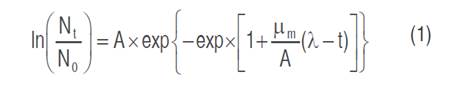The interest in plant fermentations has increased over time, since they constitute a mode of conservation that avoids the use of chemical additives or the implementation of preservation methods that may alter the physical and organoleptic characteristics of treated foods (Di Cagno et al., 2013). Brasicaceous or cruciferous have been used since ancient times in spontaneous fermentations and have become a model that allows the study of changes that occur during the process (Fusari et al., 2020; Seong et al., 2016). Spontaneous fermentations occur due to the action of natural epiphytic biota, mainly lactic acid bacteria (LAB). Plant biotype, region, climatic conditions, and growing conditions (Parkash et al., 2017; Tanyi et al., 2018) infIuence the quality of the biota, which makes it difficult to control the fermentation process and maintain the uniformity of the final product (Capozzi et al., 2017). In recent years, to avoid this drawback, the use of starters as a way to control the bioprocess has been proposed by several researchers (Johanningsmeier et al., 2007; Lillo-Pérez et al., 2021; Montemurro et al., 2021).
Several companies market products that contain selected LAB strains based on their biotechnology properties; however, they are not fated for specific vegetables or fruits (Di Cagno et al., 2013). Some food companies linked to the marketing of fermented vegetables have taken the decision to design and use native starters. This process involves the isolation and selection of strains with specific metabolic characteristics from a selected plant, for later use, under controlled fermentation conditions on the same substrate (Gu et al., 2012; Kim et al., 2019; Lee et al., 2020).
Kimchi, a traditional food of Korean culinary culture is the most cited and studied example in the use of native starters in controlled vegetable fermentations. Homemade kimchi is elaborated based on the spontaneous fermentation of Chinese cabbage where other vegetables such as radish, garlic, leek, pepper, and ginger are added (Jung et al., 2014).
In recent years its consumption increased worldwide, not only due to its organoleptic characteristics but also because of the beneficial effects on consumer health (Özer and Yıldırım, 2019). This phenomenon has increased the interest of Korean state authorities and food industries in introducing standardized protocols that include indigenous ferments to avoid the random quality of spontaneous fermentations (Kang and Lee, 2020). The main genera, among LAB, responsible for kimchi fermentation are the Leuconostoc, Lactiplantibacillus, and Weissella (Di Cagno et al., 2013; Jung et al., 2014). Therefore, these genera have been intensely studied to achieve a greater understanding of the influence it exerts on the quality of the product and the nutritional benefits. Among genus Leuconostoc, several species have been isolated from kimchi; some of them are marketed under trademarks as specific starters for the fermentation of this traditional Korean food. Leuconostoc miyukkimchii, Ln. kimchii and subspecies of Ln. mesenteroides have been used with success on an industrial scale (Chun et al., 2017; Lee et al., 2012; Oh et al., 2010).
Ln. mesenteroides ssp. jonggajibkimchii has been accepted as new subspecies since 2017, after the analysis of its complete genome sequence, and is currently marketed through Daesang Company, the patent owner (Jeon et al., 2017). The strain is recommended as a starter in kimchi elaboration due to the development of significantly excellent sensory properties compared to spontaneous fermentation. It is acid-resistant and synthesizes significant mannitol concentrations, a compound that increases the refreshing taste.
This work reports the first isolation of a Ln. mesenteroides ssp. jonggajibkimchii strain from the intestinal content Parona leatherjacket (Parona signata). The biochemical profile was compared with the type strains Ln. mesenteroides ssp. jonggajibkimchii DRC1506 and Ln. mesenteroides ssp. suionicum DSM20241. Exopolysaccharides production and the infIuence of temperature, salinity, and pH on bacterial growth were also studied. The evolution of bacterial population and pH was monitored in controlled fermentation of white cabbage and Chinese cabbage, and compared with the results obtained with Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1, a strain isolated from the spontaneous fermentation of red cabbage.
