Citrus plants are one of the most important fruit crops all over the world (Wu et al., 2018). Citrus fresh fruits and orange juice are common components of daily diets due to their high levels of vitamin C, folate, flavanones, hesperidin, and naringin, and numerous reported health benefits (Inglese and Sortino, 2019). Production of citrus in the world in 2018 was around 150 million t where oranges accounted for 50%, mandarins 22%, lemons, and limes 12% and other citrus fruits 6%. In Colombia, production in 2018 was estimated at 75000 t distributed in 20% oranges, 13% mandarins, 14% limes, lemons, and 52% other citrus fruits (FAOSTAT, 2020). In the Córdoba department, citrus fruit production, in the same year, was 1150 t, being "Valencia" orange production more than 90% (Agronet, 2021). A worldwide shortage in citrus plant supply is happening due to strict regulations during the propagation process to prevent the spread of diseases such as CTV (Citrus tristeza virus), CEVd (Citrus Exocortis Viroid) and HLB (Huanglongbing) (Wang, 2021; Vashisth et al., 2020; Folimonova, 2020; ICA, 2019). Citrus orchards are generally established with grafted plants to combine rootstock and cultivar benefits, to ensure fruit quality and uniformity, and to reduce the time for harvesting. (Talon et al., 2020; Barón et al., 2019). Minigraft is a clonal propagation technique that uses young rootstocks to be grafted with small size scion/bud parts to obtain younger plants fully adapted to field conditions, avoiding the maintenance of large size plants for scion production and the ex vitro acclimatization stage of the micropropagation process (Siqueira et al., 2016). Out of the hundreds of grafting techniques, citrus plants are usually grafted by T-budding, a time-consuming process where a bud is removed from the desired cultivar and inserted underneath of the rootstock cortex to promote callus growth and vascular connection between the two parts; the whole process may last for 24-36 months for plants to be ready for field planting (Alves et al., 2019; Widaryanto et al., 2019). Recent studies on citrus propagation focus on speed up the propagation process and increasing the number of plants produced while complying with the official regulations (Pokhrel et al., 2021; Solonia et al., 2020). The present research aimed to evaluate two types of minigrafts, cleft and T-budding, their viability and success level on the propagation of "Valencia" orange plants as a way to obtain a faster and cost-effective citrus propagation protocol.
MATERIALS AND METHODS
The experiment was carried during the year 2019-2020 in a shade house of the Institute of Applied Biotechnology for the Caribbean of the Universidad de Córdoba (Monteria, Colombia), located at 8° 31'N and 75° 58' W with an elevation of 12 masl.
Rootstock growth
Seeds for rootstock production were extracted from horticulturally ripened Cleopatra mandarin (Citrus reshni hort. Ex Tanaka) fruits harvested from field grown trees at the Universidad de Córdoba - Berastegui Campus (8°40'26" N 75°46'44" W). Fruits were washed twice with distilled water, hand-squeezed and seeds separated with a plastic sieve. The extracted seeds were profusely washed with sterile-distilled water, air-dried overnight on filter paper, and stored in sterile closed glass flasks for 4 weeks in a conventional fridge at 8 °C. Germination occurred after seeds were sown in plastic tube containers (15×5 cm) filed with peat as substrate. Seedlings were maintained under shade house conditions with a 50% saran light for 6 months with fog irrigation twice a day for 1 min each.
Bud and scion selection
Grafting material was isolated from 2-year-old grafted plants of Valencia orange (Citrus x sinensis Osbeck) obtained from an authorized citrus plant distributor (Reg. ICA 25290-06V). Plants were maintained in a shade house (50%) with fog irrigation twice a day for 1 minute each.
Minigrafting and plant growth
To evaluate grafting success percentage, two types of graft, cleft, and T-budding, were performed on 6-month-old rootstocks and, approximately, 20 cm high. For cleft grafting, the rootstock was decapitated at 5-7 cm high, and a vertical downward cut was done in the center of the decapitated stem using a sterile scalpel. The scion, 2-3 cm long tender tissue containing at least one node, was cut from both sides at the basal end with the scalpel into a gently sloping wedge (~0.5 cm) where the cambium vascular tissue was observed. The scion was properly inserted in the rootstock cut, firmly tied with Nescofilm®, and top covered with a plastic 1.5 mL Eppendorf tube for two weeks. For inverted T-budding, a 1-2 cm vertical downward cut was done with a scalpel in the rootstock stem at about 5-7 cm high, and down terminated with a perpendicular horizontal cut. The bud was removed from young-tender stem shoots by cutting at about 0.3 cm below the bud with the scalpel and making a slicing cut down under the bud finishing about 0.5 cm beyond the but point. The bud piece was inserted by pushing it upward under the two flaps of the rootstock cut and thereafter firmly tied with Nescofilm®. Two weeks after the grafting, for plants where the scion was viable (green), the rootstock stem was chop-down about 1 cm above the grafting, and once the scion bud began to grow the rootstock stem above de scion was completely removed. Grafted plants were maintained in a shade house (50%) with fog irrigation twice a day for 2 min each. The experiment consisted of a one-way factor with two treatments (Cleft and T-budding minigrafts) and 100 replicates per treatment for a total of 300 experimental units, which were distributed with a completely randomized design. Six weeks after the grafting, for each treatment, the number of successfully grafted plants, the average length of the grafted scion shoot, and the average number of fully expanded leaves were registered and analyzed with a T test (α=0.05).
