Bio fungicide production is important for phytosanitary crop management in agronomy and forestry because bio fungicides act as alternatives to chemically synthesized fungicides (Chandrashekara and Manivannan 2012). Trichoderma is one of the most widely used antagonist fungi to control different agronomic diseases, representing around 60% of the biological control agents registered worldwide (Coban and Sargin 2019). Trichoderma is a natural inhabitant in soil and has been widely cultivated industrially as a biological control product. Several commercial Trichoderma spp. products are available for plant disease management, containing various propagules as active components, such as conidia, chlamydospores, and vegetative mycelium (Coban and Sargin 2019). However, conidia are the most used propagules in biocontrol programs due to their ability to withstand extreme conditions, including high and low temperatures (Cumagun 2017). Conidia are asexual reproductive structures and a primary survival and dispersal mechanism for this genus (Cumagun 2017).
Conidial biomass can be produced by implementing either submerged or solid substrate cultivation techniques. Trichoderma conidia obtained from solid-state fermentation exhibits a higher tolerance to abiotic stress compared to propagules or biomass derived from liquid fermentation (Sriram et al. 2011). Solid-state fermentation (SSF) has allowed the productive conidia scaling of various species of the Trichoderma genus (Rayhane et al. 2020). Additionally, solid-state fermentation improves bioactive production with biofungicidal potential, which generates bioproducts (Torres et al. 2015; Rayhane et al. 2020) including enzymes (lipases, cellulases, and chitinases) (Urbina et al. 2019) metabolites (mycotoxins, lactones such as 6- pentyl- alpha-pyrone(6-PP) (Ramos et al. 2008). SSF reduces production costs, increases process efficiency, is resistant to contamination, and is an easily scalable method (Gonzalez and Vicente 2016).
Studies have shown several physicochemical factors that promote conidiogenesis. They include concentration and sources of macronutrients, like carbon (Alarcon and Utia 2020) and sources of nitrogen (Gezgin et al. 2020). Moreover, adding mineral salts like NaCl, KCl, and CaCl2 has been found to have a positive effect on conidia production (Dastogeer et al. 2018), along with maintaining a pH range of 5.5-6.5 (Raut et al. 2013). Culture conditions, such as light and temperature, have also been noted as important factors (Steyaert et al. 2010). It is also essential to consider the type of containers and culture spaces to allow for proper gas exchange. Some studies have shown that mycelial lesions can affect conidiogenesis processes due to the accumulation of volatile organic compounds (VOCs) fungi produce (Adnan et al. 2019). To improve conidia production in Trichoderma through liquid and solid fermentations, it is essential to identify specific inoculation parameters and add microelements. This will enable the provision of practical recommendations that can benefit Colombian agriculture by enhancing conidia production.
This study aimed to assess the impact of the type of inoculation on conidia production through solid-state fermentation evaluating different concentrations of conidia and inoculum volumes, of conidia production. Based on the findings, the best conditions were used to evaluate the impact of adding microelements (CaCO3, KH2PO4, MgSO4*7H2O, and (NH4)2SO4) on the conidiogenesis of two Trichoderma asperellum strains (GRB-HA01 and GRB-HA01) in solid and liquid state fermentations.
MATERIALS AND METHODS
Microorganisms
This study included two Trichoderma asperellum (GRB-HA01 and GRB-HA02) strains Universidad de Medellin Biodiversity, Biotechnology and Bioengineering Research Group (GRINBIO) donated.
General culture conditions
To activate the strains and prepare conidia inoculum, fungi were cultivated on potato dextrose agar (PDA) at a concentration of 39 g L-1 and pH=6.0 previously sterilized at 12 °C, 15 psi, 15 min. Conidia inoculum was obtained by surface washing using an aqueous solution containing sterile Tween 80 at 0.01% v/v from activated cultures on PDA agar with 8 days of growth. The concentration was adjusted to test conditions using a hemocytometer.
For solid-state fermentation, Bae et al. (2016) proposed a modified methodology in which 200 g of unsupplemented rice were placed in polyethylene bags and the substrate was hydrated with distilled water at a 2:1 ratio (rice: water). Then, the bags were sealed with cotton and spring to allow gas exchange, and sterilized at 121 °C, 15 psi, 15 min. The cultures were incubated under laboratory conditions for 12 days with alternating photoperiods of 12-hour diffused light and 12-hour darkness at 28±2 °C. The polyethylene bags were hand-shaken every 24 h during cultivation.