MATERIALS AND METHODS
Bacterial strains
The bacterial strain Tw234 was isolated from the intestinal content of Parona leatherjacket (Parona signata), collected in the Port Rawson bay, Chubut, Argentina (latitude -43.30016; longitude -65.10228). The strain Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1 (GenBank accession numbers: MT702992) belonging to Laboratorio de Biotecnología Bacteriana (Facultad de Ciencias Naturales y Ciencias de la Salud, Universidad Nacional de la Patagonia San Juan Bosco, Trelew) was used for comparative studies.
Phenotypic identification
The strain Tw234 was subjected to the following biochemical tests: Gram stain, catalase activity, oxidase activity, and gas production (CO2) from glucose (Björkroth and Holzapfel, 2006). The carbohydrate fermentation patterns were studied using the API 50 CHL system (BioMérieux, Lyon, France), following the manufacturer's recommendations.
InfIuence of temperature, pH and salinity on growth
The growth of the Tw234 strain was evaluated against different conditions of temperature, pH, and salinity. The growth was determined in Man Rogosa Sharpe broth (MRS) (Biokar, France) during 48 h of incubation at different temperatures (8, 10, 15, 20, 25, 30, 37, 40 and 45 °C) and pH values (3.0 to 9.0, at intervals of 0.5 pH units). Tolerance to NaCl was determined in MRS broth using concentrations between 1 and 8%, after 48 h of incubation at 30 °C.
Exopolysaccharides production
Exopolysaccharide (EPS) production was assessed on Brain Heart agar (Biokar, France) supplemented with Congo Red 0.8 g L-1 and sucrose 50 g L-1 (Freeman et al., 1989). The plates were incubated at 18 °C for 5 days. Exopolysaccharide production is detected by the black color development of colonies in the medium.
The solidification of fermentation milk supplemented with 3, 6, 9, and 12% (w/v) sucrose was used as a complementary assay (Wang et al., 2019). Skim milk without sucrose was used as a control. Incubation was carried out at 30 °C for 48 h and 8 and 10 °C for 5 days.
Genotypic identification
The strain was incubated in MRS broth at 30 °C for 18 h and was centrifuged at 12,000 g at 4 °C for 5 min; the total genomic DNA was extracted using Wizard Genomics kits (Promega, Madison, Wisconsin, USA) following the manufacturer’s instructions. For identification of isolated strain, two universal primer pairs, 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTACCTTGTTACGACTT-3') were used for amplifying the 16S rRNA gene in a Multigene Gradient thermal cycler (Labnet International Inc., USA). The amplified DNA was sequenced using the Macrogen sequencing service (Macrogen Inc., Seoul, Korea). The 16S rRNA gene sequence was compared with 16S rRNA gene sequences in NCBI’s GenBank using BLAST (Basic Local Alignment Search Tool https://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al., 1990).
Phylogenetic analysis
The phylogenetic tree was constructed using the Neighbor-Joining method (Saitou and Nei, 1987) with software package MEGA version 6.0 (Tamura et al., 2013). Tamura-Nei substitution was the model used, with a bootstrap value of 1,000 replicates. The resultant 16S rDNA sequence of the isolate was aligned against representative sequences of collection strain obtained from the database Ribosomal Database Project (RDP) and the National Center for Biotechnology Information (NCBI). The Weissela confusa sequence was chosen as an outgroup strain due to its phylogenetic relationship with the Leuconostoc genus.
Plant material and fermentation process
The Chinese cabbage (Brassica rapa L. var. glabra, Regel) and white cabbage (B. oleracea L. ssp. capitata, Metzg.) were obtained from the farm of the Valle Inferior del Río Chubut, Patagonia Argentina (latitude -43.14, longitude -65.19, 11 masl). Before the preparation of vegetables for fermentation, dry outer leaves of the first group of bulbs were removed. The cleaned bulbs were chopped in a shredder into 2 mm thick strips and supplemented with NaCl 3% (w/w). Shredded cabbages were autoclaved at 121 °C for 3 min.
