In recent years, berries in Peru have become very important in the food trade. Such is the case of the blueberry, which includes several species and hybrids of the genus Vaccinium (García et al. 2013). The latter is home to about 500 species with a wide geographic distribution, reported mainly throughout the Americas (Mendoza et al. 2020).
The Peruvian native blueberry (Vaccinium sp.) is a perennial species of the Ericaceae family (Mostacero et al. 2015). Currently, this fruit presents a growth in its consumption due to its nutraceutical qualities, mainly its high percentage of antioxidants and its low levels of sugars (Tejada Alvarado et al. 2019). It also possesses bioactive compounds that prevent the risk of degenerative and cardiovascular diseases (Hernández et al. 2017). These aspects allowed it to take on greater importance, amplifying its development prospects, which has motivated the establishment of plantations in the Amazon region.
According to Mendoza et al. (2020), blueberry propagation can be done by botanical seed and vegetative seed. Regarding the first method, this species presents limitations, in terms of seed availability, added to the high dormancy of seeds (Suárez-Ballesteros et al. 2018; Jiménez-Bonilla and Abdelnour 2018). On the other hand, with vegetative seeds, it turns out to be a more advantageous technique, since seeds can be available all year round and genotypes with superior agronomic characteristics can be propagated (Mendoza et al. 2020).
In vegetative propagation, there are endogenous and exogenous factors that favor root formation in cuttings (Castro-Garibay et al. 2019). Saini et al. (2013), mention that auxins have important functions in cuttings, these act in biosynthesis, transport, and signaling during the rooting phase. The most widely used auxin is indole butyric acid (IBA), due to its stability and easy translocation in plants, and can be applied in wide ranges of doses in a large number of species (Báez-Pérez et al. 2015).
Another relevant factor in rooting is the substrate, thanks to its textural properties that allow it to retain temperature, humidity, and porosity during root formation and growth (Yukari et al. 2013). According to Castrillón et al. (2008), the most used substrates for rooting stakes are agricultural soil, sand, peat, vermiculite, pumice, compost, coconut fiber, and rice husk, all with different effects depending on the species. Frías-Moreno et al. (2021) mentioned that to propagate species of the Ericaceae family, it is necessary to use substrates rich in organic matter (Frías-Moreno et al. 2021).
In research conducted on blueberries, Tejada Alvarado et al. (2019), reported that auxin AIB at a concentration of 100 to 200 mg L-1, generated better rooting and root growth percentage. In the same line, Tejada-Alvarado et al. (2021), tested the effect of AIB concentrations and different genotypes of native blueberry, achieving and higher rooting with the concentration of 2,000 mg L-1.
To find effective techniques for the multiplication of native species in a vulnerable state, the aim of this study is to evaluate the influence of indole butyric acid and the type of substrate on the vegetative propagation of the native Peruvian blueberry (Vaccinium sp.) grown in microtunnel.
MATERIALS AND METHODS
Collection of biological material
Blueberry cuttings were collected from remnant plants in natural forests in the province of Rodriguez de Mendoza. These plants had an average height of 0.40 m and were branched. In the early morning hours, apical shoots of 10 cm in length were cut. They were then covered with kraft paper and placed in a properly labeled and conditioned technopor box. In addition, the samples were moistened with a hand sprayer to keep the vegetative material turgid and immediately transferred to the laboratory.
Preparation and sowing of planting material
In the laboratory, the cuttings were standardized to a size of 7 cm. They were then subjected to a disinfection process with a fungicide (mancozeb + propineb at 1g L-1 of water) and left to rest for 3 minutes. Subsequently, the cuttings were prepared for auxin treatment by removing the basal leaves to expose the stem to 1.5 cm. A straight cut was made and the cuttings were immersed in an auxin solution of Indole-butyric acid with a purity of 98% diluted in 96 ° alcohol at various concentrations (1, 2, and 3 g L-1) along with a control (0 g L-1). The excess solution was removed, and the cuttings were allowed to volatilize for 6 to 10 min at a constant temperature of 20 °C.
At the same time, the substrates were prepared (sand 100%, sand + coconut fiber 1:1 v/v, and coconut fiber 100%), disinfected in an autoclave at 121 °C for 3 h, then allowed to dry and eliminate the excess water, until leaving it in field capacity; these substrates were placed in germination trays of 72 cavities, properly distributed according to design.
The cuttings were placed by introducing the auxin-treated base at a depth of no more than 1.5 cm, then a slight pressure was applied to the substrate to put it in direct contact with the cuttings and also to avoid leaving air chambers.
