Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Actualidades Biológicas
Print version ISSN 0304-3584
Actu Biol vol.30 no.89 Medellín July/Dec. 2008
BIOTECNOLOGÍA
TOTAL POLYPHENOLS ANALYSIS OF MATURE SEEDS AND TISSUE CULTURES OF SOME COLOMBIAN COCOA VARIETIES
ANÁLISIS DE POLIFENOLES TOTALES DE SEMILLAS MADURAS Y CULTIVOS CELULARES DE ALGUNAS VARIEDADES DE CACAO COLOMBIANAS
LUISA F. ROJAS1; JULIÁN LONDOÑO2; ADRIANA M. GALLEGO3; ANDREA L. HERRERA4; CAROLINA AGUILERA5; LUCÍA ATEHORTÚA6.
1Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección electrónica: <luisarojash@gmail.com>
2Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección electrónica: <jalondo@gmail.com>
3Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Ccolombia. Dirección electrónica: <adrianyzzz@yahoo.com.ar>
4Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección electrónica: <lore.herrera@gmail.com>
5Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección electrónica: <caroaguilerag@gmail.com>
6Sede de Investigación Universitaria (SIU). Torre I–210, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección electrónica: <latehor@gmail.com>
Abstract
The aim of this research was to establish cocoa (Theobroma cacao) cell suspensions culture to analyze the total polyphenols content for two Colombian cocoa varieties and to compare the results with the total polyphenols content from the same field varieties. The final results showed that it is possible to produce big amount of cocoa cell biomass able to synthesize the metabolites without loosing its organoleptic properties (smell, color, and flavor), and to produce an acceptable content of total polyphenols compared with the natural seeds. This preliminary study is a promising perspective for future production of the antioxidants and to supplement with them the cocoa by–products
Keywords: plant biotechnology, polyphenols, secondary metabolites, Theobroma cacao
Resumen
El propósito de esta investigación fue el establecimiento de suspensiones celulares de cacao (Theobroma cacao) para analizar el contenido de polifenoles totales en dos variedades de cacao Colombianas y comparar estos resultados con el contenido total de polifenoles para las mismas variedades de campo. Los resultados finales mostraron que es posible producir gran cantidad de biomasa de cacao capaz de sintetizar los metabolitos sin perder sus propiedades organolépticas (olor, color y sabor), y producir un contenido aceptable de polifenoles totales comparado con las semillas naturales. Este estudio preliminar es una perspectiva promisoria para la producción futura de antioxidantes y suplementar con ellos los productos derivados del cacao.
Palabras clave: biotecnología vegetal, metabolitos secundarios, polifenoles, Theobroma cacao
INTRODUCTION
Cocoa (Theobroma cacao L.) has been used in Mesoamerica for beverages since 1.000 years B. C. (Henderson et al., 2007). Scientific reports indicate that cocoa and chocolate are potential sources of antioxidants (Cooper et al., 2008). It had been found that cocoa is a rich source of antioxidant which reduces inflammation and it is correlated with reduction of heart disease risk, thus increasing both its popularity and use (Cooper et al., 2008; Keen et al., 2005).
The antioxidant properties are due to the polyphenols found in cocoa beans which are stored in the cotyledon tissue of the seeds, changing their color according to the anthocyanins content from light red–yellow purple to dark purple which belongs to the flavonoids group. In cocoa, the main polyphenols are catechins (37%), anthocyanins (4%) and proanthocyanidins (58%). The main catechin is (–)–epicatechin, which is nearly 30% of the total polyphenols content, and other few catechins are (+)–catechin, (+)–gallocatechin, and (–)–epigallocatechin. For anthocyanin the main fraction consist on cyanidin–3–arabinose and cyanidin–3–D–galactose; and for procyanidins the most abundant polyphenols are those of dimeric, trimeric, and oligomeric units of epicatechin and flavan–3,4–diol (Romanczyk,1997).
In Colombia, cocoa is one of the main tropical industrial crops and there is a great interest in promoting field culture for elite varieties to improve national competitiveness as well as productivity toward the industrial sectors. To contribute with this national commitment, the research in plant biotechnology through the plant cell suspension culture could offer interesting possibilities to study the cocoa polyphenols production and other cocoa by–products.
