarpum, the incidence of foliar Anthracnose was inversely correlated with mean temperature (P<0.05). Although the analysis showed a significant negative correlation with solar radiation and wind speed, and a positive correlation with relative humidity, the coefficients were not high. For severity, a significant negative correlation with mean temperature was observed, but with a low coefficient (-0.391; Figure 4-5; Table 7). In P. pinnatum, this type of analysis did not allow to identify significant correlations between epidemiological variables of the disease and meteorological variables (Figure 5-7; Table 7).
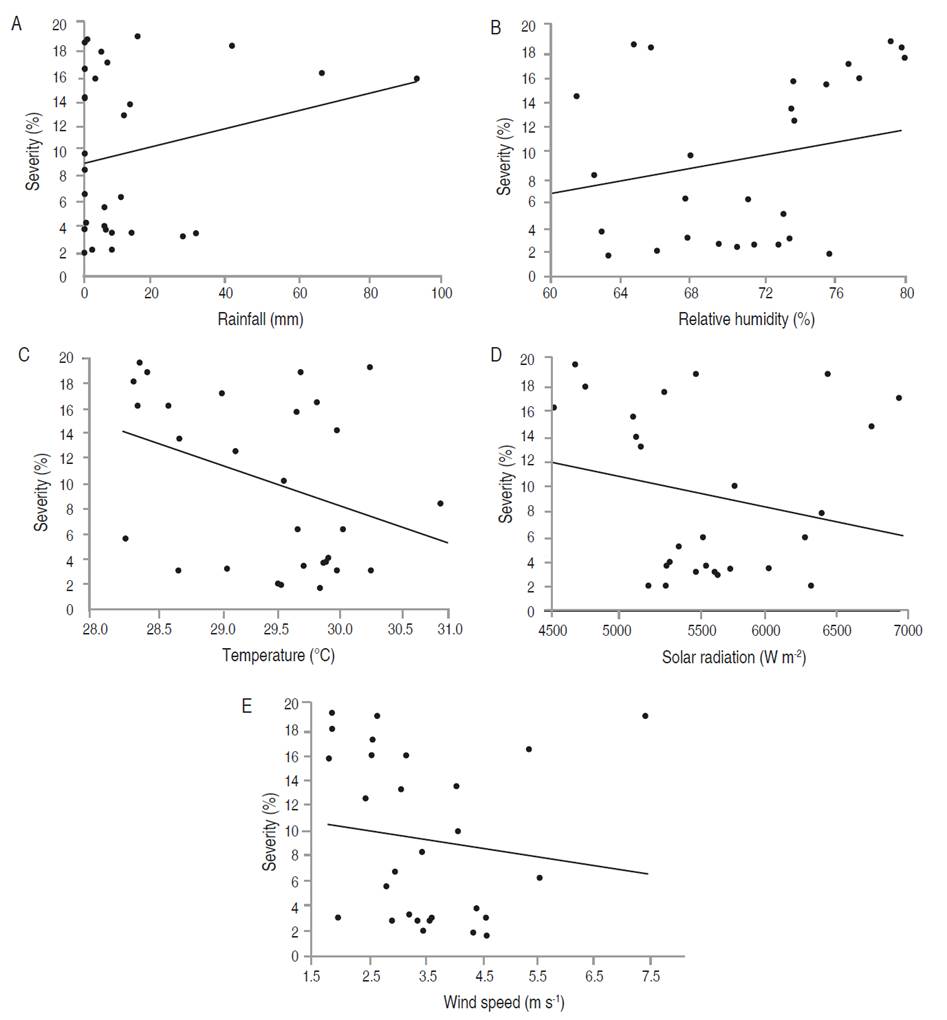
Figure 4 Relationship between the meteorological variables with the severity of foliar Anthracnose in E. cyclocarpum. Severity with (A) rainfall, (B) relative humidity, (C) mean temperature, (D) solar radiation, and (E) wind speed.
