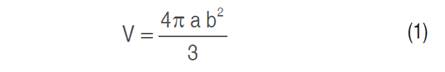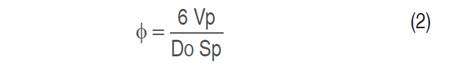Mauritia flexuosa is an oil palm found in the Colombian Andean Amazon region.It is a species recognized in Amazonian ecosystems for serving as a biological corridor for biodiversity and carbon sinking, in addition to its ecological and cultural value. Its fruits are covered by small scales that change color from reddish to violet when they reach maturity. Are oleaginous in character and are characterized by a high nutritional and medicinal value and are recognized by studies in the Brazilian Amazon as a potential source of carotenes, tocopherols, natural antioxidants, and phytosterols, also as one of the fruits with the most expressive nutraceutical properties (Barboza et al., 2022; De Oliveira and Franca, 2017); in Ecuador is considering highly nutritious (Abreu-Naranjo et al., 2020). In turn, they have high concentrations of oleic acid and five times more β-carotene than carrots, and their bioactive components possess important biological activity. The oil has photoprotective properties, healing and anti-inflammatory effects, and high oxidative stability. It is also a potential source of fatty acids with agro-industrial potential (Nastur et al., 2016). The characterization of food raw materials is the fundamental basis for establishing the qualities and possible use in the industry; in the Colombian Amazon there is little information of the canagucha that allow identifying its potential use and transformation.
Continuous consumption of fruits and vegetables is recommended for the high level of antioxidants, which limit the action of free radicals in the human body, and in turn, they prevent chronic diseases, such as: cancer, diabetes and cardiovascular and neurodegenerative diseases. Exotic fruits have an important industrial role because of components with physiological activity that contribute to preventing some diseases, in addition to formulation of new foods and cosmetic use (Araujo-Díaz et al., 2017).
Current consumer trends are oriented towards acquiring exotic products that also fit the context of functional foods. In this sense, the objective of the present work is to evaluate the morphometric, physical, and chemical characteristics, as well as the yield of the canangucha fruit in two ecotypes: I (EI) and II (EII) existing in the Colombian Amazon and contribute to the development of the value chain of the fruit.
MATERIALS AND METHODS
Collection of the fruits and pulp
Canangucha fruits (Mauritia flexuosa L. f) were collected at random from ninety adult individual trees in natural populations located in the municipalities of Florencia, Morelia, and Belén de los Andaquíes in the state of Caquetá - Colombia. The study area presented an altitude of 242 masl, a warm-humid climate characteristic of tropical humid forest, an average temperature of 28 °C and annual rainfall of 3840 mm (IDEAM, 2020). The fruits of ecotypes I (elliptical) and II (round) were selected according to the degree of maturity, identifying No. four (Sinchi, 2018). The pulp was obtained by immersing the fruits in water at room temperature for 12 hours to facilitate the detachment of the pericarp. The pulping was done manually, obtaining pulp, pericarp, and seed, and then, its percentage distribution was determined from 60 fruits per ecotype (6 replicates of 10 fruits). The pulp was cut into small pieces and stored in plastic bags at -18 °C until analysis.
Characterization methods
Morphometric characterization of the fruit and seed was performed based on the longitudinal diameter (LD) and equatorial diameter (ED) (100 samples for each ecotype) and a 78440 Staley digital Vernier caliper was used. The volume (V) and the sphericity (ɸ) were determined according to equations 1 and 2 respectively, described by Mc Cabe et al. (1993), in addition, the mass (g) was evaluated using a precision analytical balance (PCE-BSH 6000).
Where, V: Volume (cm3); a: Larger diameter (cm); b: Minor diameter (cm).
Where, Φ: Sphericity; Vp: Particle volume (cm3); Sp: Surface area for the particle (cm2); Do: Equivalent sphere diameter.