Cocoa cell culture has been developed in solid media (Janick and Pence, 1980, 1981; Tsai and Kinsella, 1981) as well as in liquid media (Gurney et al., 1992; Jalal and Collin, 1979; Leathers and Scragg, 1989; Tsai and Kinsella, 1981, 1982; Wen and Kinsella, 1992) for different purposes: effect of the culture media on cell growth (Leathers and Scragg, 1989; Tsai and Kinsella, 1981, 1982), induction of somatic embryogenesis (Janick and Pence, 1980, 1981), effect of temperature and growth pattern associated to the lipid and fatty acid content (Leathers and Scragg, 1989), and only few studies have focused in the secondary metabolites production, mainly flavonoids (Jalal and Collin, 1979) and purinic alkaloids (Jalal and Collin,1979; Gurney et al., 1992).
In relation to the polyphenols production in liquid culture, Jalal and Collin found that percentage of their production is smaller during the exponential phase, but increase during the stationary phase. They also analyzed the catechin content in callus as well as cell suspension culture, finding similar levels or even relatively lower values for the variety studied by them (Jalal and Collin, 1979).
Since polyphenols content is a premium feature for the industrial sector, there is a great interest in analyzing the total polyphenols content in different cocoa varieties under different environmental conditions, and the factors that can affect their production and preservation in its by–products. This research deals with the analysis of the total polyphenols content in callus and suspension cultures of two Colombian cocoa varieties (BIOA and BIOD) compared with the total polyphenols content from the same field varieties.
MATERIALS AND METHODS
Plant source. Two Colombian varieties (BIOA and BIOD) of T. cacao from Experimental Station of Compañía Nacional de Chocolates (CNCH) were selected for this study. Cocoa cobs were sampled in field using a stratified–randomized methodology.
Callus induction and growth. Explants of inmature cocoa cotyledons between 3 and 4 months of age were cultured aseptically on DKW solid medium supplemented with vitamins and zeatin (0.1–1.0 mg/l). Cultures were incubated in darkness at 22 ± 1 °C and sub–cultured every 4 weeks for friable callus formation.
Establishment of cell suspension cultures. 5.0 g (FW) of friable callus tissue was inoculated into a flask containing 100 ml of DKW liquid medium supplemented with vitamins, antioxidants such as cysteine, and ascorbic acid (10–50 mg/l), and zeatin (0.1–1.0 mg/l). The pre–inoculum was incubated in darkness at 22 ± 2 °C in a Gufa orbital shaker at 90 rpm. The suspension cultures were sub–cultured every 15 days until reaching an adequate culture establishment.
Kinetics of both cell growth and substrate consumption. For this study only the variety BIOA as a model was used. Initially, three different concentrations of inoculum were studied: 0.3, 0.5, and 1.0 gr (FW) cells in 10 ml of culture medium. Batch cultures were initiated with the cells from pre–inoculum and were transferred into a 100 ml flask containing 10 ml of the same liquid medium under the same operative conditions. The flasks were incubated during 18 days. The growth rate was monitored every 3 days by measuring fresh weigth (FW) and dry weight (DW) during 36 hours at 45 °C, to avoid polyphenol degradation (Wollgast and Anklam, 2000)
The final kinetic study was carried out using the best inoculum size and the same nutritional and operational conditions. DNS method for reducing sugars was used to test the substrate kinetics.
Polyphenols analysis. For both field samples and cell biomass, 10 g of material were dried at 45 °C per 36 hours, and refluxed into 500 ml of hexane for one hour for degreasing the tissue. The extraction process was done with acetone:water (60:40). The samples were placed in a rotary evaporator to eliminate traces of acetone and after that, they were kept in amber vials to pursue the spectrophotometric analysis and to quantify total polyphenols expressed as Cathechin Equivalent through Folin–Ciocalteu method.
Statistical methods. In the field, twelve cobs for each variety were collected for this study. The cobs were harvested under CNCH protocols according to the size and maturity features. Mean and standard deviation values were calculated for total polyphenols content and population variance was estimated in order to find out the dispersion of the variability.
RESULTS
Field sample analysis. 12 cocoa cobs were harvested from maturity stage of BIOA variety. They were taken from 15 years old trees, nearly 3 m in height and although the trees were generally healthy, some of the cobs were affected by the insect monalonion and Xyleborus sp. (smuggler). The average size of the seeds was 2.5 cm, dark purple color. For the BIOD variety, growing conditions and plant features were similar, but trees were younger (4 years old) and the seeds color were lighter than variety BIOA.
