Qualitative and quantitative losses of fresh products occur in all stages (from harvest to final delivery to the consumer) (Benichou et al., 2018). To reduce these losses, producers and operators must prioritize the biological and environmental factors involved in degradation and adopt postharvest techniques that delay aging and maintain the best possible quality. Apricots have a short postharvest life and are very sensitive to damage due to their soft and juicy texture. This fruit has a high nutritional value, but due to the rapid softening of the fruit texture, it has a short shelf life and loses its quality in a short time (Moradinezhad and Dorostkar, 2020). The increased demand for fruits in the international markets implies continuous research and improvement of technologies to maintain quality and extend the shelf life of produce (Benichou et al., 2018).
It is well known that calcium plays a major role in maintaining the quality of fruit and vegetables (Singh et al., 2021; Oliveira et al., 2016; Boshadi et al., 2018). The immersion method can be more useful than spray to apply postharvest treatments (Ghasemi et al., 2021). Postharvest calcium has been demonstrated to maintain membrane integrity and firmness of fruit tissue, which increases the shelf life of fresh produce (Moradinezhad et al., 2019; Li et al., 2020). Manganaris et al. (2007) examined the postharvest effect of calcium salts on peach fruit. They showed that pulp calcium increased by 74% and reduced the severity of cold injury symptoms, ethylene levels, pectin polygalacturonase, and pectin methylesterase. Another study showed that the postharvest application of 3% calcium chloride on apricot fruit not only maintained sensory properties but also increased the strength and durability of the cell wall and also significantly reduced the microbial load (Sartaj et al., 2013).
Shahroudi apricot cultivar is one of the important commercial cultivars of Iran. However, there is scarce information about the effect of postharvest calcium salts solution dipping on the physicochemical properties of apricot fruit cultivar 'Shahroudi'. Therefore, this study aimed to investigate the effect of postharvest application of different calcium salts (calcium chloride, calcium nitrate, and calcium sulfate) on the quality of 'Shahroudi' apricot fruit.
MATERIALS AND METHODS
Plant material
Apricots of the cultivar Shahroudi were harvested in the east region of Iran at the commercial maturity stage (total soluble solids about 11% and firmness 20N). This research was conducted late in May 2018. The fruits were then transferred to the postharvest laboratory, Faculty of Agriculture, University of Birjand. 200 fruits (weighing about 55 g) were considered for this experiment. After sorting, the fruits were washed with distilled water to remove any dust.
Treatments
Apricot fruits were divided into seven groups and then dipped in water or solutions of calcium salts at 25 °C for 2 min as follows:
[1] Distilled water (control).
[2] Calcium chloride solution 1% (CaCl2 1%).
[3] Calcium chloride solution 2% (CaCl2 2%).
[4] Calcium nitrate solution 1% (Ca(NO₃)₂ 1%).
[5] Calcium nitrate solution 2% (Ca(NO₃)₂ 2%).
[6] Calcium sulfate solution 1% (CaSO₄ 1%).
[7] Calcium sulfate solution 2% (CaSO₄ 2%).
All treated fruits were allowed to air dry at room temperature (25 °C) for 1 h. Thereafter, the apricots were placed in polyethylene containers (dimension: 9×9×12 cm) with 500 mL volume and wrapped with double-layer cellophane, and then stored in a cool room at 2±1 °C with 85±5% relative humidity for three weeks. Important parameters such as temperature and relative humidity that directly affect the results were controlled during the experiment. All traits (except shelf life) were measured and evaluated after 3 weeks.
Firmness, total soluble solids (TSS), and titratable acidity (TA)
To measure the firmness of fruits, a digital penetrometer (Fruit Hardness Tester, Model FHT 200, Extech Co., USA) and a 2 mm probe were used. Data were presented in Newton units. Total soluble solids in fruit juice were measured using a hand-held refractometer (RF 10, Brix 0-32%, Extech Co., USA), and data were expressed as percentages (%). The titration method was used to evaluate the titratable acidity expressed as the percentage of malic acid.