RESULTS AND DISCUSSION
Minigrafting and plant growth
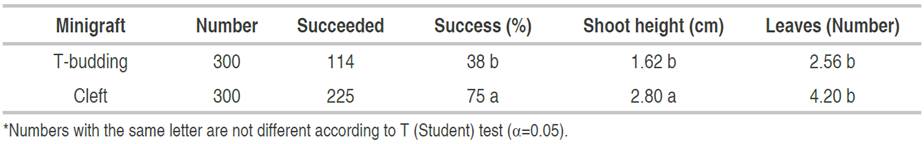
The success of grafting was observed in plants propagated using cleft and T-budding minigrafts (Figure 1). Valencia orange plants propagated with cleft minigraft showed a 75% success while plants propagated by T-budding minigraft resulted in 38% success; the T-test showed that the number of successful cleft minigrafts was statically higher (P=7.77×10-6) than the number of T-budding successful minigraft. In the same way, plants propagated using cleft minigraft showed a significant (P=0.0483) increase in shoot height, and a statistically (P=0.0005) higher number of leaves, compared to plants propagated using T-budding minigrafts (Table 1). Grafted recovered plants showed no morphological abnormality or deficient growth during the evaluation period.
Table 1 Percentage of success, shoot height and leaf number of Valencia orange plants propagated using T-budding and cleft minigrafts.
Grafting is a plant propagation technique used for centuries, especially on evergreen plants (Barón et al., 2019). Massive propagation of citrus plants for crops is based on T-budding 40-50 cm rootstocks with scion buds of specific cultivars, in an 18-24 months process to obtain plant material ready to plant in field crops, after mother plants have been carefully selected (Kamanga et al., 2017). Attempts to speed up the process include the use of tissue culture techniques such as rootstock production through micropropagation (Vashisth et al., 2020) and in vitro micrografting; however, low multiplication rates and time for ex vitro plantlet recovery are still a challenge (Chamandoosti, 2020; Sangma et al., 2020). The success of grafting is founded on a vascular reconnection between rootstock and scions, a process that involves hormones, molecular factors, and even whole genome transfer at the grafting area (Rasool et al., 2020; Gautier et al., 2019). Xylem tissue formations, callus proliferation at the graft union, and vascular bundles fiber growth are reported to be regulated by auxins, cytokines, and gibberellins during graft formation (Sharma and Zheng, 2019). An increased accumulation of stilbene metabolites at the graft union as a result of a re programming of the metabolome at the graft interface to support wounding stress, callus cell proliferation and the healing process were observed when grafting grapevine plants (Prodhomme et al., 2019). Therefore, tissue regeneration that supports grafting healing, decreases with plant aging due to a lack, or reduced, expression of several transcription factors that promote the expression of products, especially auxins, that contribute to callus formation at the wound site (Ibañez et al., 2020).
Demand for citrus plants for orchard plantation is increased worldwide due to difficulties to comply with strict regulations implemented to avoid the spread of diseases or for replanting dead or declining trees (Bhandari et al., 2021). In Colombia, legal measures enforce that mother plants to provide bud and scions must be isolated with anti-aphid fabric mesh to avoid incidence of pests (ICA, 2019). These measures significantly increase the cost of plant production leaving middle and small plant propagation operations out of business. Evidence of this situation is the ICA database of nurseries where in the Córdoba department appears only one nursery reported in 2021 (ICA, 2021), indicating that new citrus crops in the area are established with non-locally produced plant material. A lower disease incidence and better field performance are usually reported when crops are planted with locally produced grafted plants (Ramírez-Jiménez et al., 2020; Noor et al., 2019). Minigrafting has been used to accelerate plant propagation in fruit species (Belmonte-Ureña et al., 2020), for the diagnosis of plant diseases (Spano et al., 2020), as a strategy for molecular biology studies (Bartusch et al., 2020; Tsaballa et al., 2021) and as a mechanism for somatic embryos rescue (Raharjo and Litz, 2005). The use of minigraft in the propagation of citrus cultivars has not been previously reported. In the present study, it was observed that propagation of Valencia orange using cleft and T-budding minigraft was possible, with a 75 and 38% of success, respectively; a higher percentage of success in cleft grafting could be the result of a better contact of cambial tissues of the rootstock and the scion compared to the contact between the inserted bud and the inner layer of the rootstock cortex in T-budding; however histological analysis is recommended to completely guarantee it. Percentages of success obtained from this study are prone to increase with some adjustments when massive propagation is implemented. The minigraft technique may provide nurseries and propagators with a suitable mechanism to propagate citrus plants at the local level, reduce costs for mother plant maintenance, and speed up the propagation process by using younger rootstocks; however, it is recommended to evaluate the performance of propagated plants at the nursery and field crop level.
CONCLUSION
The results of the present study of the propagation of "Valencia" orange plants using Cleft minigraft showed a higher percentage of success compared to T-budding, and the plants recovered by this method developed a significant higher number of shoots than plants propagated by T-budding. Cleft minigraft is a viable alternative for plant propagators to produce grafted citrus plants while complying with official regulations.
