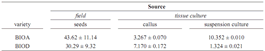
Total polyphenol content for variety BIOA was 43.62 ± 11.14, while for variety BIOD was 30.29 ± 9.32, both in Cathechin Equivalents Units. Perhaps, these differences are due to the genetic variability of each variety expressed by the color of the seed that is darker for BIOA than for BIOD, and for the age of the trees. The concentration of flavonoids in cocoa was analyzed taking into account the phenotype of each variety and their environmental growing conditions. However, both varieties were growing in the same environmental conditions but they expressed differences in the total polyphenol content, perhaps due to the age of the plants and their genotypes. In fact, the color of seeds was different and, according to Cakirer and collaborators, flavonol accumulation was directly proportional to the intensity seed color (Cakirer et al., 2003).
Callus culture growth. The callus was initiated after four weeks of incubation for both varieties. The development of the callus for BIOA variety is shown in figure 1. The color changes of calluses were associated with the physiological maturity stage of the tissue and polyphenols accumulation as well as in the seeds. The friability of the calluses was reached around the sixth month in the variety BIOA and at the fourth month in the variety BIOD.
Figure 1. Serial sequences of callus development according to variety BIOA in DKW solid medium supplemented with vitamins, zeatine, and antioxidants. A. White callus starts growth at third month on cotyledonal tissue. B. At fourth month callus tissue is beige with granular appearance. C. Brown friable callus is obtained approximately at sixth month
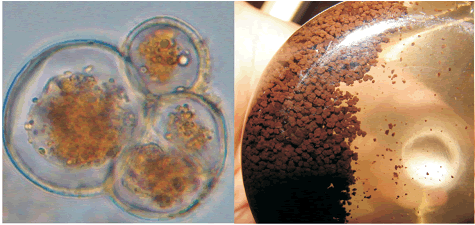
Cell suspension culture growth. Callus culture was transferred into DKW liquid medium and the suspension culture was established after 45 days for both varieties. After the third sub–culture, the cells proliferated into small and disaggregated cells and were suitable to initiate a batch culture. The percentage of viability was around 70% and the cells were round (figure 2A). The variety BIOA disaggregated faster than variety BIOD. Cell suspension cultures were dark brown (figure 2B). Younger cells changed their color from beige to light brown depending on the culture phase.
Figure 2. Details of cocoa cell suspension culture for BIOA variety on liquid DKW medium supplemented with vitamins, zeatine, and antioxidants. A. Cocoa cells in exponential phase. B. Cell suspension cultures of four weeks after establishment
Inoculum size effect. According to the results of inoculum size assay, cultures for next studies were inoculated with 0.5 g (FW) cells in 10 ml of culture medium. The cell growth corresponds to a normal pattern as has been described in literature (Street, 1973). The other inoculum sizes tested did not show a normal growth pattern and they were very unstable with consecutive increases and decreases in the rate of biomass production.
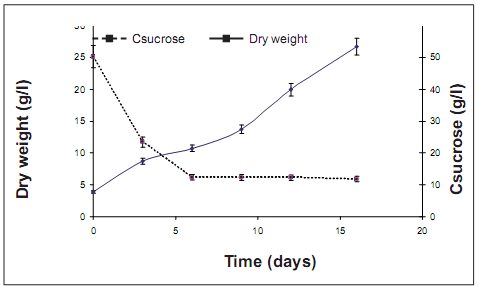
Kinetics of both cell growth and substrate consumption. Batch cultures were initiated with 0.5 g of fresh cells and 6–fold of biomass was reached after 16 days of culture (figure 3). Lag phase was not observed and cells started exponential phase at the first day of culture. The pattern of the substrate consumption seems not to be related with the rate cell growth, since decreases quickly around 6th day and stay invariable for the next days. This pattern suggests that carbon source is not the limit substrate, and perhaps were other nutrient factors that could stimulate the cell growth. The biomass at the final of the culture was filtered and dried to evaluate some organoleptic properties like color, smell and flavor. During this preliminary evaluation it was observed that cocoa biomass was characterized by a brown color, with a soft smell and flavor similar to the cocoa field seeds. Those results indicate that the total polyphenols obtained by cell culture could be used as raw material for cocoa
Table 1: Total polyphenol content expressed by Cathechin Equivalents (mg by gram of sample; data are shown as media ± SEM)
figure 3. growth bioA cells variety and substrate consumption in batch suspension culture
by–products, but there is still a lot work to be done before the industrial level. Bromatologic, citotoxic, mutagenetic, carcinogenic analysis as well as those for cell and polyophenols stability, among others, are mandatory in order to supplement cocoa by–products.