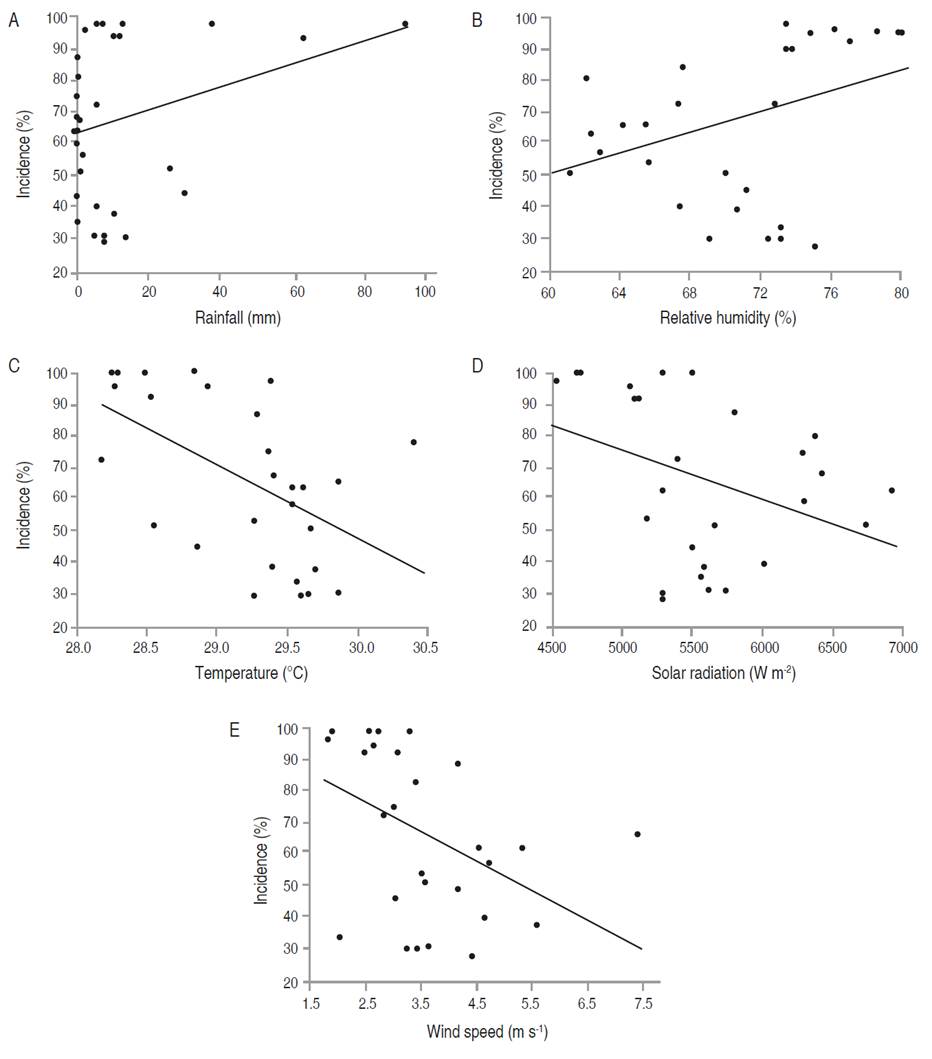
Figure 5 Relationship between the meteorological variables with the incidence of foliar Anthracnose in E. cyclocarpum. Incidence with (A) rainfall, (B) relative humidity, (C) average temperature, (D) solar radiation, and (E) wind speed.