The strains Tw234 and Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1 were individually used as starters, according to the method described by Xiong et al. (2014) with some modifications. The strains were cultivated in MRS broth for 18 h at 30 °C to reach a concentration of 109 CFU mL-1, later were centrifuged (4,000 g, 15 min). The cell pellets were washed twice and resuspended in distilled water. The vegetables were individually inoculated with the starters reaching a final population of approximately 104 CFU g-1 and incubated at 18 °C for 96 h.
Fermentation parameters
The pH and LAB growth were monitored at 0, 2, 12, 18, 24, 36, 48, 72, and 96 h. The pH determination was carried out with a calibrated pH-meter (Orion 410). The growth was monitored on MRS agar using serial dilutions of sauerkraut brine samples; the plaques were incubated at 30 °C for 48 h (Lanza et al., 2020). The growth data of the strains RCTw1.1 and Tw234 obtained during the controlled fermentation were fitted using the Gompertz equation (1):
Where Nt is the number of microorganisms (CFU mL-1) at time t (h), N0 is the number of microorganisms at the inoculation time. Bacterial growth parameters are: A asymptotic value, µm the maximum specific growth rate (h-1), and λ the lag time (h) (Biesta-Peters et al., 2010). The experimental data were fitted by nonlinear regression using STATISTICA software (Statsoft, Tulsa, Oklahoma, USA). The coefficient of determination (R2 adj) and root mean square error (RMSE) were used as criteria for adequacy of fit.
Statistical analysis
The fermentations were carried out in duplicate. The results were subject to a one-way analysis of variance (ANOVA). The pairs of means of the treatments were compared applying the Tukey test (P≤0.05), using the statistical package STATISTICA software (Statsoft, Tulsa, Oklahoma, USA).
RESULTS AND DISCUSSION
The general biochemical characteristics exhibited by the Tw234 strain correspond to those described for the genus Leuconostoc: catalase-negative, oxidase-negative, gram-positive cocci arranged in pairs or short chains, heterofermentative and vancomycin-resistant (30 µg mL-1) (Chun et al., 2017).
The growth on MRS broth of the Tw234 strain was observed at temperatures between 8 and 40 °C, with an optimum growth temperature between 30-32 °C, but no growth was observed below 8 and at 45 °C. At 8 °C there was a remarkable difference compared with the collection strain Ln. mesenteroides ssp. jonggajibkimchii DRC1506, which does not exhibit growth at temperatures below 10 °C according to its description (Jeon et al., 2017). The strain Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1, belonging to our collection and isolated from spontaneous fermentation of red cabbage, exhibited growth at 8 °C similar to the studied strain. This metabolic feature, perhaps derived from the selective pressure exerted by the Patagonian marine environment’s low temperatures, has potential application in the food industry. For instance, the organoleptic characteristics of kimchi depend on the processing temperature and have been demonstrated to be superior when the fermentation process occurs at temperatures below 10 °C. Tw234 y RCTw1.1 strains displayed growth between 3.5-8.5 pH values while the type strain DRC1506, according to its description, showed a growth pH range of 4.0-9.0. NaCl tolerance values of Tw234 and RCTw1.1 strains were recorded in concentrations from 0 to 6%, as those values reported for the strain type DRC1506 (Jeon et al., 2017).
Exopolysaccharides have varied applications in different industries as they can be used as gelling agents, bio-fIocculants, stabilizers, or emulsifiers. In the particular case of food, its production during fermentation increases viscosity and improves the final texture of the product (Wang et al., 2019). The Tw234 strain was able to produce exopolysaccharides at 30, 10, and 8 °C, when the test was conducted in BHI agar, supplemented with sucrose and Congo red. Exopolysaccharides production was also detected when the milk solidification test was performed on all sucrose concentrations assayed. Solidification was achieved after 48 h of incubation when the test was performed at 30 °C, while the same effect was detected, after five days, when the test was carried out at 8 °C.