Soluble solids were measured using a digital refractometer (Hanna Instruments Inc. Woonsocket RI-USA-96801), and the results were expressed in °Brix. The pH was determined by direct reading on a benchtop pH meter (GPH503). Water activity (aw) was determined using a dew point hygrometer (Aqualab 3TE series, Decagon, Devices, Pullman, WA, USA). The peroxide index (PI) was performed according to Ariza et al. (2011), leaving the sample in the dark for 5 min and then measuring the absorbance at 500 nm. The color was determined in fruits with pericarp and without pericarp (50 fruits for each ecotype), according to the CIE L*a*b* and CIE L*C*H* coordinates. These were derived using a sphere spectrophotometer (SP64, X-Rite Inc, MI, USA) under conditions of illuminant D65, 10° observer, and both including and excluding the specular component.
Total phenol content (TP) was determined using a Folin ciocalteu reagent and 0.07 N Na2CO3 in aqueous solution (7.44% w v-1). The method described by Restrepo et al. (2010) measuring the absorbance at 760 nm in a spectrophotometer (Thermo scientific, Madison, USA), and the results were expressed in mg gallic acid equivalent (GAE) 100 g-1. The antioxidant capacity was determined from the DPPH and ABTS methods, using analytical grade reagents: 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-Azino bis (3 ethilbenzothiazoline-6-sulfonic acid) (ABTS) from Sigma-Aldrich Co. (St. Louis, MO, USA). DPPH was determined according to the method described by Cerón et al. (2010) that was measured at 517 nm, where the results were expressed in mg equivalent Trolox (TE) g-1. ABTS was determined according to the method described by Re et al. (1999) at 734 nm, expressing the results in mg TE g-1. For DPPH and ABTS, the percentage inhibition formula was described by Koolen et al. (2013).
The carotene content (α and β) was determined according to the methodology described by Sierra et al. (2007) and Cândido et al. (2015). An Agilent 1100 series HPLC with DAD, C18 column in reverse phase 250 cm, 4.6 mm, 5 µm, with an acetonitrile mobile phase (0.05% triethylamine): methanol: ethyl acetate (95:5:0) was used for 20 min and then adjusted to a ratio of 60:20:20, respectively, until the end. A flow of 0.5 mL min-1 and an injection volume of 10 µL were used, reading at 450 nm at room temperature. α-Tocopherol was determined according to the methodology described by Sierra et al. (2007) using an Agilent 1100 series HPLC with DAD, C18 column in reverse phase 250 cm, 4.6 mm, 5 µm, mobile phase of methanol: water (95:5), flow of 0.5 mL min-1, injection volume of 10 µL, and reading at 292 nm at room temperature. The mineral content (Ca, Cu, Fe, Mg, K, Na, and Zn) was performed by atomic absorption spectrometry method 985.35 (AOAC, 1980).
The bromatological characterization of the pulp was determined in triplicate in terms of moisture content, protein, ether extract, ash, and fiber, following the AOAC method, (1990) (920.151, 984.13, 920.39, 942.05, and 985.29 respectively).
Statistical analysis
Differences in morphological variables of fruits and seeds between the ecotypes were evaluated with t test (P<0.05) using Statgraphics 8.0 program. Principal component analysis was used to ordinate the samples using morphological variables of fruits and seeds. Biplot graphs were used to visualize the ordination and the morphological variables, using InfoStat version 2020 (Di Rienzo et al., 2008).
RESULTS AND DISCUSSION
Physical characteristics
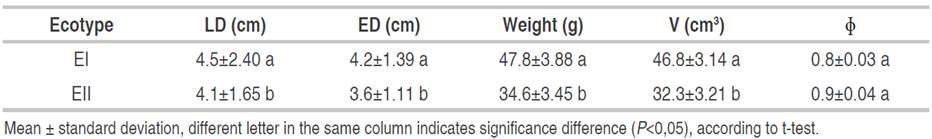
The results corresponding to the morphometric variables of the EI and EII ecotypes are presented in Table 1. All the morphometric measures except the sphericity (ɸ) presented significant differences (P<0.05) with respect to the ecotype of the fruit. Ecotype EI presented higher values for all variables evaluated in comparison to Ecotype EII however, these values were lower than those reported by Dos Santos et al. (2015) and Carvalho et al. (2013) in Brazil. The morphometric measurements from ecotype II were similar to those reported by Quispe et al. (2009) in Perú and lower than those reported by Guerra et al. (2011) in the Colombian Orinoquía. On the other hand, the total weight obtained from the EI ecotype was similar to that reported by different authors with a small fluctuation, this difference can be attributed for the soils and development conditions of the palm.