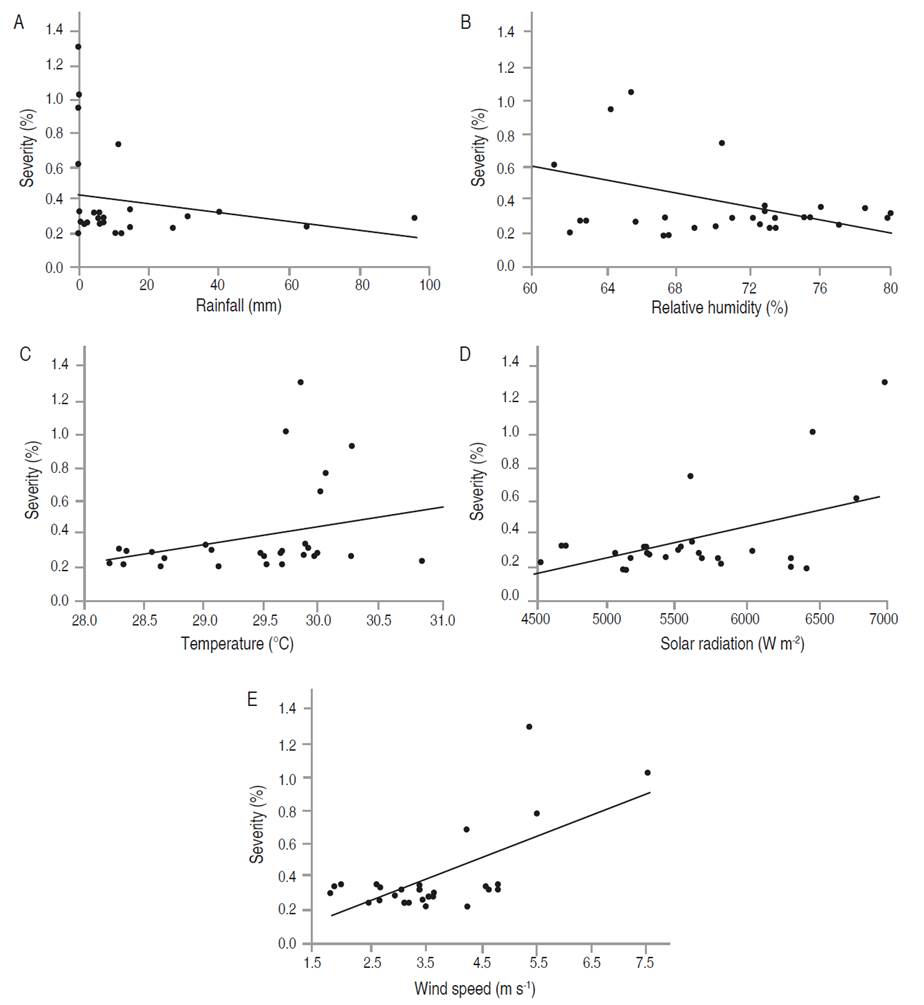
Figure 6 Relationship between the meteorological variables with the severity of foliar Anthracnose in P. pinnatum. The severity with (A) rainfall, (B) relative humidity, (C) mean temperature, (D) solar radiation, and (E) wind speed.