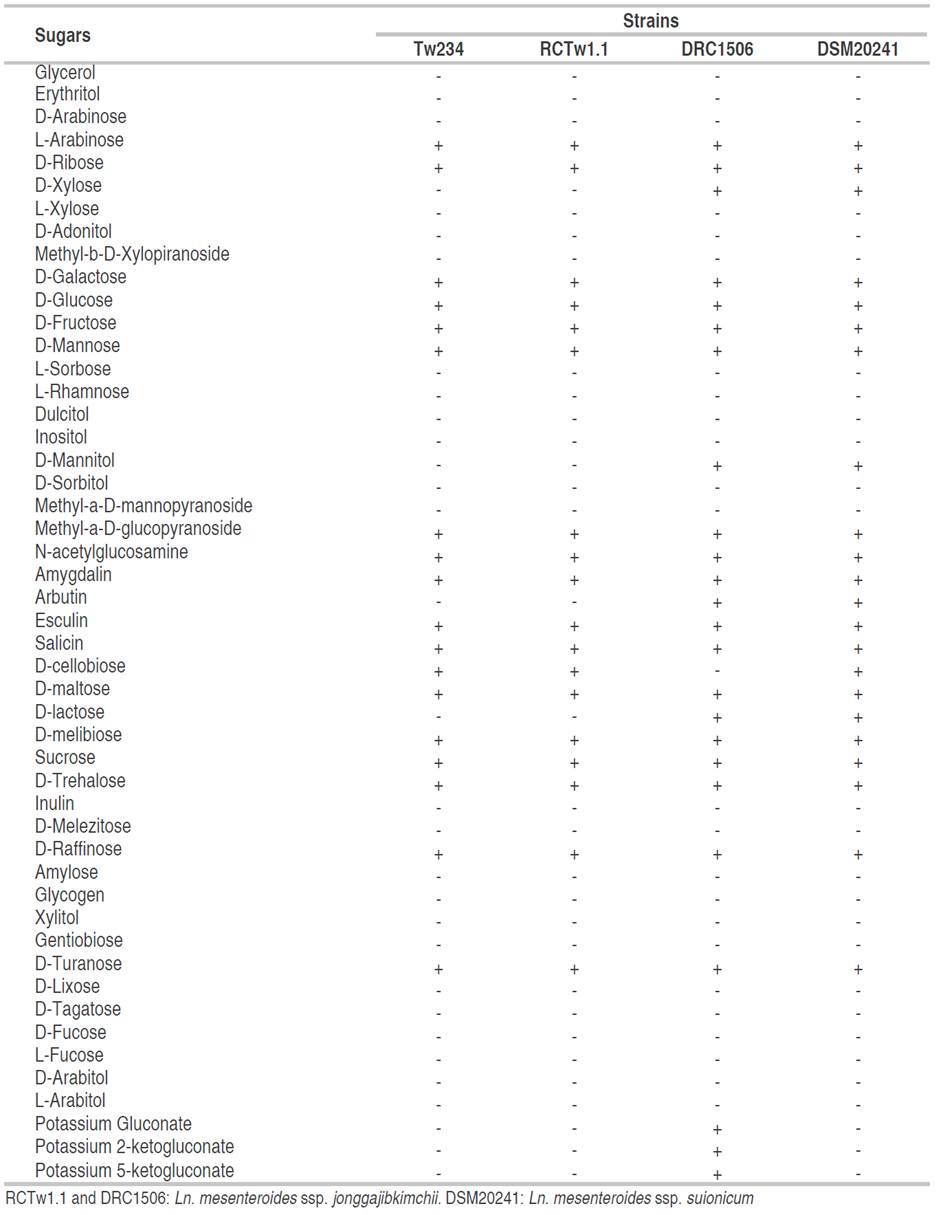
In Table 1 can be observed the fermentation profile of the Tw234 strain, Ln. mesenteroides ssp. jonggajibkimchii DRC1506, Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1, and Ln.mesenteroides ssp. suionicum DSM 20241. The latter strains were included because they are phylogenetically very close to Ln. mesenteroides ssp. jonggajibkimchii, and were reclassified as representing two different subspecies in 2017, after the complete sequencing of both genomes (Gu et al., 2012; Jeon et al., 2017).
