Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Actualidades Biológicas
Print version ISSN 0304-3584
Actu Biol vol.30 no.89 Medellín July/Dec. 2008
BIOTOXICIDAD
TRIAL EVALUATION OF TOXICITY AND PATHOLOGY IN MURINE TISSUES USING BACILLUS SPHAERICUS COLOMBIAN STRAIN
EVALUACIÓN PRELIMINAR DE LA TOXICIDAD Y PATOLOGÍA DE LA CEPA COLOMBIANA BACILLUS SPHAERICUS EN TEJIDOS DE RATÓN
Silvia C. Rivera–Rodríguez1; jenny Dussan–Garzón2
1Centro de Investigaciones Microbiológicas (CIMIC). Departamento de Ciencias Biológicas, Universidad de los Andes. Bloque J, Oficina # 207. Calle 18 # 0–03. Bogotá, Colombia. Dirección electrónica:<si–river@uniandes.edu.co>
2Centro de Investigaciones Microbiológicas (CIMIC). Departamento de Ciencias Biológicas, Universidad de los Andes. Bloque J, Oficina # 207. Calle 18 # 0–03. Bogotá, Colombia. Dirección electrónica: <jdussan@uniandes.edu.co>
Abstract
Colombian isolate Bacillus sphaericus OT4b25 was tested for toxicity and pathology on rabbits and mice as mammalian models. After a high single dose of microorganism (108–109 spores/ml) in four different tests that included dermal, inhalatory, oral, and intraperitoneal routes of administration, subsequent daily observations were performed and weekly body weight was measured. In the oral and intraperitoneal routes infectivity was evaluated by a gross necropsy at interim or final sacrifice, recovering the microorganism both from organs and from peripheral blood. In the oral test, clearance of microorganism was estimated in feces and in the inhalatory test it was estimated from lungs. In both tests a decrease of the recovered microorganism was observed in time. Even though in the oral test recovery of the microorganism from the liver, spleen and lungs and additionally in the intraperitoneal from caecum and injection site was observed, it was not recovered from blood and brain in both tests. Although a persistence of the microorganism in animals was observed after oral, inhalatory and intraperitoneal routes of administration, no animals died or showed lesions during each test, and the body weight was not affected compared with the control groups. In conclusion, at the conditions and doses tested Bacillus sphaericus OT4b25 is not toxic or pathogenic following dermal, oral, inhalatory, and intraperitoneal routes of administration.
Key words: Bacillus sphaericus, biological control, Colombian isolate, pathology, toxicity
Resumen
La toxicidad y patogenicidad del aislamiento colombiano de Bacillus sphaericus OT4B25 fue probada en conejos y ratones como modelos mamíferos. Después de la administración de una sola dosis del microorganismo (108109 esporas/ml) en cuatro diferentes pruebas que incluían rutas de administración dérmica, inhalatoria, oral e intraperitoneal, se realizó un seguimiento con observaciones diarias y la determinación del peso de los individuos semanalmente. En las dos últimas rutas de administración la infectividad fue evaluada a través de necropsias en sacrificios interinos intentando recuperar el microorganismo de órganos y sangre. En la prueba oral, se estimó la eliminación del microorganismo en heces y en la inhalatoria se hizo de los pulmones, en ambas se observó la disminución en los títulos del microorganismo recuperado a través del tiempo. aunque en la ruta de administración oral el microorganismo se recuperó principalmente del hígado, bazo y pulmones, adicionalmente a estos órganos del sitio de inyección y del ciego en la intraperitoneal, no se recuperó de sangre o cerebro en ambas pruebas. a pesar de la persistencia del microorganismo en los animales tras su administración oral, inhalatoria e intraperitoneal, no se observaron muertes o lesiones durante ninguna de las pruebas y el peso corporal no se vio afectado comparado con los grupos control. En conclusión, en las condiciones y dosis probadas, la cepa de Bacillus sphaericus OT4B25 no es patógena o tóxica a través de las rutas de administración probadas.