Total phenolic content (TPC), calcium content, and fruit shelf life
Total phenolic content was determined using the Folin-Ciocalteau method. The absorbance at 755 nm was measured using a spectrophotometer (Bio Quest, CE 2502). The final results were expressed as mg of Gallic acid (EAG) 100 g-1 of fresh apricot. The calcium content of fruit tissue was determined using the acid digestion method. The calcium ion level was measured with an atomic absorption spectrophotometer, and the results were expressed as mg Ca 100 g-1 dry weight (DW).
Fruit shelf life was assessed using visual observations and acceptability for presentation to the consumer. This parameter was expressed in terms of days (Moradinezhad and Jahanani, 2016).
Experimental design and statistical analysis
This experiment was carried out in a completely randomized design with seven treatments and four replications. Statistical analysis was performed with GenStat (Discovery Edition, version 12.1, 2009, VSN, International, UK) and Excel. Also, the LSD test at the level of 1% (P˂0.01) was used to identify significant differences between means. In the present study, mean ± standard error was used to present the data better.
RESULTS AND DISCUSSION
Firmness
Dipping apricots in different calcium salts had a significantly affected on maintaining tissue firmness compared to control samples (Figure 1). At the end of the storage period, it was found fruits treated with CaCl2 at 2% had the highest (18.1N) firmness while control fruit exhibited the lowest (10.6N) firmness. However, firmness was 20 N on the first day (see materials and methods) and these results showed that 2% CaCl2 treatment had the least change in tissue firmness compared to the day of harvest. On the other hand, evaluations showed that by increasing the concentration of all used calcium salts from 1% to 2%, the firmness of apricot fruit tissue was significantly maintained.
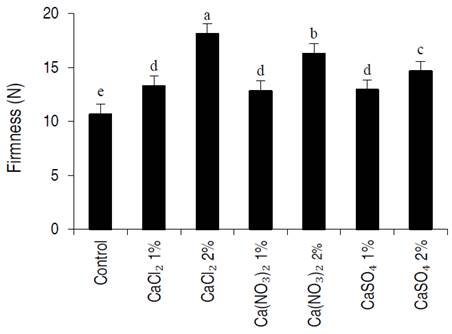
Figure 1 Effect of dipping in different calcium salts solution on the firmness of apricot fruit stored at 2±1 °C for 21 days. CaCl2 (calcium chloride), Ca(NO3)2 (calcium nitrate), CaSO4 (calcium sulfate).Error bars represent the error deviation. Symbols with the same letter are not significantly different between them at P˂0.01 (LSD test).
It has been proven that ethylene production in fruit tissue (climacteric fruits) causes the fruit to ripen and soften. In the report on apple fruit, Mohebbi et al. (2020) showed that postharvest application of calcium chloride reduced ethylene production and subsequently increased the firmness of fruit tissue; they stated that lower ethylene production in Ca-treated fruit may be due to its contribution to the maintenance of cell membranes, delaying ACO-catalyzed conversion of ACC into ethylene. An important function of Ca in plants is to increase the rigidity of the cell wall and to promote the cohesion of neighboring cells. This positive effect of calcium on firmness has been widely reported by other researchers (Ortiz et al., 2011; Moradinezhad and Jahanani, 2016).
On the other hand, the results showed that among different calcium salts, calcium chloride (2%) maintained fruit tissue firmness more than calcium nitrate, and calcium sulfate. This effect is probably due to the formation of calcium pectate through the reactions of calcium with pectic acid, calcium chloride increases the molecular bond between cell wall components more than other calcium salts and strengthens the pectin chain. This scenario was consistent with the results of Manganaris et al. (2007) using peaches.