Tw234 and RCTw1.1 strains displayed the same fermentation profile. Unlike the collection strains DRC1506 and DSM20241, both strains did not use xylose, mannitol, arbutin, and lactose as carbon sources. Tw234 and RCTw1.1 strains were able to ferment D-cellobiose, as described for the DSM20241 strain but not for the DRC1506 strain.
A remarkable data derived from the same information source indicates that the strain type DRC1506 can ferment gluconate, 2-ketogluconate, and 5-ketogluconate as potassium salts. This physiological trait is not described in any of the subspecies of Ln. mesenteroides (Chun et al., 2017). The Tw234 and RCTw1.1 strains, as well as the Ln.mesenteroides ssp. strain DMS20241 were not able to use the sugars as mentioned earlier as energy sources.
Phylogenetic tree analysis based on 16S rRNA gene sequences shows that strains Ln. mesenteroides ssp. jonggajibkimchii DRC1506, Tw234 and RCTw1.1 are grouped in the same clade and very close to the other subspecies of Ln. mesenteroides (Figure 1). 16S rRNA of the Tw234 strain (1233 bp) exhibited 100% homology with the sequence of the strain type Ln. mesenteroides ssp. jonggajibkimchii DRC1506, using the BLAST program (Basic Local Alignment Search Tool). The cited type strain can be found in the Japanese Collection of Microorganisms under the JCM 31787 denomination or in the Korean Culture Center of Microorganisms under the KCCM 43249 denomination. The partial sequence of the gene 16S rRNA of the strain under study was deposited in the GenBank under the name Ln. mesenteroides ssp. jonggajibkimchii Tw234 (access number: MN831890.1).
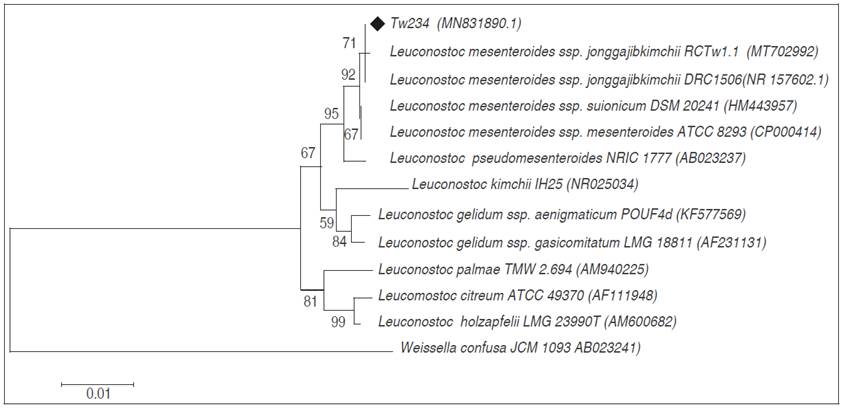
Figure 1 Phylogenetic tree constructed by Neighbour-Joining method based on the relationship between the 16S rRNA gene sequences of strain Tw234 (♦) and related species with the genus Leuconostoc. The numbers at internal nodes are bootstrap support values (≥70%). GenBank accession numbers are given in parentheses. The 16S rRNA sequence of Weissella confusa was chosen arbitrarily as the outgroup sequence (bar, 0.01 substitution per nucleotide position).
Figure 2a displays the change in pH values in Chinese cabbage and white cabbage fermentation when the process was carried out by the Tw234 strain. The values remained relatively stable for the first 12 h of the process and then dropped until stabilized at 48 h. Chinese cabbage and white cabbage reached pH values of 4.27 and 3.89, respectively, remaining stable until 96 h.
Figure 2b shows the changes that occurred when the process was performed with the RCTw1.1 strain. The pH decline began at 6 h and stabilized at 48 h, reaching values of 4.23 in Chinese cabbage and 3.69 in white cabbage. The maximum acidification rates of the Tw234 strain were 0.047 and 0.074 pH h-1 units for Chinese and white cabbage, respectively, while values of 0.037 and 0.068 pH h-1 units were determined for the RCTw1.1 strain.