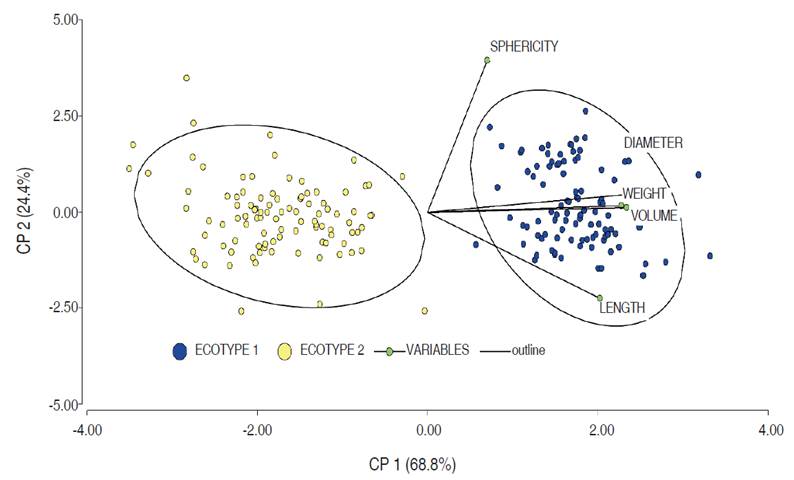
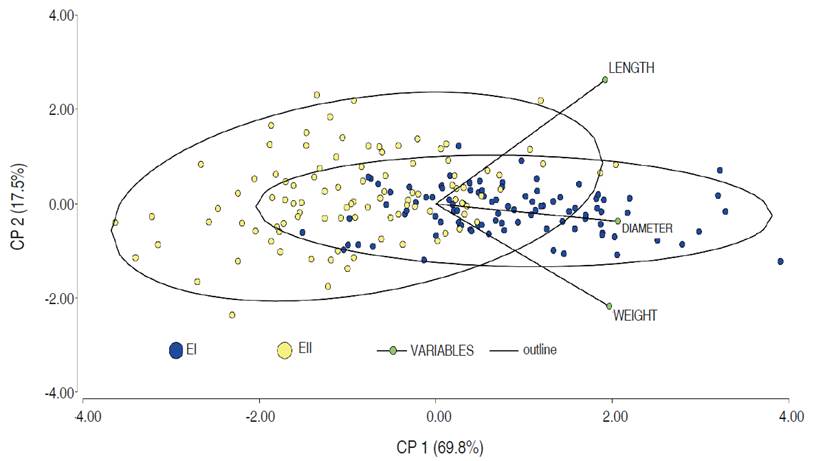
The principal component analysis (PCA) of the morphometric measurements of the E1 and E2 ecotypes are presented in Figure 1. The differences can be attributed to the weight, which is conditioned by genetic and environmental factors and can generate high variability in the fruits. On the other hand, agronomic and bioclimatic factors can influence the development of the palm and its ecotypes (Guerra et al., 2011). The variables LD, ED, weight, and V are positively related to higher values for EI in comparison to EII.
In general, the parameters allow for establishing the necessary quantities of raw material that are required in a transformation process and, in turn, choosing the most suitable transport inside and outside the plant. Likewise, sphericity allows for determining the best conditions for processing of the fruits.