Palabras clave: aislamiento colombiano, Bacillus sphaericus, control biológico, patogenicidad, toxicidad
INTRODUCTION
Among the many options available for integrated pest management, biological control achieved by microorganism has many advantages (in contrast with conventional chemical pesticides): microorganism are biodegradable, do not produce significant disequilibria in ecosystems and act with specificity on their target organisms, not representing an important risk for others and resulting in low expositions to the environment avoiding pollution problems (DeBach, 1987; Singer, 1990). Microbial pesticides that have been proved to mitigate plagues must be studied to evaluate their potential toxic and contaminant effects before being introduced to the environment, to guarantee the absence of any risk, enhance their advantages and benefits as biocontrollers.
Concerning the biosafety of microbial pesticides, one of the leading regulatory bodies is the usa Environmental protection agency (US EPA ), which together with the US Office Pesticides programs (US OPP ) has defined the policies, rules and test guidelines for assays that evaluate the risk of biological and biologically derived pesticides. according to the us Epa, microbial pest control agents require toxicological/pathological test a requirement for registration (US EPA, 2007).
The colombian isolate Bacillus sphaericus OT4b25 has been studied since it was isolated, selected by its larvicidal capacity (Dussan et al., 1996, 1997) and characterized at molecular and physiological levels (Dussan et al., 2002). additionally, viable systems for its production and scaling at industrial level have been investigated (ortega, 2004; Zamora, 2006), showing its great potential as a microbial pest control agent on third instar larvae of Culex quinquefasciatus, with an LC50 of 3,311 x102 CFU/ml when the stain was cultivated in the same media evaluated in this work (Zamora, 2006), with the goal of proposing it as a competitive option ahead of other control systems for Culex quinquefasciatus larvae. Even though there are many studies that consider B. sphaericus as a non risk organism (Mancebo et al., 2003; Shadduck et al., 1980; US EPA, 1998), the strain OT4b25 must be considered susceptible to genetic changes, and determinant factors such its concentration, dynamics and degradation under specific conditions and growth media, could influence its particular behavior (Ortega, 2004; Zamora, 2006). Thus, a necessary step for a reliable and safe application at the effective doses in the environment is the practical certainty that biosafety assays could provide.
The goal of this study is to carry out toxicity and pathology dermal, oral, inhalatory and intraperitoneal assays for B. sphaericus strain OT4b25 as a series of test relating to the first grade of toxicity evaluation of this colombian isolate, whose conditions of culture and larvicide activity have been evaluated in previous studies (Dussan et al., 1997, 2002; Ortega, 2004; Zamora, 2006).
MATERIALS AND METHODS
Strain and culture media. The strain OT4b25 of B. sphaericus was isolated and characterized in previous studies (Dussan et al., 1996, 1997, 2002; Ortega, 2004; Zamora, 2006). 50 ml of culture media (sodium acetate 5.00 g/l, yeast extract 3 g/l, MgCl2 1 x 10–3 M, CaCl2 7 x 10–4 M, and MnCl2 5 x 10–5 M) were inoculated at 1% on 250 ml erlenmeyers flasks from an ON culture in brain heart infusion broth (BHI). The erlenmeyers were incubated in agitation (140 rpm, 30 °c) for 5 days. these were monitored continuously through microscopic observations to obtain an axenic and highly sporulated culture. the determination of colony Forming units (CFUs) and spore concentration in culture was made with serial dilutions on sterile water and plated on spc agar. For spores a previous heat shock (95 ºc by 15 minutes) was made before plating.