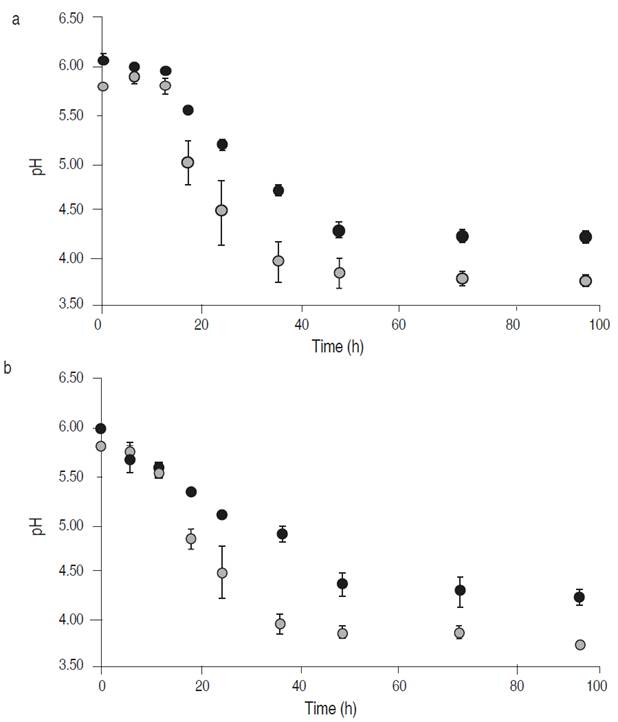
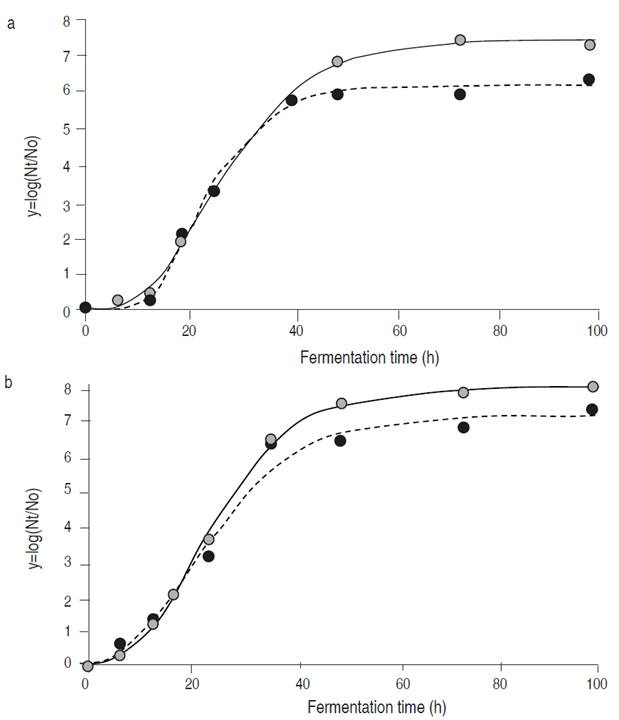
Figure 2 Changes in pH values during fermentation of white cabbage (•) and Chinese cabbage (•) inoculated with the strains Tw234 (a) and Ln. mesenteroides ssp. jonggajibkimchii RCTw1.1 (b). Each value is the mean ± standard deviation of two measurements.
The final pH values and maximum acidification rates are comparable in both strains. The differences observed when comparing the evolution of the two vegetables’ parameters are due to the different supply of sugars that are at a much higher concentration in white cabbage compared to Chinese cabbage (USDA, 2020).
The final pH and acidification rate values obtained in the fermentation of white cabbage using the Tw234 strain, are comparable to those reported by Johanningsmeier et al. (2007) when the Ln. mesenteroides strain LA 81 (ATCC 8293) was used as a starter. Previous reports recommend, to obtain good quality kimchi, the use of strains that adapt to the environment generated during the fermentation of Chinese cabbage (low temperatures, low pH, and presence of NaCl) and also, that the final pH does not drop below 4.2 (Ick, 2003; Jung et al., 2014). The Tw234 strain exhibits all these characteristics, may be of interest to the food industry, its potential use as a starter in producing foods that include the cited cruciferous.