Determination of the effect of inoculum concentration and volume on conidiogenesis in solid culture
To evaluate the effect of conidia concentration in the inoculum on the conidiogenesis of T. asperellum GRB-HA01 and T. asperellum GRB-HA02 strains, the inoculum was prepared at concentrations of 1.0x105, 1.0x106, and 1.0x107 conidia mL-1. Subsequently, to determine the combined effect of the inoculation volume, rice substrates were inoculated at ratios of (10, 30, and 50 mL per 200 g of un-supplemented rice).
Determination of the effect of microelements on conidiogenesis in solid culture
To evaluate the effect of adding microelements on conidia production for T. asperellum GRB-HA01 and T. asperellum GRB-HA02 strains, a rice substrate was supplemented before sterilizing the medium with one of the following salts (% w/w): 2.5 ammonium sulphate (NH4)2SO4; 1 calcium carbonate CaCO3; 2 magnesium sulphate; MgSO4*7H2O; or 0.5 potassium dehydrogenate phosphate KH2PO4. Additionally, the effect of mixing the salts was evaluated, and a culture without supplementation was used as a control. After sterilization, the culture was inoculated using the best result obtained during the evaluation of the concentration and volume of inoculum, 10 mL of conidia suspension, and a concentration of 1.0x107 conidia mL-1.
Determination of the effect of microelements on conidiogenesis in liquid culture
To determine how adding microelements affected conidia production for T. asperellum GRB-HA01 and T. asperellum GRB-HA02 strains, liquid fermentation was carried out using potato dextrose broth (PDB) as a culture medium at a concentration of 39 g L-1 and peptone at 0.01% (w/v). Each treatment was enriched before sterilizing the medium with (% w/v): 2.5 (NH4)2SO4; 1 CaCO3; 2 MgSO4*7H2O; and 0.5 KH2PO4, respectively. The pH values of the media were adjusted to 5.5. The combined effect of microelements a salt mixture and a control culture without supplementation was evaluated. Fermentations were conducted in a 250 mL Erlenmeyer flask using 100 mL of medium. Flasks were sealed with a cotton plug and sterilized at 121 °C, 15 psi, for 15 min. Subsequently, the cultures were inoculated with 10 mL of a mycelial suspension from activated cultures in a PDB medium with 10 days of growth. Submerged fermentations were incubated in a rotary shaker at 100 rpm for 12 days at room temperature (28±2 °C), with a photoperiod of 12-h light and 12-h darkness.
Conidia sampling, counting, and determining yield
To determine conidia production based on concentration, inoculum volume, and enrichment with microelements in solid fermentations, a sample of 1 g of colonized substrate was taken on days 3, 6, 9, and 12 for each of the treatments. The samples were dissolved in 10 mL of water sterilized with Tween 80 at 0.01% (v/v), and then, shaken at 100 rpm for 24 h. The conidia concentration for each treatment was determined using a hemocytometer. To evaluate how adding microelements affected conidiogenesis in liquid cultures, a sample of 1.5 mL was taken on days 3, 6, 9, and 12, and conidia were counted using a hemocytometer.
Yields generated in solid (Ypx-S), and liquid (Ypx-L) fermentation were determined based on the number of inoculated conidia for various inoculation strategies and adding microelements. They were calculated based on the total conidia produced per 200-g culture bag of rice, and the number of conidia produced per flask culture with 100 mL of PDB for each treatment about the control.
Statistical analysis
A completely randomized design (CRD) was used to determine the effect of inoculum concentration and volume on conidiogenesis. The factors were three levels of inoculum concentration (1.0x105, 1.0x106, and 1.0x107 conidia mL-1), three levels of inoculum volume (10, 30, and 50 mL) and the effect of microelement addition on conidiogenesis in solid and liquid media block design with six levels (CaCO3, KH2PO4, MgSO4*7H2O, (NH4)2SO4), a mixture of all microelements, and a negative control without salts was used. Each experiment was carried out using three replicates and two repetitions in time. To conduct analyses, variables were initially described using descriptive statistics and later analyzed using parametric tests. In all cases, variables were expressed as the mean standard deviation. To determine the effects of the treatments on the variables, an analysis of variance (ANOVA) was conducted, followed by a Tukey test. Before any statistical analysis, Levene’s tests were performed to determine homogeneity, and the Shapiro-Wilk test was used to evaluate data normality. The value P<0.05 was used as a statistical criterion to reveal significant differences among treatments with 95% confidence. All data were analyzed using the IBS SPSS Statistics Version 25 statistical program.