Toxicity and pathology assays. All tests were conducted according with Health Ministry resolution N.º 008430 of 1993 and Colombian Government law 84 from 1989. The assays were conducted following the Microbial Pesticide Test Guidelines of the Office of Prevention Pesticides and Toxic Substances (OPPTS) of Environmental Protection Agency (US EPA 2006, 2007): Documents OPPTS 885.3100, 885.3050, 885.3150, 885.3200, (Guidelines of acute dermal, oral, pulmonary and injection test respectively) (US EPA, 1996). A minimum of 25 female and 25 male mice (CF–W) 4 weeks old, 3 female and 3 male rabbits (New Zealand White) 70 days old were obtained from a commercial hatchery, and used for the tests free of parasites or pathogens with nulliparous and non pregnant females. Animals were maintained in an acclimatized room at 25 ºC and fed ad libitum. After a prior observation period of 3 days, administration of the bacterial inoculum was done through each route: 1 ml/animal with 1 x 109 CFU (3 x 108 spores) for the dermal test, 45 ml/animal with 5 x 109 CFU (8 x 108 spores) for the oral test, 60 ml/animal with 1 x 109 CFU (2 x 108 spores) for the inhalatory test and 0.5 ml/animal with a concentration of 2 x 109 CFU (4 x 108 spores) for the intraperitoneal test. The concentration of the original inoculum was reached by centrifugation and re–suspension in the same culture media for the first three tests. For the intraperitoneal test the microorganism was re–suspended in sterile saline solution, and the culture media without microorganism was administered to another experimental group. In all cases the microorganism was inactivated by autoclave and not by heat (because the nature of spore formation of B. sphaericus).
Clinical observations were made at least once each day on a period of 4 weeks for oral, inhalatory, and intraperitoneal; and 2 weeks for dermal tests. In the dermal test there were 2 experimental groups with equal numbers of females and males: 4 individuals to which the microorganism was administrated in the indicated concentration (inoculum group) and 2 individuals which were treated with sterile water in the same conditions (control group). In the oral and inhalatory tests, there were 3 experimental groups with equal number of females and males: 34 individuals to which the inoculum in the indicated concentration was administrated (inoculum group), 8 to which inactive microorganism was administrated (inactive inoculum group) and 8 control individuals to which no treatment was administrated (control group). The intraperitoneal test were performed with 4 experimental groups with equal number of females and males: 36 individuals to which the inoculum in the indicated concentration was administrated (inoculum group), 4 to which inactive microorganism was administered (inactive inoculum group), 4 to which culture media without microorganism was administered (culture media group), and 8 control individuals to which no treatment was administrated (control group). In the oral test, clearance of the administered microorganism was measured recovering a pool of 1 g of feces of each experimental group two times a week, and the CFU/g of feces were determined by plate count in agar (with the same composition of the culture media previously mentioned plus 1.5% agar) achieved by duplicate. Before administration of microorganism, the normal biota in feces was examined for the presence of similar microorganisms in micro and macro–morphology to the one administered. In the inhalatory test, clearance was measured extracting lungs from 3 individuals from the same sex weekly. Lungs were weighed and homogenized in PBS pH 7.4 in a 1:1 weight:volume proportion helped by a sterile scalpel. The number of microorganism recovered (CFU/g lungs) was determined by plate count in agar (with the same composition of the culture media mentioned above plus 1.5% agar). In all cases the colony morphology of B. sphaericus was confirmed by gram stain and microscopic observation. In oral and intraperitoneal tests, infectivity and persistence of the microorganism was evaluated through gross necropsy and observation of growth in organs (spread with a sterile rake on agar with the same composition as the culture media).
Statistical methods. All the statistical procedures were done in Statistix 8.0. For all tests differences in body weight between treatment groups (inoculum, inactive inoculum, culture media inoculum and control groups depending of the test) for each day measurements were evaluated with a two–way analysis of variance without interaction procedure. The proposed statistic model describes that the effect in body weight is the sum of the effects of treatments (Ci) and sex of the individuals (Dj).
For the oral test, differences in the UFC/g of feces between sex and days were evaluated with a two–way analysis of variance where the dependent variable was the UFC/g of feces that is affected by sex and day post–administration. For this analysis the exponential data were transformed with ln (natural logarithm). For oral and intraperitoneal test, the evaluation of differences between frequencies of microorganism isolation from organs in time and sexes was made with a three–way analysis of variance. Where the statistical model proposes the percentage of isolation as a dependent variable and the organ of isolation and sex as the variables whose effect influences the prior variable.
For the observation of the statistical different groups in the CFU/g of feces and frequencies of microorganism isolation from organs there was made a Tukey test as an all–pair wise comparison with á = 0.05.