Several microbial growth models are found in the literature, such as the Baranyi, Logistic, and Gompertz models (Zwietering et al., 1990). In this study was used Gompertz model, regarded as the most suitable model to describe microbial growth curves due to its simplicity and interdependence of the parameters. Growth curves of the strains Ln mesenteroides ssp. jonggajibkimchii Tw234 and RCTw1.1 were satisfactorily modeled using the Gompertz equation (1) cited above, obtaining R2 adj values between 0.98 and 0.99, and low RMSE values (0.13-0.36) (Table 2).
Table 2 Value of parameters obtained by non-linear regression of Gompertz equation (1) for the growth of the strains Ln. mesenteroides ssp. jonggajibkimchii Tw234 and RCTw1.1 under study.
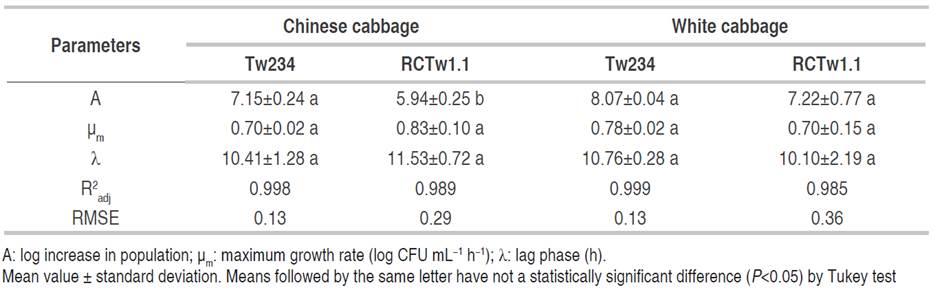
In Figure 3, experimental data and growth curves modeled for both strains can be observed during the fermentation of Chinese cabbage and white cabbage. The estimated parameters µm (specific growth rate) and λ (latency phase duration) showed no statistically significant differences between the strains studied (P>0.05). The asymptotic value (Figure 3a) showed significant differences among cultures only in Chinese cabbage (P<0.05), getting the strain Tw234 (log10 7.15±0.24) a higher increase in population than the strain RCTw1.1 (log10 5.94±0.25).
CONCLUSIONS
In recent years, the search and selection of LAB based on technological, sensory, and nutritional characteristics for application in controlled plant fermentations have been developed with intensity.
The goal of the selection of strains for the controlled production of fermented vegetables is to obtain a uniform quality product that avoids variations in spontaneous processes. The patent for Ln. mesenteroides ssp. strains jonggajibkimchii DRC1506 and Ln. mesenteroides ssp. suionicum DSM20241 marketed by Korean company Daesang for kimchi production is an example of this trend. In this work, the first isolation of a strain Ln. mesenteroides ssp. jonggajibkimchii from the intestinal content of Parona leatherjacket (Parona signata) were reported. The strain exhibits comparable characteristics with the cabbage isolated strain RCTw1.1 and the type of strain Ln. mesenteroides ssp. jonggajibkimchii DRC1506. This study also demonstrated that the strain Tw234 exhibits the metabolic characteristic of interest to the food industry as growing at low temperatures and exopolysaccharides production. Moreover, the evaluated kinetic parameters in vegetable matrices were similar between the RCTw1.1 and Tw234 strains, achieving this latter a better fit to the kinetic model. These properties make Ln. mesenteroides ssp. jonggajibkimchii Tw234 a potential candidate to be used as a starter in controlled fermentations. Leuconostoc and related species start the first stage of vegetable fermentation; therefore, only the metabolic traits related to this phenomenon were investigated in this work. However, new technological features such as synthesis of antimicrobial compounds, increase of the antioxidant activity, behavior at industrial scale, and combination with other strains in two-stage controlled fermentation should be approached in future investigation.