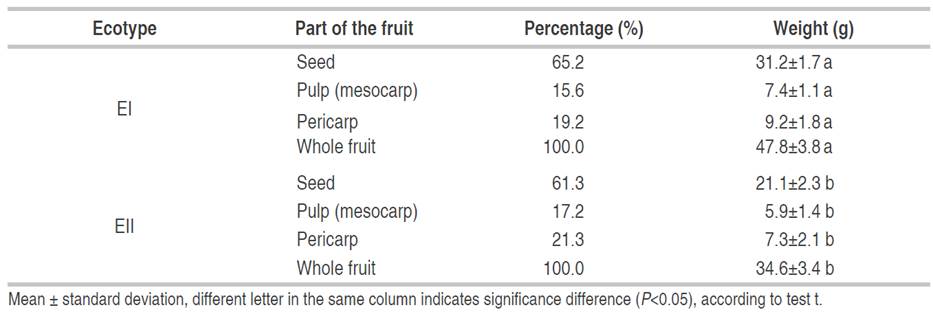
The percentage distribution of the fruit parts and their weights are presented in Table 2. It is observed that the greatest proportion is the seed, followed by the pericarp and finally the pulp. The values obtained from seeds in both ecotypes are higher than those reported by Quispe et al. (2009) in Perú and Imbrozio et al. (2010) in Brazil with 57.5%, and 49.51%, respectively. For the pulp, the values obtained in both ecotypes were lower than those reported by Quispe et al. (2009): 21.2% and Vásquez et al. (2010): 27.0%.
Various investigations report similar percentage distribution of the components of the canangucha fruit for EI with 62.5%, 17.6%, and 19.9% for the seed, pulp, and pericarp respectively. In Brazil, Imbrozio et al. (2010) reported higher values for pulp and pericarp with 24.2% and 22.0%, respectively. On the other hand, Quispe et al. (2009) reported similar values in the pericarp (21.3%) for EII and lower for ecotype I. For ecotype II, the investigation reported lower values in the seed (54.8%) similar in the pericarp (19.6%), and higher for the pulp (25.6%) (Dos Santos et al., 2015). Similarly, Batista et al. (2012) reported higher values for the pulp and pericarp with 63.8% and 25.1%, respectively, and lower values for the pulp (11.0%).
Regarding the weight of the different fruit parts, the seed was less than that reported by Guerra et al. (2011) and Imbrozio et al. (2010) with 43.5 g and 41.8 g, respectively. In turn, it was found that EI presented higher fruit and seed weight (%) and less pulp (%) and pericarp (%) than EII. The differences in the percentage distribution of the fruit components have been attributed to the fruit characteristics, climatic changes, and soil conditions, among other factors (Dos Santos et al., 2015; Guerra et al., 2011; Imbrozio et al., 2010).
The morphometric variables of the seeds from ecotypes EI and EII presented similar values for LD and ED. For these variables, Vásquez et al. (2010) and Freitas-Alvarado et al. (2011) report higher LD and lower ED values than those found by the present investigation. The weight of the seeds presented significant statistical differences (P<0.05) with respect to the ecotype, determining the EI ecotype to be higher than EII. It was also noted that the seed weight of ecotype I is higher than that found by Freitas-Alvarado et al. (2011) and lower for ecotype II. Figure 2 shows the PCA for seed morphometry for EI and EII. It can be observed that the variables weight and LD are more related to ecotype EI and ecotype EII presented lower values for the afore mentioned variables.
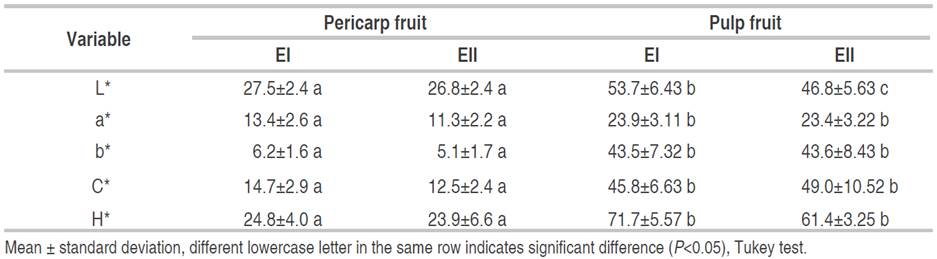
The CIE-L*a*b* color coordinates for the whole fruit with pericarp and without pericarp (pulp) of ecotypes E1 and E2 are presented in Table 3. Significant differences (P<0.05) were found in all the variables regarding the sample type and the ecotype; however, it is observed that the color parameters of the fruit with pericarp did not present significant differences (P>0.05) with respect to the ecotype. The Canangucha pulp only shows significant differences (P<0.05) in the L* with respect to the ecotype.