Total soluble solids (TSS)
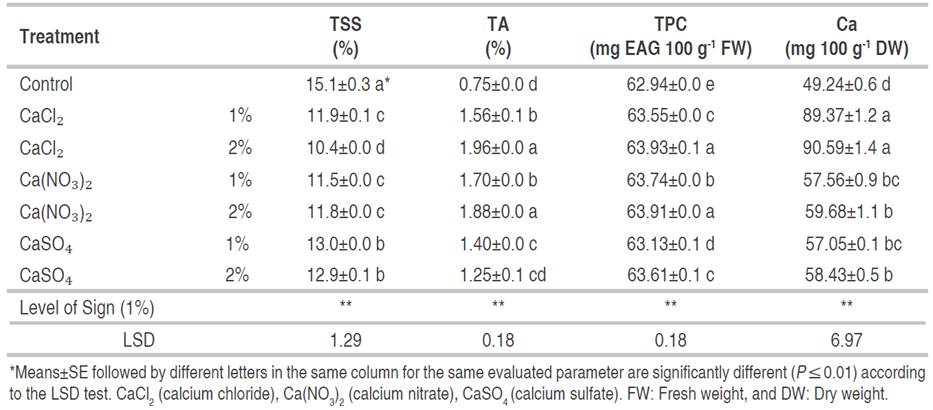
According to Table 1, calcium salts treatment had significant effects on the TSS. In all treatments, TSS was lower compared to control fruits, and the lowest TSS was obtained from the 2% CaCl2 treatment.
Table 1 Effect of dipping in different calcium salts solution on total soluble solids (TSS), titratable acidity (TA), total phenolic content (TPC), and calcium content of tissue of apricot fruit stored fruit at 2±1 °C for 21 days.
During storage and ripening, starch present in the fruits converts slowly and gradually into sugar. Sajid et al. (2019) reported that calcium changes the function of enzymes such as pectinase, methylesterase, and polygalacturonase. It seems that calcium treatment can delay ripening, senescence, and respiration rate, which reduces the amount of total soluble solids. Since calcium slows down respiration and metabolism and also slows down the hydrolysis of polysaccharides to monosaccharides, it leads to the delay of ripening and reduces the TSS of fruit during storage (Ranjbar et al., 2018).
Titratable acidity (TA)
After 21 days of storage, the TA in all treated fruit increased compared to the control samples. The results showed that the postharvest application of calcium salts had a significant effect on the TA of apricot fruit. The highest TA value was obtained from CaCl2 2% (1.96%) and Ca(NO₃)₂ (1.88%) treatments and, the lowest value was observed in control samples (0.75%) (Table 1).
The titratable acidity is directly related to the concentration of organic acids present in the fruit, which are an important parameter in maintaining the quality of fruits. In apricot, malic acid is the principal acid. It has been proved that acidity decreases during ripening due to the breakup of acid into sugars. Liu et al. (2017) reported that calcium chloride maintains the titratable acidity of the fruit; they stated that calcium treatment could reduce acid oxidation. Similarly, Ranjbar et al. (2018) on apple and Ishaq et al. (2009) on apricot fruit reported that the calcium chloride application maintained acidity during storage.
Total phenolic compounds (TPC)
As shown in Table 1, all postharvest calcium salts dips had a significant effect on the TPC of apricot fruit. The highest TPC values were obtained using CaCl2 and Ca(NO3)2 treatments at 2%.
Phenolic compounds in fruits and vegetables can protect cells against oxidative injury through scavenging free radicals. Jacobo-Velázquez et al. (2011) suggested a model or the mechanism of action of calcium on phenol content. They stated that cytosolic ATP is released in stressed and damaged cells. This ATP is received by membrane receptors. This process signals and produces activated oxygen, which in turn increases the rate of mitochondrial respiration. Increased reactive oxygen activates phenol synthesis pathways. Accumulation of phenolic molecules in plant tissues is done to neutralize and scavenger free radicals (antioxidants). Also, Ranjbar et al. (2018) reported that calcium increases the amount of total phenol in apple fruit; since calcium rises the polysaccharide content in the fruit cell wall and the permeability of the membrane, therefore, the wall strength, and cell membrane can be maintained, which lead to preventing the oxidation of phenolic compounds. Similar to the present study, CaCl2 (2%) treatment significantly enhanced total phenols content in apricot fruits, suggesting that CaCl2 (2%) and Ca(NO3)2 (2%) might be an efficient strategy to improve total phenols content in fruits such as apricot, which increases the nutritional value of this fruit.