Polyphenol analysis. Comparison of total polyphenols content among seeds, callus, and cell suspension cultures of both varieties are shown in table 1. The total polyphenol content in seed from BIOA variety is higher than BIOD; although both varieties were cultured under the same agroecological conditions (soil, precipitation, humidity, and temperature), they came from trees of different ages. On the other hand, comparing calluses culture from the two varieties, the total polyphenols content was higher for the BIOD variety, but in cell suspension culture, the results show the opposite results, being the highest for the BIOA variety (table 1).
DISCUSSION
For the field seeds, there are some plausible explanations for the results were obtained. Phenolic compound biosynthesis and accumulation are affected by biotic and abiotic conditions (Dixon and Paiva, 1995), especially by UV irradiation and nutrient starvation (Giorgi et al., 2009), among others. One of the main functions of flavonoids in plants is the protection against stress conditions (Winkel–Shirley, 2002). The change in the increase in total polyphenol content for BIOA from callus (3.267) to suspension culture (10.352) could be explained by the stress caused when the cells passed from solid to a liquid media. However that explanation does not seem to apply to BIOD variety, which suddenly decreased the polyphenol content in suspension culture; this result was probably due to genotype expression and the age of the trees, from which explants came from.
Flavonoids are secondary metabolites produced in plant due to stress generated by biotic or abiotic conditions with many biological functions, apparently including roles in stress protection (Winkel–Shirley, 2002). Plant cell culture are promising potential alternative sources for the production of these metabolites, for this reason and even when the polyphenol content into cell suspension culture was lesser than the content in the seeds of thus study, there are still some interesting strategies available to increase the secondary metabolite production, including elicitation, immobilization, and manipulation of some nutrient as well as to improve yield (Ramachandra and Ravishankar, 2002). Cysteine and ascorbic acid were used in the media culture, to activate cell growth; however the presence of these antioxidants seems to inhibit polyphenol production as shown in the callus and cell suspension culture total polyphenol content compared with the seed content (table 1).
Antioxidants use and effect. Exposure to environmental stress results in increased production of oxidative species such as superoxide, hydrogen peroxide, and nitric oxide in plants (Delledonne et al., 1998). The ability to survive to these cellular toxins depends on the metabolic responsiveness of detoxification mechanisms since Reactive Oxygen Species (ROS) and reactive nitrogen species have both direct and indirect effects on the cellular redox state and the expression of various stress–related genes, including those involved in antioxidant defense (Durner et al., 1998).
Ascorbic acid has a pivotal role in plant cells as an antioxidant molecule that prevents oxidative stress caused by photosynthesis, oxidative metabolism or exposure to pollutants (Loewus, 1999). In addition, an increase in the synthesis of phenolic compounds is another common response to environmental stress in plants (Dixon and Paiva, 1995). The accumulation of anthocyanin pigments in vegetative tissues is a hallmark of plant stress. In many cases, these compounds may provide antioxidant activity as part of a general stress response, however, there is also evidence that flavonoids may function in plants to screen harmful radiation, bind phytotoxins, and help to regulate the stress response by controlling auxin transport (Jacobs, 1988).
Currently, there is not any available support to explain polyphenols content in cell suspension culture that could be due to the gene expression of each genotype, as well as the age of the tissue from the original explants the samples came from, but may be other causes that are not possible to explain here, due to the lack of data and more scientific studies and research. However, to know polyphenol production mechanisms that have been widely studied in plants could be used as another tool to increase the synthesis in cell suspension cultures.
ACKNOWLEDGEMENTS
The authors thank to Servicio Nacional Aprendizaje (SENA), Universidad de Antioquia, and Compañía Nacional de Chocolates for the financial support and technical assistance.