The trays were finally placed in a microtunnel of 3.0x1.0x0.90 m, in which the temperature (>18 °C) and relative humidity (>80%) were controlled and a nebulized irrigation system with forget-type nozzles was incorporated.
Variables studied
After 120 days, the following variables were evaluated: rooting, number of roots, and root size for each factor and the interaction of these.
Experimental design and data analysis
The experiment was conducted under a completely randomized design with a bifactorial. Factor A corresponded to the substrate, with three levels: sand, sand + coconut fiber, and coconut fiber. Factor B was the IBA dose, with four levels: 0, 1, 2, and 3 g L-1. The data complied with the assumptions of normality and homogeneity of variances, therefore, an analysis of variance (α=5%) was performed. Processing of all data was performed with InfoStat Statistical Software version 2020.
RESULTS AND DISCUSSION
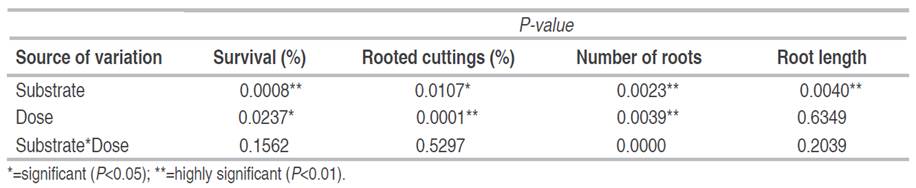
In the overall analysis, significant differences were identified between substrates in rooting (P<0.05), as well as in survival, number, and length of roots (P<0.01). Both the percentage of rooted cuttings and the number of roots showed a high significant difference (P<0.01) between the different doses of AIB; in addition, a significant difference (P<0.05) was observed in survival. However, no significant effects were found in the interaction between both factors (Table 1). On the other hand, Yukari et al. (2013) mentioned that the factors substrate and AIB doses act independently in the rooting process, suggesting that the type of substrate and AIB doses do not affect the percentage of rooted cuttings of Rubus spp. and that the application of AIB is even unnecessary.
Table 1 Probability P value in the rooting of native blueberry mini-shoots with different substrates and AIB concentrations.
The 5% Tukey test (Table 2) was performed for both factors. In the variables of the substrate factor, two ranges of significance were registered; where the sand + coconut fiber substrate registered a statistical difference concerning the other treatments, presenting a higher percentage of survival and rooting; with 94.7 and 63.8%, respectively, as well as a greater number (6.9) and length of roots (9.2 cm). Research conducted by Frías-Moreno et al. (2021) in raspberry, showed that organic matter substrates presented greater efficiency in propagation with an increase in the survival of the number of plants, and also mentioned that substrates with a high content of organic matter and adequate nutrient composition favor vegetative growth. Regarding AIB doses, a statistical difference was observed in the survival percentage at the 3 mg L1 concentration compared to the other doses, also showing the lowest survival percentage (72.92%), which contrasts with the results of Marangon and Biasi (2013), who suggest that an increase in AIB concentration results in a reduction in the percentage of dead cuttings. To rooting percentage, two significant ranges were identified that can be associated with two groups: doses of 0 and 1 g L-1 form one group, while 2 and 3 g L-1 constitute another group. Although no statistical differences were found within each group, both groups differed from each other, with the second group (2 and 3 g L-1) showing the best rooting results. Therefore, the choice of dose for rooting will depend on factors internal or external to the study.
Table 2 Multiple comparisons of means according to Tukey post-hoc test (α=5%) for rooting variables of native blueberry mini shoots.
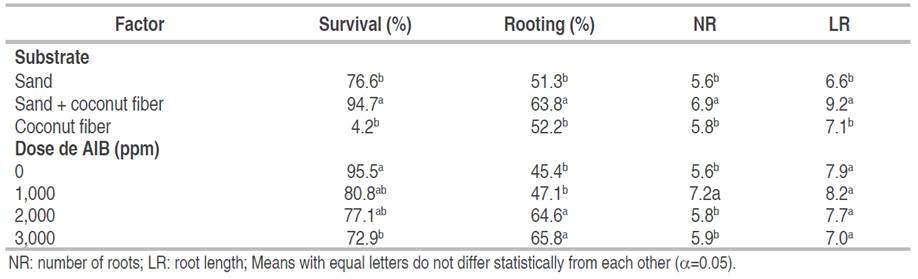
In support of these findings, Marangon and Biasi (2013) note that the highest rooting percentages in different blueberry varieties were obtained with concentrations of 2 g L-1. This concentration also yielded promising results in microscopic cutting of the apical portion of branches of the Climax variety (Schuch et al. 2007). Regarding root number, the 1 g L-1 concentration of AIB showed the best results with a significant statistical difference. However, no significant effects on root length were observed (Table 2).