RESULTS AND DISCUSSION
Evaluation of the volume and concentration of inoculum to produce conidia of T. asperellum strains in solid fermentation
The production kinetics of conidia for T. asperellum GRB-HA01and GRB-HA02 (Figure 1A and 1B) showed differences in conidia production responses resulting from the application of different volumes and concentrations of conidia in the inoculum. In this study, the highest conidia production and the best yields were achieved at 12 days when the cultures were inoculated with 1.0x107 conidia mL-1 at a volume of 10 mL, both for T. asperellum GRB-HA01 (6.9x109±5.7x102 conidia g-1, Ypx-S of 700 conidia per conidia inoculated) and for GRB-HA02 (1.3x109±1.4x102 conidia g-1, Ypx-S of 130 conidia per conidia inoculated). The positive effect of a rise in initial conidia concentration in Trichoderma inoculum has been reported by authors like Coban and Sargin (2019). Their study evaluated the effect of conidia inoculum concentration for Trichoderma harzianum at concentrations of 1.0x104, 1.0x105, 1.0x106, 1.0x107, and 1.0x108 UFC mL-1, achieving a maximum concentration of 1.0x109 CFU mL-1 at a 1.0x108 UFC mL-1 inoculum concentration. However, conidia production values are similar compared with those found in this study for T. asperellum GRB-HA01 (6.9x109±5.7x102 conidia g-1) and GRB-HA02 (1.3x109±1.4x102 conidia g-1).
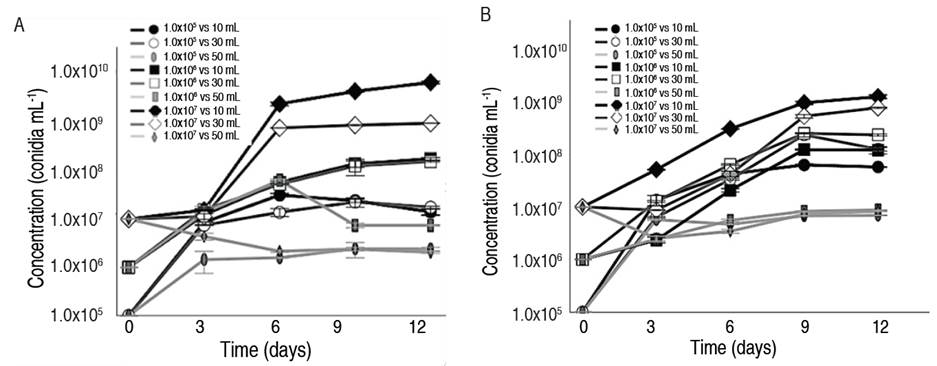
Figure 1 Conidia production kinetics with different inoculum volumes and concentrations of A. T. asperellum GRB-HA01, B. T. asperellum GRB-HA02.
When comparing the results obtained using concentrated inoculum 1.0x107 conidia mL-1 using 10 mL with those obtained at concentrations of 1.0x106 conidia mL-1 (GRB-HA01: 1.3x108±1.1x102 conidia g-1; Ypx-S: 130 conidia per conidia inoculated; GRB-HA02: 1.2x108±2.1x102 conidia g-1; Ypx-S: 120 conidia per conidia inoculated) and 1.0x105 (GRB-HA01: 4.0x107±3.1x102 conidia g-1; Ypx-S: 400 conidia per conidia inoculated; GRB-HA02: 7.6x107±2.8x102 conidia g-1; Ypx-S: 760 conidia per conidia inoculated), it was observed that conidia production decreased 10 times for GRB-HA01 and GRB-HA02 when the concentration was 1.0x106 conidia mL-1 and decreased 100 times for GRB-HA01 and GRB-HA02 for 1.0x105 conidia mL-1. Statistical data analyses of the inoculum concentration and volume for T. asperellum GRB-HA01 and GRB-HA02 showed that inoculum concentration significantly and positively affects conidia production (P<0.05). However, inoculum volume did not have any significant effect. When comparing the means of the inoculum concentration using a Tukey analysis, 1.0x107 conidia mL-1 was the concentration that best promoted conidia production compared to concentrations of 1.0x105 and 1.0x106 for each of the evaluated volumes. Results were similar to those reported by Alarcon and Utia (2020), who evaluated three doses of Tricho-D based on Trichoderma harzianum (0.2, 0.25, and 0.3 kg ha-1) during field tests on potato crops. They observed that the highest dose (0.3 kg ha-1) allowed greater substrate colonization; therefore, a higher presence of conidia, improving crop productivity and reducing the degree of severity generated by Rizhoctonia solani by 75% compared to a control that showed a 60% reduction.