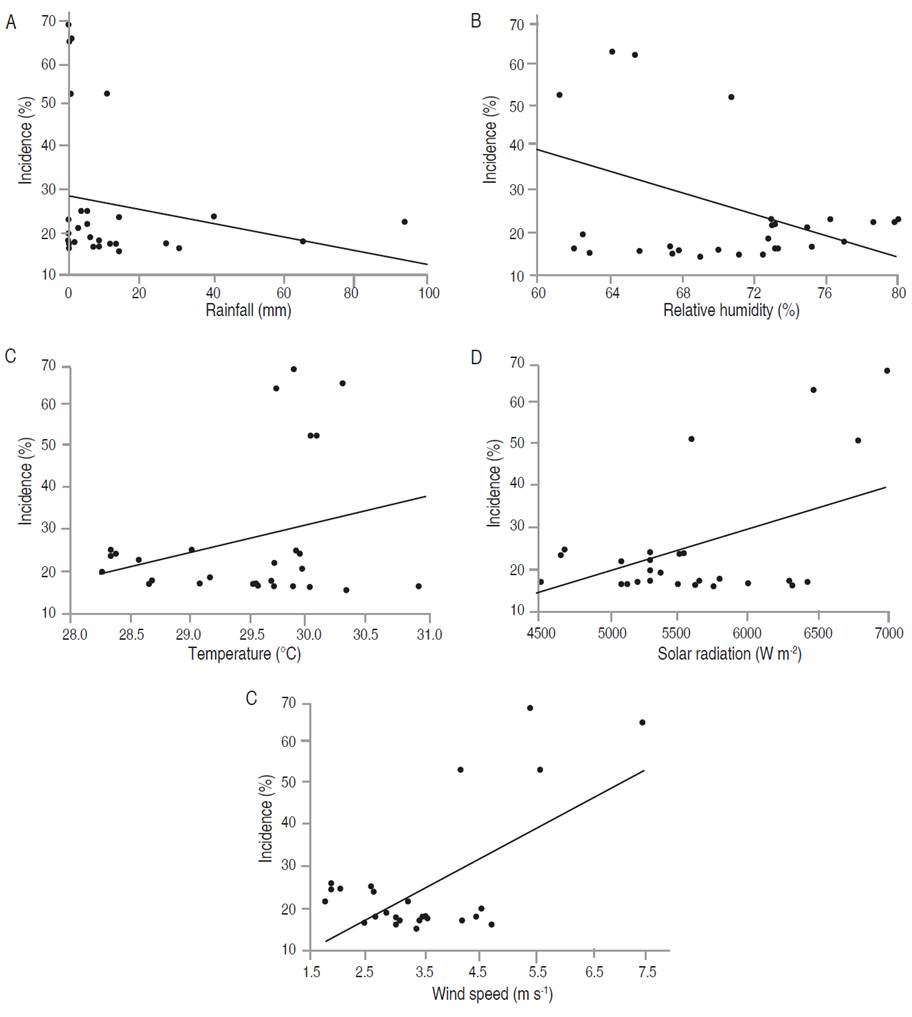
Figure 7 Relationship between the meteorological variables with the incidence of foliar Anthracnose in P. pinnatum. Incidence with (A) rainfall, (B) relative humidity, (C) mean temperature, (D) solar radiation, and (E) wind speed.
Table 7 Correlation between the meteorological variables and diseases values (incidence and severity) of foliar Anthracnose in the foliage of E. cyclocarpum and P. pinnatum, recorded from 26-03-2016 to 06-11-2016. (n = 29).
The multiple regression analysis by ordinary least squares confirmed that mean temperature was the only variable related to the incidence of foliar Anthracnose in E. cyclocarpum, explaining the incidence behavior by 39.72% with the whole model (Table 8). In the highest temperature values (between March and June) with an average of 30 °C (Table 2), the incidence remained at values close to 45% (Figure 2B); however, the temperature decreased slightly, except for certain events with maximum peaks, coinciding with the increase in incidence. On the other hand, severity could not be explained from the behavior of any of the analyzed meteorological variables. This raises the hypothesis that there are factors intrinsic to the pathogen or the host that influence a lesser or greater aggressiveness of the disease, under the meteorological conditions of the study area.
Table 8 Multiple regression between meteorological variables and the incidence of Colletotrichum spp. in the foliage of E. cyclocarpum, registered from 26-03-2016 to 06-11-2016.