RESULTS
During the assay period there were no animal deaths, signs or symptoms of disease, effects on skin and fur, eyes and mucous membranes, respiratory system, circulatory system, somatomotor activity and behavior pattern. The null hypothesis that the means of weights of individuals inside each treatment group separated by sex for each day of measure is equal is accepted in all cases. Statistical values F and P for each hypothesis are shown in figures 1–4.
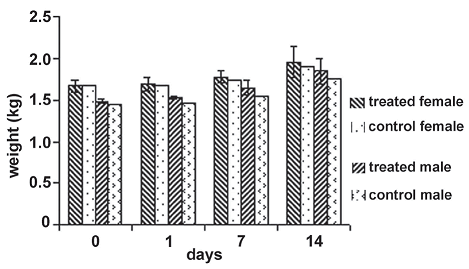
Figure 1. Weight average behavior of individuals in the experimental groups during the toxicity and pathology dermal assay. The statistical values for the null hypothesis that the means of the weights of individuals inside each treatment group separated by sex for each day of measure is equal, is accepted in all cases with day 0: F = 0.16, P = 0.7246; day 1 : F = 0.66, P = 0.5029; day 7: F = 0.78, P = 0.4710; day 14: F = 0.23, P = 0.6782
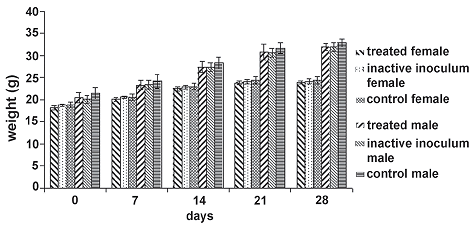
Figure 2. Weight average behavior of individuals in the experimental groups during toxicity and pathology oral assay. The null hypothesis that the means of the weights of individuals inside each treatment group discriminate by sex for each day of measure is equal, is accepted in all cases (day 0: F = 2.88, P = 0.0668; day 7: F = 1.90, P = 0.1632; day 14: F = 1.70, P = 0.1979; day 21: F = 1.21, P = 0.3156; day 28: F = 2.73, P = 0.0896)
Toxicity and pathology dermal test. For the assay period, there were no skin lesions, irritation or any apparent changes on it. Skin remained intact and healthy as in the control group. As not even a small adverse effect was observed, it was decided that the dissection of animals was not necessary.
The weight of individuals showed a similar pattern along the assay period for the two experimental groups without statistical differences (figure 1); both groups treated with the inoculum and the control group increased in a similar way.
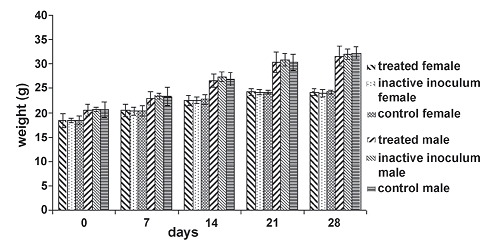
Figure 3. Weight average behavior of individuals in the experimental groups during toxicity and pathology inhalatory assay. The null hypothesis that the means of the weights of individuals inside each treatment group discriminate by sex for each day of measure is equal, is accepted in all cases (day 0: F = 0.08, P = 0.9277; day 7: F = 0.08, P = 0.9215; day 14: F = 0.48, P = 0.6213; day 21: F = 0.09, P = 0.9186; day 28: F = 0.24, P = 0.7893)
Toxicity and pathology oral assay. During this assay, there was no mortality of any experimental unit; none of the animals exposed to the inoculum or the inactive inoculum showed behavioral or clinical abnormalities.