When inoculum was increased to 30 mL for the highest conidia concentration (1.0x107 conidia mL-1), conidia production decreased to 7.0x108±2.3x102 conidia g-1 (GRB-HA01) at 6 days, and 8.0x108±2.7x102 conidia g-1 (GRB-HA02) at 9 days, decreased the Ypx-S to 10 conidia per conidia inoculated, which is 10 times lower. The highest inoculum volumes (50 mL), regardless of the initial conidia concentration, generated the lowest final conidia production, with values of 8.0x106 (1.0x105 conidia mL-1), 6.0x106 (1.0x106 conidia mL-1), and 2.0x106 conidia g-1 (1.0x107 conidia mL-1), and Ypx-S of 80, 6 and 0.2 conidia per conidia inoculated for GRB-HA01 and values of 1.0x106 (1.0x105 conidia mL-1), 1.0x107 (1.0x106 conidia mL-1) and 2.0x107 conidia g-1 (1.0x107 conidia mL-1). However, reducing conidia concentration at high volumes improved the conidia production response in GRB-HA01. When comparing the poor results achieved by higher inoculum volumes and those generated at concentrations of 1.0x107 conidia mL-1 and 10 mL, conidia production per gram of inoculated substrate was 100 times greater in the latter for T. asperellum GRB-HA01 and GRB-HA02. An analysis of the different volumes using a Tukey test demonstrated that the use of concentrated inoculum at volumes of 10 and 30 mL achieved the highest conidia production. In contrast, inoculum volumes of 50 mL least favored the conidiogenesis processes in both strains of T. asperellum. Studies such as Domingues et al. (2000) support the results rendered in this study, showing that inoculum size plays an important role in fungi morphology and is related to metabolite growth and production. This effect has also been reported by Chakravarthi et al. (2020) who show that high concentrations of conidia inoculum led to the formation of filamentous mycelium. The same effect was observed in this study when volumes of 50 mL were used showing an increase in the formation of filamentous mycelium by increasing inoculum concentration. Chakravarthi et al. (2020) show that small inoculums produce pellets that help dispersion in the substrate and improve growth, protein production, and conidiogenesis. This behavior is influenced by various endogenous signals and environmental factors, which affect fungi physiology, biochemistry, and development.
Effect of microelements on conidiogenesis of T. asperellum GRB-HA01 and GRB-HA02 in solid culture
There were important macroscopic differences in rice-grain color and coverage in T. asperellum GRB-HA01 and GRB-HA02 cultures after adding different microelements (CaCO3, KH2PO4, MgSO4*7H2O, (NH4)2SO4 and micronutrient mixture). Conidia counts generated in the culture indicated that CaCO3 and KH2PO4 increased conidiogenesis in T. asperellum GRB-HA01. However, T. asperellum GRB-HA02 was not as sensitive to the presence of MgSO4*7H2O, (NH4)2SO4, and all the salt mixtures, for which the results matched the control. In this study, CaCO3 was the microelement that generated the greatest rice-grain coverage and promoted green tone generation for T. asperellum GRB-HA01 and T. asperellum GRB-HA02, which is a good conidiogenesis process indicator. Conidia production kinetics (Figure 2A and 2B) confirmed that CaCO3 was the molecule that had the most positive impact on conidia production of T. asperellum GRB-HA01 (3.0x1011±2.5x102 conidia g-1 and a Ypx_S: 30,000 conidia per conidia inoculated), and T. asperellum GRB-HA02 (8.0x1010±1.1x101 conidia g-1 and a Ypx_S: 8,000 conidia per conidia inoculated).