The canangucha pulp presented greater clarity (>L*) than the fruit with pericarp, the latter being smooth. The pulp has a porous surface, which can present a greater homogeneity of the surface refractive index, due to the air content in the pores and less light absorption that makes it appear clearer (Carvalho et al., 2013). For the pulp, the EI ecotype presents greater L* than EII, which could be attributed to greater matrix content of dark pigments contributed by the carotenes (Cândido et al., 2015). These were enhanced and presented the most orange tone (<H*) (main contribution of carotene pigments) and the highest intensity or color saturation (>C*) for ecotype II.
The pulp color coordinate is in the first quadrant of the chromatic plane a*b*, where the behavior or correlation of the color parameters is not very clear. This is because chromaticity b* is mostly associated with the carotene content and is similar in both ecotypes, a situation that confers the search for other factors, such as porosity, composition, among others.
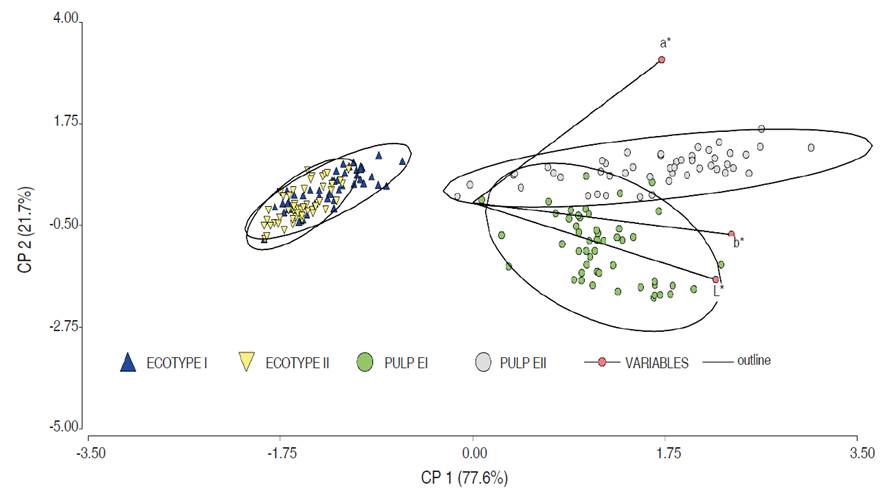
The PCA of the color parameters for the fruit pericarp and pulp in the EI and EII ecotypes are presented in the Figure 3. The results illustrate that the fruit pericarp for both ecotypes were similar, where the pulp only denotes differences in the L* and was greater in the E1 ecotype. For their part, the values of a* were independent of L*. However, both a* and b* and b* and L* were positively related.
Chemical Characteristics
The pH presented significant differences (P<0.05) between ecotypes I and II, where the values fluctuated between 4.5±0.2 and 3.7±0.7, respectively. The °Brix did not present significant statistical differences (P>0.05), fluctuating between 16.4±1.4 and 15.2±1.0 respectively, and these results were similar to those reported by Vásquez et al. (2010). The pH value found for EI is higher than that reported by Guerra et al. (2011) (3.74) in Venezuela and lower than that reported by Milanez et al. (2018) (4.9) in Brazil. This acidic characteristic can give the fruit a greater advantage against bacterial deterioration, improving its conservation (Guerra et al., 2011). Some Amazon fruits have similar characteristics, such as arazá, cocona, copoazu, camu camu, and caimarona grape.
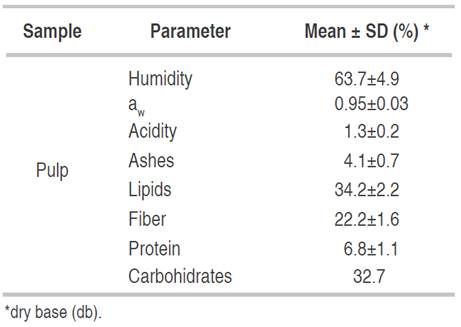
The proximal analysis of the Ecotype EI of the canangucha fruit is presented in the Table 4, this was selected due to the availability of fruit in the study area with respect to EII, observing that the fruit contains an ideal moisture content with its corresponding high aw, which makes it a favorable substrate for microbial growth and deterioration reactions. The humidity of the fruit was similar to that reported by Vásquez et al. (2010) (63.96%) in the Shambo variety. On the other hand, it was higher than reported by Sinchi, (2017), Vásquez et al. (2010) and Quispe et al. (2009) with 57.0%, 51.3%, and 54.3%, respectively. The values found for the canangucha were within the range for oleaginous fruits, which range from 54 to 80% according to Vásquez et al. (2010).