Clearance of the microorganism detected in feces (table 1) was observed only in the inoculum group and was not detected in either the control or the inactive inoculum groups. In the recovery of the first samples (day 0), one order of magnitude less (108 CFU) than the administered microorganism (109 CFU) was recovered in the inoculum treated groups. The null hypothesis that the UFC/g of feces between sex is equal is
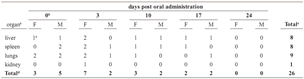
Table 1. Plate count of CFU (Colony Forming Units) in feces during observation period (CFU/g) in the oral test [IG = inoculum group; a = only the treatment group where the microorganism was detected is shown (inoculum group). In the inactive inoculum and control groups, microorganism was not detected. The values are the means of two replicas of plate counts for each determination day; b = day 0 corresponds to the administration day, the samples were taken 4 hours after administration; c = number of CFU/g of feces]. Statistical values are explained inside text
accepted (F = 29.21; P = 0.0003). Although, the bacterial counts were maintained for 7 days after administration and they diminished in time until day 24 when the microorganism was not detectable under a 10–3 dilution (detection limit), there were no statistical differences in the UFC/g of feces inside the first 14 days post–administration, and only the measures taken on days 17 and 21 had statistical difference belong to a different homogeneous group.
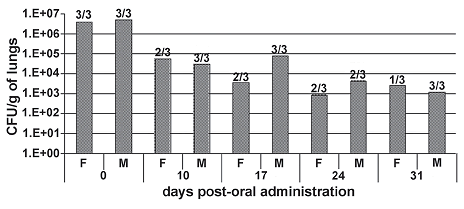
Figure 4. clearance of microorganism in lungs (CFU/g) in the inhalatory test and fraction of positive cases of isolation. Day 0 corresponds to the determination 4 hours after administration. F to female and M corresponds to male. number of cases of positive isolation (growth on organ) over 3 identical organs (from 3 individuals of the same sex in each case) showed over each bar. it is shown the average of UFC/g of lungs of the cases of positive isolation
In the gross necropsy evaluation there were no observable lesions in organs, but the microorganism was eventually isolated from the liver, spleen, lungs, and kidneys (table 2). The lungs, spleen and liver had high frequencies of positive cases of isolation over the entire experimental period, and had no statistical differences in the percentage of the positive cases of microorganism isolation during all the experiment period (F = 0.11; P = 0.8959). B. sphaericus OT4b25 being found only once in kidneys, was never isolated from the blood or brain. The detection of B. sphaericus OT4b25 in all the organs where this bacteria was isolated had no statistical differences between sexes (F = 1.21; P = 0.2798). Even though, the frequency of appearance in organs tends to diminish in time, only in the 3rd week when no growth was observed was there a statistical difference from the other weeks in the Tukey test (F = 6.11; P = 0.0094).
The weight of individuals (which was determined weekly) showed a similar behavior in time for all experimental groups (figure 2), with no statistical differences between inoculum, inactive inoculum and control groups for each day measurements.
Toxicity and pathology inhalatory assay. There was no evidence of adverse effects occurring from intranasal instillation of the high single dose of B. sphaericus OT4b25 together with microbially produced substances in the culture media. In subsequent observations, there were no deaths, observable toxic or pathogenic effects such as behavioral or clinical abnormalities. The null hypothesis that the means of the weights of
Table 2. Isolation of microorganism from organs in the oral test [ a = only organs where the microorganism was detected are shown. in blood and brain the microorganism was never isolated; b = day 0 corresponds to the determination 4 hours after administration; c = number of cases of positive isolation (growth on organ) over 3 identical organs (from 3 individuals of the same sex in each case); d = sum of cases of positive isolation in organs in each determination day over 12 different organs examined (from 12 individuals of the same sex each day); e = sum of cases of positive isolation per organ (during all the determination period) over 30 identical organs from 30 individuals of both sexes; F = female; M = male]. statistical values are explained inside text
individuals (figure 3) inside each treatment group discriminate by sex for each day of measure is equal, is accepted in all cases (statistical values are shown in figure 3).
Recovery values and detection of the microorganism in lungs (figure 4) was estimated in the tested animals sacrificed during the study and was only recovered in the inoculum group (it was not detected in either the control or the inactive inoculum groups). Although the microorganism was isolated in almost all evaluated cases at each interim sacrifice during all the experimental period, the average of CFU/g in lungs shows a decrease in the levels of microorganism recovered in time that diminish from 107 (order of magnitude isolated 4 hours after administration) to 103. The loss of the original inoculum concentration (compared with the microorganism recovered after 4 hours of administration) is probably due to the deviation of the microorganism to the digestive system after intranasal instillation.