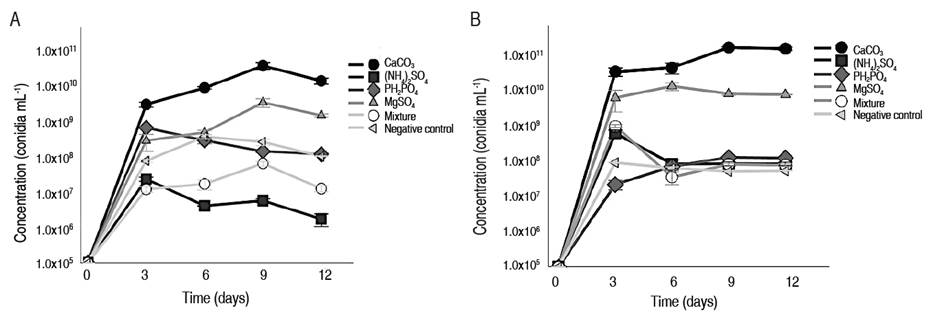
Figure 2 Conidia production kinetics with different microelements. A. T. asperellum GRB-HA01, B. T. asperellum GRB-HA02 in solid cultures.
Although KH2PO4 also increased conidia production of T. asperellum GRB-HA01 (1.2x109±1.9x102 conidia g-1 and a Ypx_S: 120 conidia per conidia inoculated) and T. asperellum GRB-HA02 (4.0x109±4.9x103 conidia g-1 and a Ypx_S: 400 conidia per conidia inoculated), the conidiogenesis was 10 times better for T. asperellum GRB-HA01. Adding CaCO3 to the culture media improved conidia production in GRB-HA01 ranging from 1.0x106 to 1.0x102 when it was compared with (NH4)2SO4, MgSO4*7H2O, a microelement mixture, and negative control, for which production of 1.4x106; 1.4x108; 1.2x107 and 1.0x108 conidia g-1 and a Ypx_S: 0.1, 14, 1.2, and 10 conidia per conidia inoculated was achieved, respectively. In this study, statistical analysis demonstrated that the use of microelements in solid fermentation had a significant effect on conidiogenesis processes (P<0.05) for T. asperellum GRB-HA01 and GRB-HA02. Comparing the means of data using a Tukey test showed that CaCO3 is a microelement that improves conidia production. It reduces the times in which T. asperellum GRB-HA01 and GRB-HA02 achieved complete substrate colonization.
According to Martinez (2007), it has been possible to demonstrate that the presence of CO2 is a determining physical-chemical factor that causes variation in the Trichoderma population. In addition, CaCO3 is a cofactor that does not lead fungus metabolism to generate conidia. However, the presence of calcium ions produces an increase in osmotic pressure in fungal cells inducing sporulation (Krystofova et al. 1995). Šimkovič et al. (2008) showed this effect and they evaluated the effect of calcium ion (0-1.0 mol L-1) concentration, observing an increase in conidiogenesis (3.0x108 conidia mL-1) by adding 0.1 mol L-1 Ca2+ and represented another path to conidia Trichoderma viride formation.
Adding different microelements to the substrate during the kinetics of conidia production gave a maximum conidia production after 9 days of culture, with values for T. asperellum GRB-HA01 of 3.0x1011, 1.4x106, 1.4x108, 1.2x109, 1.2x107, and 1.0x108 conidia g substrate-1 when CaCO3, (NH4)2SO4, MgSO4*7H2O, KH2PO4, a mixture of all salts and negative control were added, respectively. Afterwards, values only varied by 3.3x1011, 3.0x106, 2.4x108, 3.5x109, 1.9x107, and 1.4x108 conidia g substrate-1 when the exponent was not changed. A similar behavior was observed for T. asperellum GRB-HA02 obtaining values of 8.0x1010, 5.9x107, 1.3x107, 4.0x109, 2.5x107, and 2.4x107 conidia g substrate-1 when CaCO3, (NH4)2SO4, MgSO4*7H2O, KH2PO4, a mixture of all salts and a negative control were added, respectively, and their values only varied by 8.7x1010, 6.9x107, 1.2x107, 4.5x109, 2.6x107, and 2.8x107 conidia g substrate-1 without changes in the exponent. The cultivation time in which the maximum coverage of the rice grains was reached was 9 days, which is ideally the moment to start the drying process favoring reduced production times.