The lipid content found for the pulp was similar to that reported by Sinchi (2017) for EI in the Caqueta foothills (37.0%). On the other hand, it was higher than that reported by Quispe et al. (2009) (18.1%) and Vásquez et al. (2010) (23.1%), and lower than that reported by Imbrozio et al. (2010) (38.0%). Lipid values can vary according to the ecotype, the stage of fruit maturity, and the seasonality of the harvest.
In Peru, Quispe et al. (2009) found that the main component of canangucha oil was oleic acid (78%), in turn, Milanez et al. (2018), mentioned that this oil has high concentrations of carotenes, tocopherols, and acids such as oleic and palmitic, which help prevent cardiovascular diseases. Similarly, some authors indicated that the oil has functional properties due to the high concentrations of monounsaturated fatty acids, conferring hypocholesterolemic action. Other studies have indicated that canangucha has higher content of omega 3, 6 and 9 fatty acids than sacha inchi (Plukenetia volubilis), which are important for the lipid metabolic process and decrease the risk of cardiovascular diseases (Sinchi, 2017).
Fiber is a component that has functional properties, preventing conditions such as constipation, irritable colon, and obesity. Therefore, the consumption of canangucha can be considered healthy. The result found was on the order of 22.2%, lower than that reported by Sinchi (2017) (37.7%) and higher than that reported by Quispe et al. (2009) (10.1%).
The pulp protein content was similar to that reported by Quispe et al. (2009) with 2.32%, and lower than that reported by Sinchi (2017) with 3.62% and Vásquez et al. (2010) with 5.50%. Protein intake from the canangucha pulp is relatively low. However, it is a high-quality plant-based protein that contains aromatic amino acids, such as tyrosine and phenylalanine, and sulfur amino acids, such as cysteine, methionine, and tryptophan (De Oliveira and Franca, 2017).
For carbohydrates (32.7%), the value found was higher than that reported by Sinchi (2017), Vásquez et al. (2010) and Guerra et al. (2011) with 21.2%, 5.6% and 18.1%, respectively. Canangucha can be considered an energy source with respect to its carbohydrates and lipids (Sotero et al., 2013).
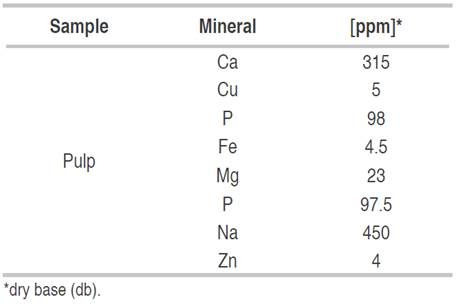
Minerals are important in the diet since they act as cofactors in different metabolic reactions that are carried out in the body. In turn, they are essential regulatory and structural components that must be consumed in the diet, according to Sotero et al. (2013). In this sense, the mineral content in the canangucha pulp is presented in Table 5, highlighting higher levels of Ca than that reported by Pereira et al. (2016) and lower than those reported by Sotero et al. (2013) and Vásquez et al. (2010) with 354 ppm and 1197 ppm, respectively, the Ca is important for the prevention of diseases, such as osteoporosis and rickets.
On the other hand, the Cu level was similar to that reported by Sotero et al. (2013) with 6 ppm, and it was higher than that reported by Vásquez et al. (2010) (4.6 ppm). In the case of P, Fe, and Mg levels, these were lower than those reported by Sotero et al. (2013) and Vásquez et al. (2010), and higher than those reported by Pereira et al. (2016). For the Na content, the values were higher than that reported by Vásquez et al. (2010) and Sotero et al. (2013), with 134 ppm and 126.9 ppm, respectively, therefore it´s consumption should be limited in hypertensive people. Regarding Zn, the values were lower than those reported by Sotero et al. (2013) and Vásquez et al. (2010) with 10.8 ppm and 726 ppm, respectively. This variability observed for the minerals in the canangucha fruit of the Colombian Amazon region when compared to those grown in Peru, Brazil, and Venezuela may be due to edaphoclimatic, genetic conditions, and the state of maturity of the fruit.