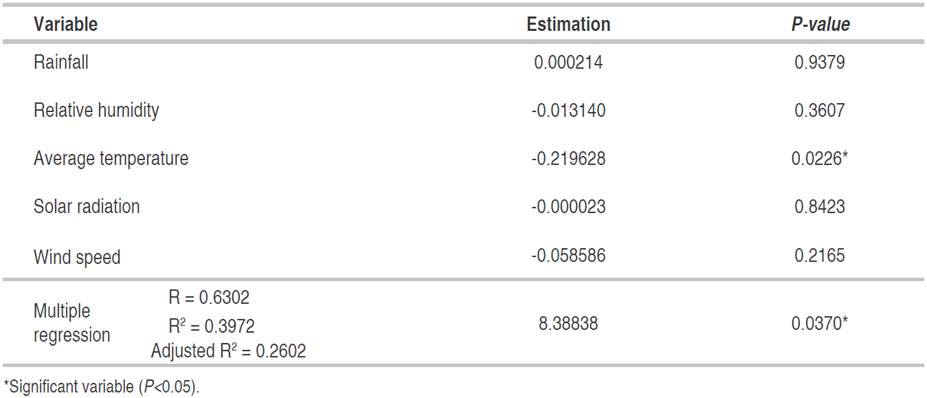
The multiple regression analysis by ordinary least squares confirmed that mean temperature was the only variable related to the incidence of foliar Anthracnose in E. cyclocarpum, explaining the incidence behavior by 39.72% with the whole model (Table 8). In the highest temperature values (between March and June) with an average of 30 °C (Table 2), the incidence remained at values close to 45% (Figure 2B); however, the temperature decreased slightly, except for certain events with maximum peaks, coinciding with the increase in incidence. On the other hand, severity could not be explained from the behavior of any of the analyzed meteorological variables. This raises the hypothesis that there are factors intrinsic to the pathogen or the host that influence a lesser or greater aggressiveness of the disease, under the meteorological conditions of the study area.
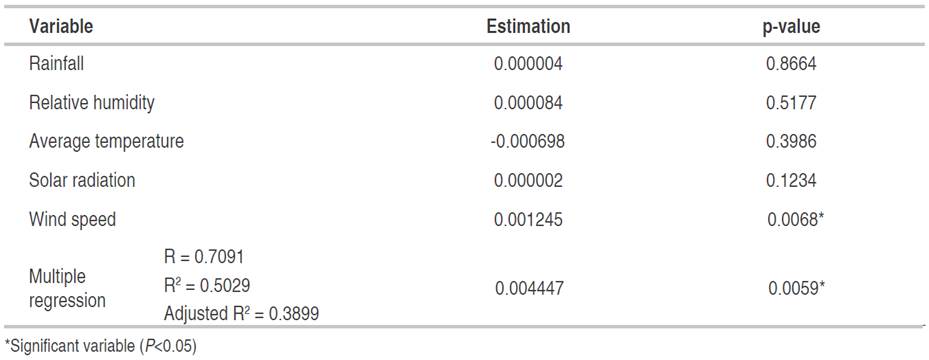
In P. pinnatum, the multiple regression analysis indicated a relationship of incidence and severity of foliar Anthracnose with wind speed, explained in 44.59 and 50.29% of its behavior with the whole model, respectively (Tables 9 and 10). Figures 6E and 7E show that the maximum values of wind speed, which correspond to March-April, coincided with the highest values recorded in both incidence and severity. Nevertheless, it is noted that these variables were not correlated (Table 7).
Table 9 Multiple regression between meteorological variables and the incidence of Colletotrichum spp. in the foliage of P. pinnatum, registered from 26-03-2016 to 06-11-2016.
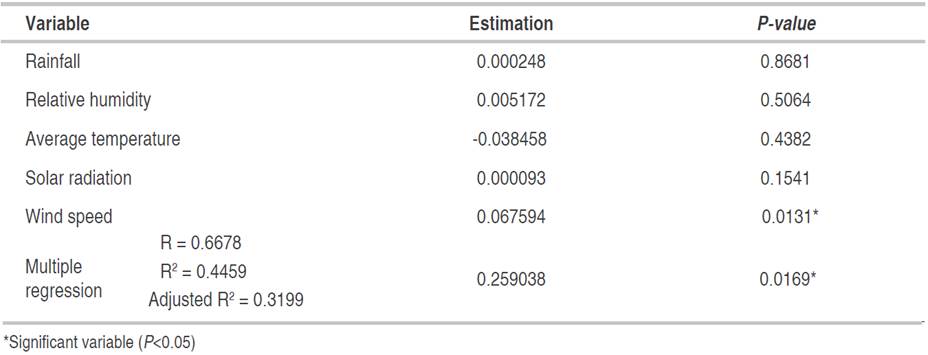
Table 10 Multiple regression between meteorological variables and the severity of Colletotrichum spp. in the foliage of P. pinnatum, registered from 26-03-2016 to 06-11-2016.