The infectivity of the microorganism was evaluated periodically during the test and at the conclusion of the test by a gross necropsy evaluation.
Toxicity and pathology intraperitoneal assay. When the skin was bypassed as a barrier by the microorganism or by a microbially produced substance, as in the intraperitoneal administration, there were no deaths, behavioral or clinical abnormalities observed in all tested animals. In this case, the culture media without microorganism was evaluated for its possible toxic effects alone, and as in the other tests (when it was administered together with the microorganism) it does not show any adverse effects on the treated animals.
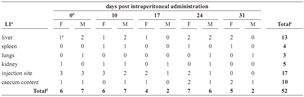
No pathological changes were observed in the gross necropsy in all animals examined. The clearance of B. sphaericus OT4b25 was estimated recovering it from blood, tissues and organs. Detection frequencies are shown in table 3. There were no differences between days (F = 2.77; P = 0.0491) and between sexes (F = 0.68; P = 0.6115) during all the experimental period; but statistical differences were observed between organs (F = 8.19; P = 0.0001): the most frequent location from which the microorganism was recovered was the site of injection showing its presence in all cases until the second week and its persistence for almost 24 days after administration. The second most frequent organ was the liver not
Table 3. Isolation of microorganism in the intraperitoneal toxicity/pathogenicity test [LI = location of isolation; a = only organs and sites of isolation where the microorganism was detected are shown. in blood and brain the microorganism was never isolated; b = day 0 corresponds to the determination 4 hours after administration; c = number of cases of positive isolation (growth on organ) over 3 identical organs (from 3 individuals of the same sex in each case); d = sum of cases of positive isolation in organs in each determination over 8 different organs examined (from 3 individuals of the same sex each day); e = sum of cases of positive isolation per organ (during all the determination period) over 30 identical organs from 30 individuals of both sexes; F = female; M = male]. statistical values are explained inside text
showing a pattern of occurrence in time. The caecum content evidenced that the microorganism was present on it, and it was almost as frequent as isolation from the liver; this occurrence could be due to the proximity with the site of injection. All last three sites of isolation belong to the same homogeneous group in the Tukey test. Apparently, the spleen, lungs and kidneys are similarly involved in this particular system of elimination of B. sphaericus OT4b25 due to the frequency of isolation and the homogeneous group where Tukey test situate them. As in the case of the liver, there was no obvious pattern in time on the frequency of microorganism isolation on these organs. As in the oral test, the microorganism was never isolated from brain and blood in all cases examined.
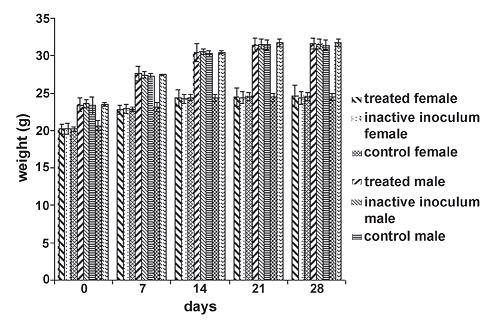
There were no statistical differences in body weights for each day measurements taken between the experimental groups (figure 5). Due to the absence of pathologic observable effects in all tests, we conclude that B. sphaericus OT4b25 is not pathogenic or infectious and it does not cause any apparent symptom of disease after any route of administration tested in this work.