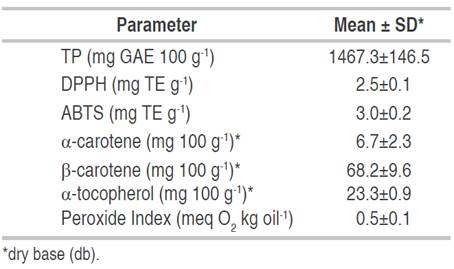
The results of TP, ABTS, DPPH, α-carotene, β-carotene and α-tocopherol in the canangucha pulp are presented in Table 6, in addition to relating the peroxide index value. These components conferring physiological activity are important because they protect the organism from the damaging effects of free radicals (Koolen et al., 2013) and counteract and prevent various cardiovascular diseases, in addition to slowing down lipid oxidation processes in food products.
The TP content was higher than those reported by Best et al. (2022), Carmona-Hernández et al. (2021), Abreu-Naranjo et al. (2020), Milanez et al. (2018), Schiassi et al. (2018), Nogueira (2017), Cândido et al. (2015), Dos Santos et al. (2015), Vásquez et al. (2010), and Koolen et al. (2013) with 28.8, 235.9, 435.08, 110.7, 47.2, 270.6, 435.1, 281.0, 187.5, and 378.0 mg GAE 100 g-1, respectively, in countries, such as Brazil, Peru, and Venezuela. Likewise, Koolen et al. (2013) mentioned that the antioxidant activity of the canangucha pulp is important, due to the phenolic compounds. In turn, Tauchen et al. (2016) report 87 mg GAE g-1 in liquid extract obtained from canangucha pulp.
On the other hand, Resende et al. (2019) report TP contents in canangucha by product flours that vary between 93.2 to 934.6 mg GAE 100 g-1, in other Amazonian fruits some authors reported, such as asai (Euterpe oleracea M), camu camu (Myrciaria dubia McVaugh), and acerola (Malpighia emarginata S) values of 454, 1176, and 1063 mg GAE 100 g-1, respectively. Other investigations carried out by Koolen et al. (2013) with phenolic extracts of canangucha indicate that these possess a strong capacity to inhibit the growth of pathogens. In addition, Milanez et al. (2018) mentioned that it has high concentrations of antioxidants that prevent oxidative stress. For DPPH, the values found are higher those reported by Tauchen et al. (2016) with 0.131 mg TE g-1 in pulp extract and higher than that reported by Nogueira. (2017) with 0.58 mg TE g-1. In other studies, Schiassi et al. (2018), and Koolen et al. (2013) reported 951.5 EC50 g fresh weight g-1 and 19.8 mg mL-1, respectively.
For ABTS, the results were higher than those reported by Schiassi et al. (2018) with 1.51 mg TE g-1 and Nogueira (2017) with 2.18 mg TE g-1 in Brazil. However, they were lower than those reported by Cândido et al. (2015) with 8.26 mg TE g-1 in Brazil. In some Amazonian fruits, such as arazá (Eugenia stipitata), copoazú (Tehobroma grandiflorum), sacha inchi (P. volubilis), asai (E. oleracea) and camu camu (M. dubia) levels are found of 5.05 mg TE g-1, 2.4 mg TE g-1, 2.47 mg TE g-1, 3.77 mg TE g-1 and 38.3 mg TE g-1, respectively. Studies carried out by Camelo-Silva et al. (2021) in Brazil report values for ABTS of 293.7-411.4 µmol TE g-1, and for DPPH of 0.07-10.45 µmol TE g-1.
The present peroxide index for canangucha was higher than that reported by some authors in Brazil. These values are important due fat content can trigger oxidation reactions, and this can cause the product to deteriorate, generating an unpleasant taste and odor. For their part, Quispe et al. (2009) reported a value of 4.6 and 4.8 meq O2 kg-1 for extraction of canangucha oil at 25 and 60 °C in Peru.