Calcium content of fruit
As expected, all calcium salts treatments increased the calcium content of apricot fruit compared to the control. The highest value of calcium was obtained in CaCl2 (1 or 2%) treatment. Also, in other treatments, the calcium content of fruit increased with an increment in concentration from 1 to 2% of applied salts (Table 1).
It has been proved that calcium is an essential substance in maintaining the structure of the cell wall, also; it is well known that it reduces ethylene and delays the ripening process of products (Mohebbi et al., 2020). Calcium treatment is one of the safe treatments that, in addition to maintaining the quality of the fruit, it is also non-toxic. Similarly, Madani et al. (2016) reported that using calcium (spray) on papaya fruits significantly increased the calcium content of the pulp and peel of the fruit. They exposed a marked increase in calcium content in papaya fruit suggesting that exogenous calcium might be able to cross the fruit epidermis and incorporate it into the cell wall matrix of fruit cells.
Shelf life
The shelf life of apricot fruits was evaluated during cold storage. As shown in Figure 2, the shelf life in all calcium treatments was extended compared to the control. However, the highest shelf life was obtained using CaCl2 at 2% treatment (35.33 days), while the shelf life of the control samples was only 15.67 days.
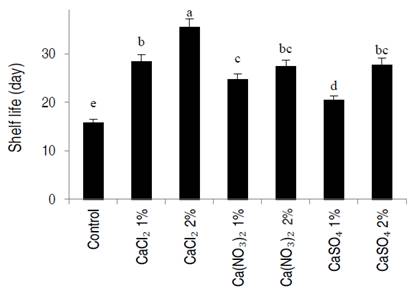
Figure 2 Effect of dipping in different calcium salts solution on the shelf life of apricot fruit stored at 2±1 °C for 21 days. CaCl2 (calcium chloride), Ca(NO3)2 (calcium nitrate), CaSO4 (calcium sulfate). Error bars represent the error deviation. Same letter are not significantly different between them, at P˂0.01 (LSD test).
Fruit shelf life is dependent mainly on firmness and cell wall composition. During apricot postharvest storage, firmness gradually decreases while pectin is gradually depolymerized and degraded (Gao et al., 2020). Liu et al. (2017) reported that apricot treatment with 1% calcium chloride followed by cold storage at 5 °C extends shelf life, maintains a firmer texture, and reduces the degradation of cell wall polysaccharide main and side chains in apricot fruit. The results of this study showed that treatment with three forms of calcium salts had a positive effect on the postharvest quality and prolonged shelf life of apricots. Also, the shelf life results agreed well with those from tissue firmness and decay, suggesting that the short shelf life was related to tissue cell wall endurance. Previously, Moradinezhad and Jahani (2016) have also described similar views that calcium application delays extended shelf life and retards decomposition during cold storage in apricot fruit.
CONCLUSION
In conclusion, we found the positive effect of calcium chloride (CaCl2) and calcium nitrate (Ca(NO₃)₂) postharvest application on enhancing the total phenols of apricot fruit 'Shahroudi' cv. was accompanied by increased firmness, the calcium content of pulp, and titratable acidity. Also, results showed that the application of the mentioned calcium salts (2% CaCl2 and 1% Ca(NO₃)₂) significantly extended the apricot fruit shelf life. Therefore, these treatments may be recommendable for practical use in this kind of fruit.