Microelement evaluation for T. asperellum GRB-HA01 suggests that adding a salt mixture s and (NH4)2SO4 (Figure 2A and 2B) reduced the conidiogenesis process to values of 1.2x107±1.6x102 and 1.4x106±1.2x102 conidia g-1 with Ypx_S: 1.2 and 0.1 conidia per conidia inoculated, respectively. These two treatments rendered the lowest values when compared with other treatments, including a negative control where values of 1.0x108±1.5x102 conidia g-1 Ypx_S: 10 conidia per conidia inoculated (substrate without microelements) were achieved. While the lowest values of conidia production using T. asperellum GRB-HA02 were observed in treatments with MgSO4*7H2O, (NH4)2SO4, and salt mixtures, achieving similar values to those obtained in the control (2.4x107±1.0x102 conidia g-1; Ypx_S: 2.4 conidia per conidia inoculated). On a macroscopic scale, metabolism was directed towards biomass production. According to Šimkovič et al. (2008), the presence of Mg2+ ions inhibit conidiogenesis in fungi because they decrease osmotic pressure in cells. The macroscopic scale shows that adding (NH4)2SO4 does not help sporulation processes. It is possible that ammonium (NH4) is used as a source of nitrogen and redirects cell metabolism toward biomass generation. Finally, the mixture of all microelements did not allow GRB-HA01 and GRB-HA02 to achieve good growth and sporulation, possibly due to the generation of an osmotic imbalance in cells. Studies such as Adnan et al. (2019) and the results obtained in this study show that conidial responses vary greatly among species because each species has specific nutritional requirements that define metabolism and growth. However, there are metabolic adaptations specific to each species’s environment that change conidia production.
Effect of adding microelements on conidiogenesis in liquid culture
Large differences were found in fungi conidia production when different microelements were added to the culture. T. asperellum GRB-HA02 was the microorganism that showed the highest conidia production in media containing the salt mixture of (3.1x109±2.8x103 conidia mL-1) (Figure 3B), with yields of 310 conidia per conidia inoculated. In contrast, GRB-HA01 treatments that used microelement mixtures presented lower values (1.2x108±9.4x103 conidia mL-1), with a Ypx-L of 12 conidia per conidia inoculated. CaCO3 (3.0x107±1.1x102 conidia mL-1) was the only treatment that was below the negative control (4.3x107±6.9x107 conidia mL-1), with a Ypx-L of 3.0 conidia per conidia inoculated. The maximum conidia production for T. asperellum GRB-HA01 (Figure 3A) was achieved by adding KH2PO4 (1.3x109±2.8x102 conidia mL-1), giving a Ypx-L of 130 conidia per conidia inoculated.

Figure 3 Conidia production kinetics in T. asperellum. A. GRB-HA01 and B. GRB-HA02 in liquid fermentation.
In general, when the salts weren’t appropriate or when they were not added to the culture media, conidia production of the T. asperellum GRB-HA01 and GRB-HA02 strains decreased by 80-98% achieving Ypx-L of 0.7-48 conidia per conidia inoculated. For other treatments, using GRB-HA01, a maximum conidia concentration (conidia mL-1) achieved values of 1.9x108±8.9x103 ((NH4)2SO4), 2.6x108±8.8x104 (MgSO4), 3.0x107±1.1x102 (CaCO3), and 1.2x108±9.4x103 (mixture of all microelements) with Yp-L: 19, 26, 3 and 12 conidia per conidia inoculated, respectively. In T. asperellum GRB-HA02 cultures, the effect of reducing conidia production was higher and the maximum concentration had values of (conidia mL-1) 9.6x107±2.7x103 (KH2PO4), 3.3x108±8.4x104 ((NH4)2SO4), 4.8x108±4.7x102 (MgSO4), 6.6x108±1.4x102 (CaCO3), and 1.3x107±8.4x104 (control) with Ypx-L: 9.6, 33, 48, 66, and 1 conidia per conidia inoculated, respectively.
In this study, the highest conidia production in T. asperellum GRB-HA01 and GRB-HA02 strains was achieved in 6 days, after production was kept constant or reduced. For instance, with GRB-HA01, media supplemented with NH4SO4 changed from 4.8x108 to 1.8x108 conidia mL-1, and in cultures enriched with microelement mixture, concentration decreased from 2.6x108 to 1.2x108 conidia mL-1.