Physiologically active compounds (PAC) act as protectors of the immune system, participate in defense reactions, and improve biochemical and metabolic processes in the body, and these compounds include vitamins, antioxidants, minerals, among others, frequent consumption of these decreases the risk of suffering from some cardiovascular and chronic diseases (Milanez et al., 2018). The results obtained for α-carotene are high to those reported by Hamacek et al. (2018) (2.3 mg 100 g-1) in Brazil, while α-carotene reached levels of 1.5 mg 100g-1. Furthermore, Milanez et al. (2018) reported values of 28.8, 42.9, and 45.1 mg 100 g-1 in green, semi-ripe, and ripe canangucha fruits, respectively, while Sotero et al. (2013) reported 29.6 mg 100 g-1 in canangucha oil. For β-carotene, the results found are higher those reported by Abreu-Naranjo et al. (2020), Hamacek et al. (2018) Schiassi et al. (2018) and Sotero et al. (2013) with 19.5, 21.6, 17.0 and 10.4 mg 100 g-1, respectively, and close to than those reported by Cândido et al. (2015) and Vásquez et al. (2010), with 52.9, 34.2 mg 100 g-1, respectively.
In Brazil, Cândido et al. (2015) indicated that, among the carotenes in canangucha, β-carotene (100% activity of pro-vitamin A) is the majority component (13.7 mg 100 g-1), On the other hand, Dos Santos et al. (2015) also reported 65% for the β-carotene/oleic acid ratio when evaluating the canangucha pulp. These variations may be due to the degree of maturity, variety, agronomic factors, and extraction process. The results for α-tocopherol were lower than those reported by Hamacek et al. (2018) and Vásquez et al. (2010) with 44.9 and 68.3, respectively, these results can be due to genetic variation and development of the palm.
According to Beltrán et al. (2012), 1 µg of retinol equivalent activity (RAE) is equal to 12 µg of α-carotene and 6 µg of β-carotene; therefore, in the present study, canangucha has a contribution of 11,927.7 µg RAE 100 g-1 in dry base and 4449.2 µg RAE 100 g-1 on a wet basis. In turn, the value found is higher than reported by Hamacek et al. (2018) with 1899.33 µg 100 g-1 RAE. Some authors mentioned that canangucha is an important source of provitamin A, which could be used as a supplement in populations with deficiencies for this compound. In turn, Koolen et al. (2013) mentioned that these levels are 20 times richer in provitamin A than carrots, acerola, and papaya. For their part, some investigations proposed the consumption of canangucha as a supplement in the treatment of testoterone replacement therapy (TRT), due to its contribution in antioxidants, tocopherols, β-carotene, phytosterols, and oleic acid in patients with hypogonadism.
Finally, some authors indicated that the variety and the state of fruit maturity has great influence on the chemical composition, level of antioxidants and bioactive compounds of the canangucha, this fruit has the potential to application in functional foods and as a source of antioxidants and bioactive compounds (Abreu-Naranjo et al., 2020). Furthermore, the highest contents of TP antioxidants, and vitamin C were found in the green state, while β-carotene was more prevalent in the mature state. The results found for this study were specific to the Colombian Amazon.
CONCLUSION
The canangucha fruit of ecotype I presented higher LD, ED, mass, and volume with respect to ecotype II, which allows for establishing the differences between them. On the other hand, both present great morphometric variation with respect to fruits from Brazil and Peru, which can be attributed to the genetic variability, edaphoclimatic conditions of the growth zones, and the species. The ecotypes I and II pulp coordinates were in the first quadrant of the color plane with reddish-orange colorations corresponding to maturation degree four. The pulp percentage was higher for EI when compared to EII, which presented a higher percentage of seeds.
Canangucha is characterized by its contribution of lipids, fiber, total phenols, and β-carotene as a source of provitamin A, in addition to significant contribution of equivalent activity of retinol and these can be used as a supplement for people with vitamin A deficiency. Due to its nutritional and antioxidant characteristics canangucha is considered a species with great food and pharmaceutical potential, as well as a promising and sustainable value chain for the Colombian Andean Amazon region.