REFERENCES
Bhojwani SS, Razdan MK. 1996. Plant tissue culture: Theory and practice, a revised edition. Elvesier Science. Amsterdam. [ Links ]
Cooper K, Donovan J, Waterhouse A, Williamson G. 2008. Cocoa and health: a decade of research. British Journal of Nutrition, 99(1):1–11. [ Links ]
Cakirer M. 2003. Color as an indicator of flavonol content in the fresh seeds of Theobroma cacao. The Pennsylvania State University. <http://guiltinanlab.cas.psu.edu/Publications/Cocoa/Melisthesis.pdf>. Fecha de consulta: 21 de febrero de 2007. [ Links ]
Dixon RA, Paiva N. 1995. Stress–induced phenylpropanoid metabolism. Plant Cell, 7(7):1085–1097. [ Links ]
Delledone M, Xia Y, Dixon R, Lamb C. 1998. Nitric oxide functions as a signal in plant disease resistance. Nature, 394(6693):585–588. [ Links ]
Durner J, Gow A, Stamler J, Glazebrook J. 1998. Ancient origins of nitric oxide signaling in biological systems. Proceedings of the National Academy of Sciences, 95(25):14206–14207. [ Links ]
Giorgi A, Mingozzi M, Madeo M, Speranza G, Cocucci M. 2009. Effect of nitrogen starvation on the phenolic metabolism and antioxidant properties of yarrow (Achillea collina Becker ex Rchb.). Food Chemistry, 114(1):204–211. [ Links ]
Grace SC, Logan BA. 2000. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philosophical Transactions of the Royal Society B: Biological Sciences, 355:1499–1510. [ Links ]
Gurney K, Evans L, Robinson D. 1992. Purine alkaloid production and accumulation in cocoa callus and suspension cultures. Journal of Experimental Botany, 43(6):769–775. [ Links ]
Hammerstone JF, Schmitz HH. 1998. Cocoa components, edible products having enhanced polyphenol content, methods of making same and medical uses. Patent Cooperation Treaty (PCT) WO 98/09533, Mars Incorporated, U.S.A. [ Links ]
Henderson J, Joyce R, Halls G, Hurst J, McGovern P. 2007. Chemical and archaeological evidence for the earliest cocoa beverages. Proceedings of the National Academy of Sciences, 104(48):18937–18940. [ Links ]
Jacobs M, Rubery P. 1998. Naturally occurring auxin transport regulators. Science, 241:346–349. [ Links ]
Jalal M, Collin H. 1979. Secondary metabolism tissue culture of Theobroma cacao. New Phytologist, 83(2):343–349. [ Links ]
Janick J, Pence VC. 1980. Method of non–agricultural production of cotyledons. US Patent 4.204.366. Purdue Research Foundation, West Lafayette, IN. [ Links ]
Janick J, Pence VC. 1981. Plant tissue produced by non–agricultural proliferation of cocoa embryos. US Patent 4.301.619. Purdue Research Foundation, West Lafayette, IN. [ Links ]
Kim H, Keeney PG. 1984. (–)Epicatechin content in fermented and unfermented cocoa beans. Journal of Food Science, 49:1090–1092. [ Links ]
Keen C, Holt R, Oteiza P, Fraga C, Schmitz H. 2005. Cocoa antioxidants and cardiovascular health. American Journal of Clinical Nutrition, 81(1):298S–303S. [ Links ]
Leathers RR, Scragg AH. 1989. The effect of different temperatures on the growth, lipid content and fatty acid composition of Theobroma cacao cell suspension cultures. Plant Science, 62(2):217–227. [ Links ]
Loewus FA. 1999. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry, 52:193–210. [ Links ]
Ramachandra S, Ravishankar G. 2002. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnology Advances, 20:101–153. [ Links ]
Romanczyk LJ, Hammerstone, JF, Buck MM, Post LS, Cipolla GG, Micceland CA, Mundt JA, Schmitz HH. 1997. Cocoa extracts compounds and methods for making and using the same. Patent Cooperation Treaty (PCT) WO 97/36497, Mars incorporated, U.S.A. [ Links ]
Street HE. 1973. Plant Tissue and Cell Culture. Blackwell Scientific Publications. London, England. [ Links ]
Tsai C, Kinsella J. 1981. Initiation and growth of callus and cell suspensions of Theobroma cacao L. Annals of Botany, 48:549–558. [ Links ]
Tsai C, Kinsella J. 1982. Tissue culture of cocoa bean (Theobroma cacao L.): Incorporation of fatty acids into lipids of cultured cells. Lipids, 17(12):848–852. [ Links ]
Winkel–Shirley B. 2002. Biosynthesis of flavonoids end effects of stress. Current Opinion in Plant Biology, 5:218–223. [ Links ]
Wollgast J, Anklam E. 2000. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Research International, 33:423–447. [ Links ]
Yamasaki H, Sakihama Y, Ikehara N. 1997. Flavonoidperoxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiology, 115:1405–1412. [ Links ]
Yamasaki H, Grace S. 1998. EPR detection of phytophenoxyl radicals stabilized by zinc ions: evidence for the redox–coupling of plant phenolics with ascorbate in the H2O2–peroxidase system. FEBS Letters, 422:377–380. [ Links ]
Recibido: octubre 2008.
Aceptado para publicación: diciembre de 2008.