Statistical data analyses showed that microelements significantly affected conidia production (P<0.05), achieving maximum production after 6 days of culture. However, Tukey’s analysis demonstrated that KH2PO4 had a significant positive effect on conidia production for T. asperellum GRB-HA01 (P<0.05), achieving values of 1.3x109±2.8x102 conidia mL-1, while for T. asperellum GRB-HA02 the mixture of all treatments favored this conidia production process (P<0.05), with values of 3.1x109±2.8x103 conidia mL-1. These results were similar to those Gonzalez and Vicente (2016) obtained. They evaluated different nitrogen sources and achieved maximum conidia production (1.16x109 conidia mL-1) using 5 g of KH2PO4 and 1.3 g of MgSO4*7H2O, and the lowest production was using (NH4)NO3, and (NO4)*2SO4 at concentrations in the order of 1.0x107 and 1.0x108 conidia mL-1, respectively. This is similar to the results achieved in this study for T. asperellum GRB-HA01 using (NH4)2SO4 (1.9x108±8.9x103 conidia mL-1), MgSO4 (2.6x108±8.8x104 conidia mL-1), CaCO3 (3.0x107±1.1 x102 conidia mL-1), and the mixture with all microelements (1.2x108±9.4x103 conidia mL-1), and for T. asperellum GRB-HA02 with the addition of KH2PO4 (9.6x107±2.7x103 conidia mL-1), (NH4)2SO4 (1.4x108±5.1x102 conidia mL-1) and MgSO4 (4.8x108±4.7x102 conidia mL-1).
When comparing the results of fermentation in liquid state and solid media, important differences were observed in conidia production for T. asperellum GRB-HA01 and GRB-HA02. The addition of micronutrients improved conidia production, reaching the highest values at 9 days of culture in solid fermentations in media enriched with CaCO3 (GRB-HA01: 3.0x1011±2.5x102 conidia g-1; GRB-HA02: 8.0x1010±1.1x101 conidia g-1). In contrast, conidia production in liquid fermentations generated a reduction in conidia concentration (GRB-HA01: 3.0x107±1.1x102 conidia mL-1; GRB-HA02: 6.6x108±1.4x102 conidia mL-1), with values similar to the control. The difference in conidia concentrations in solid and liquid fermentations allowed an increase of 10,000 times more for GRB-HA01 and 100 times for GRB-HA02 in a solid medium. A different response was evidenced at 6 days of culture during conidia production in liquid fermentations. Hence, the highest conidia concentration in GRB-HA01 was reached with the media supplemented with KH2PO4 (1.3x109±2.8x102 conidia mL-1), while for GRB-HA02 it was achieved in the media containing micronutrient mixture (3.1x109±2.8x102 conidia mL-1). However, the study showed values were lower than those in solid fermentations. Hölker et al. (2004) have also observed this effect, and they report that conidia obtained in solid fermentations are more resistant to desiccation and more stable in a dry state. In contrast, the germination capacity of spores obtained in submerged cultures decreases rapidly. In addition, in solid-state fermentations, there is less catabolic repression, less demand for water, and greater conidia productivity.
The results of this study indicate that Trichoderma sporulation processes are the result of biotic and abiotic factors. It is important to carry out a similar evaluation for all Trichoderma species to enhance conidia production. Monga (2001) and Adnan et al. (2019) observed that the production of chlamydospores, hyphae, and conidia or biomass production of Trichoderma species are less dependent on exogenous media and that generating conidia from a wide range of Trichoderma species like T. harzianum, T. viride, T. koningii, T. saturnisporum and T. polysporum has rendered that it depends on environmental acidity and nutrient-deficient systems.
CONCLUSIONS
This study highlights the importance of inoculation as a method to evaluate the conidiogenesis processes of T. asperellum GRB-HA01 and GRB-HA02. Results demonstrate that higher conidia production in T. asperellum can be achieved by inoculating cultures with 1.0x107 conidia mL-1 and a volume of 10 mL. Adding CaCO3 to a culture medium in solid fermentations also led to the highest conidia production for both strains, while adding (NH4)2SO4 and a salt mixture had a negative effect on conidiogenesis. Under liquid culture conditions, the highest conidia production was observed in T. asperellum GRB-HA02 after six days of culture in media containing a mixture of all salts, while T. asperellum GRB-HA01 produced the highest conidia production after nine days of culture adding KH2PO4. Nonetheless, conidia production in liquid culture was less than that of conidia production obtained in solid fermentations for both strains. Overall, this study highlights the importance of optimizing inoculation and microelement addition in both solid and liquid fermentation to enhance conidiogenesis in Trichoderma asperellum, which could have significant implications for the development of more efficient sustainable processes to produce bioactive compounds and biocontrol agents.