The temperature was the only variable that influenced the development of the disease in E. cyclocarpum, showing a negative correlation. Although an optimal temperature range of 25-30 °C is proposed for Colletotrichum spp. (Miles et al., 2013), the different fungal species present specific requirements (Lima et al., 2015). In this study, when the temperature was more favorable for the activity of the pathogen, an increase in the disease was evidenced. Nonetheless, to better understand the effect of temperature on the development of the disease, it would be necessary to carry out complementary research on the specific optimum temperature of the Colletotrichum species found in the forests of the Caribbean region.
In other interactions, in addition to temperature, relative humidity affects the expression of disease, such as those observed in Anthracnose caused by Colletotrichum spp. in avocado (Márquez, 2016), and in mango inflorescences (Páez et al., 2003). Likewise, correlations have been described between solar radiation and the number of lesions of C. gloesporioides in Stylosanthes scabra (Pangga et al., 2011), on the survival and production of conidia of Colletotrichum acutatum (Fracarolli et al., 2016), or the beginning of Colletotrichum lindemuthianum infections in beans (Pérez-Vega et al., 2010). Nevertheless, in the present study, this variable was not determining for the disease behavior.
For P. pinnatum, although the regression analysis indicated a relation between wind speed and level of disease (incidence and severity), the correlation was not significant. In contrast, disease incidence in E. cyclocarpum was correlated with wind speed. The effect of wind speed on diseases caused by Colletotrichum spp. is mainly related as a complementary mechanism of spore dissemination (Siddiqui and Ali, 2014). However, the correlation between disease increase and wind speed is not clear. According to Guyot et al. (2005), although strong winds can favor an increase in infections, this phenomenon does not necessarily produce a notable dispersal of spores.
In this study, no relationship was found between rainfall and disease progress under conditions of the Universidad del Magdalena campus. Similar results have been observed in the epidemiology of Colletotrichum spp. in mango (Páez et al., 2003) and in Heliconias cultivars (López-Vásquez et al., 2013). Some studies show that high rainfall does not necessarily favor the dispersal of Colletotrichum spp. spores. (Guyot et al., 2005). Even heavy rainfall can have a negative effect on the severity of Colletotrichum spp., due to the washing of the inoculum caused by the rains (Huertas-Palacios et al., 2009).
CONCLUSIONS
Foliar Anthracnose associated with Colletotrichum spp. in forest species varied with the period of the year and the host. During the months with the highest rainfall, the highest incidence and severity values were presented in E. cyclocarpum; however, in P. pinnatum, the maximum values were observed during the dry period. Likewise, the values of the development rate and the area under the disease progress curve indicated different behaviors in each pathosystem.
The progress of the disease was related to relative humidity, temperature, solar radiation and wind speed for E. cyclocarpum. In P. pinnatum, no correlation was found between environmental factors and disease level. It is highlighted that the phenological cycles of the host, which define defoliation and successive regrowth, influenced the initiation and development of multiple infective processes. It would be necessary to clarify the influence of the disease/pathogen presence in the host’s defoliation in further investigations.
Finally, the initial hypothesis was confirmed, since the behavior of foliar anthracnose caused by Colletotrichum spp. varied according to the forest species. This research provides the first epidemiological studies of Colletotrichum spp. associated with forest trees in the Colombian