According to Castrillón et al. (2008), the most important factors in seedling formation are: sources of vegetative material, rooting media, treatments with rooting stimulators, and environmental conditions. In the Tukey test in the interaction of factors (substrate * AIB dose), the best results in the variable number of roots were obtained with the 1 g L-1 dose of AIB in the Sand + coconut fiber substrate, possibly due to its ability to retain adequate moisture and help in the interaction with AIB. Less encouraging results were observed in the sand substrate without the application of AIB, while the average in the coconut fiber substrate decreased more than the control group at the 2 g L-1 AIB dose (Figure 1).
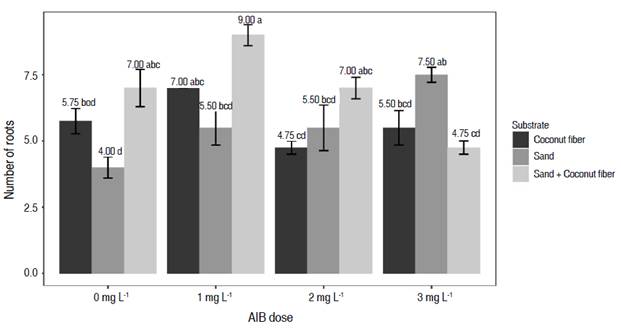
Figure 1 Effect of the interaction between substrate and AIB dose factors on the number of roots of Vaccinium sp.
In the root length variable, a significant effect was observed in the Sand + coconut fiber substrate with a dose of 1 g L-1 of AIB, showing better results. In the Sand + coconut fiber substrates, no statistical differences were found in any AIB dose (Figure 2). However, Báez-Pérez et al. (2015) indicate that the rooting process occurs at the expense of the vegetative material, i.e., the presence or absence of nutrients in a substrate will not affect the rooting power of a cutting, but is subject to the vigor of the mother plant.
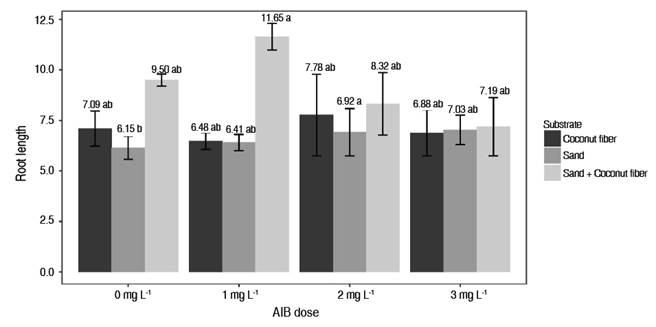
Figure 2 Effect of the interaction between substrate factors and AIB dose on root length of Vaccinium sp.
The substrate sand + coconut fiber gave the best results in the variables number and length of roots at a concentration of 1 g L-1 (Figures 1 and 2). This substrate presents characteristics of good aeration, structure, moisture retention capacity, and drainage, among others (Picolotto et al. 2013); which maximize the effects of the AIB hormone. Likewise, Kumar et al. (2022) mentioned that exposure to high auxin concentrations produces inhibitory effects on root number and length, which would explain the downward trend in the means of the variables with increasing doses of AIB. On the other hand, the sand substrate, having a lower moisture retention capacity and therefore higher porosity, seems to suppress the effects of AIB, presenting an increasing trend with increasing doses of AIB; that is, in the results shown, the substrate variable is a factor that conditions the effects of Auxin.
Figure 3 illustrates the clonal propagation process of native blueberries, depicting the blueberry mother plants in the field and the rooting of minicutting at 120 days, utilizing various substrates and AIB doses. These findings underscore the importance of meticulously selecting substrates and AIB doses to ensure a successful rooting process during the clonal propagation of native blueberries. Considering these variables is crucial for achieving optimal plant development and ensuring success in the clonal reproduction of this species.
CONCLUSION
The substrates used in the study had different effects on the native blueberry mini-stakes. The sand-based substrate favored survival and, in combination with coconut fiber, favored the rooting of the cuttings. AIB auxin, used at a concentration of 2 g L-1, considerably improved the rhizogenic capacity of the cuttings. This study demonstrates the feasibility of propagating native blueberries using available substrates and effective auxins. The developed technique turns out to be a promising alternative for the mass reproduction of native species for the conservation and rehabilitation of degraded ecosystems.
