Figure 5. Weight average behavior of individuals in the experimental groups during toxicity and pathology intraperitoneal assay. the null hypothesis that the means of the weights of individuals inside each treatment group discriminate by sex for each day of measure is equal, is accepted in all cases (day 0: F = 0.32, P = 0.8135; day 7: F = 0.20, P = 0.8951; day 14: F = 0.01, P = 0.9988; day 21: F = 0.06, P = 0.9827; day 28: F = 0.11, P = 0.9555)
DISCUSSION
The administration of the strain B. sphaericus OT4b25 by dermal, oral, inhalatory, and intraperitoneal routes does not cause any mortality events or observable lesions when organs were examined. The microorganism does not generate behavioral or clinical abnormalities, and does not influence the body weights of individuals, as there were no statistical differences in weight means between treatments. These results are in concordance with Siegel and Shadduck (1990), who used more critical tests to evaluate the possible pathogenic opportunistic behavior of different strains of B. sphaericus (SII–I, 1404–9, and 1593–4). They injected these strains in mice, rats and rabbits by intracerebral, intraocular, subcutaneous, and intraperitoneal routes, showing no deaths or clinical illness after administration of viable or inactivated microorganism.
The microorganism was rapidly excreted, because detection in feces after 4 hours of administration showed bacteria recovery one order of magnitude less (108 CFU) than the concentration administered (109 CFU) (table 1). Similar observations were made by Mancebo et al. (2003) who recovered B. sphaericus 2362 from rat feces almost as soon as our study (3 hours after administration), but at two orders of magnitude less (106 CFU) than the concentration administered (108 CFU). The microorganism was found in feces during the first 21 days of the experiment but on days 24 and 28 it was not detected in 10–3 and 10–2 dilutions respectively.
As in the oral test, in the inhalatory test the microorganism had a long time of residence inside the animals, and albeit its concentration diminished in time, it persisted on lungs for 4 weeks not causing any clinical or pathological problem.
In contrast to the observations made by Mancebo et al. (2003), who administered the microorganism by dermal (in albino rabbits) and oral (in rats) routes, our strain had a longer period of elimination or clearance after oral administration and though its isolation in organs was scarce (compared with the total cases examined), it was found more frequently than Mancebo et al. (2003). In the present study, lungs had the highest frequencies of positive cases of isolation across the experimental period (9 of 30 cases), and 2 of 3 cases were positive 4 hours after oral administration. This was probably due to the return of the microorganism from the stomach to the upper airways eventually passing to the lungs.
Detection in the liver 4 hours after oral administration (1 out of 3 cases) was possibly due to a first stage of an elimination route or clearance of the microorganism other than the feces or digestive elimination and this hypothesis is encouraged by the high frequency of occurrence of the microorganism in this organ during the intraperitoneal test. This is supported by more positive cases found in later determinations (table 2) in the oral test and after intraperitoneal administration.
In the oral test, the spleen was another organ where the microorganism was detected frequently, but it was not as frequent as in the intraperitoneal injection where the organs are more directly exposed to the microorganism. The unique detection in the kidneys in the oral test (1 case out of 30 organs examined during all the test period) could be considered as a non representative event, but it is mentioned because the kidneys could be involved as part of an alternative, less frequent route of elimination after intraperitoneal administration with a similar occurrence as spleen.
In summary, we show that certain organs such as the liver are more relevant and more related in the process of elimination of B. sphaericus OT4b25 than others (evaluated here). Even if the microorganism was isolated from the organs mentioned above there were no observable macroscopic alterations or lesions on them. Albeit the long period of elimination and the frequency of positive isolation from organs, the strain of B. sphaericus OT4b25 diminished through time in feces and organs until it was undetectable around the fourth week.
Mancebo et al. (2003) showed a scarce presence of the bacteria in tissues and organs, finding it, in a few cases, until 7 days post oral administration in the kidneys, spleen, lymphatic nodes and liver. They compared their results with those found by Siegel and Shadduck (1990) who demonstrated the persistence of B. sphaericus 2362 after intraperitoneal injection and its appearance on spleens, which were proposed as organs related with the clearance route of B. sphaericus. Similarly to Mancebo et al. (2003), in our study we support our findings on the theory proposed by Siegel and Shadduck (1990), where the release of “… nonmultiplying bacteria into the general circulation from extra splenic sites and the subsequent filtration and entrapment of these bacteria by the spleen” (Siegel and Shadduck 1990). Therefore the dynamic of B. sphaericus clearance from mammals could have a different behavior to inert particles, and implies persistence through different organs. Unlike previous studies, we show a high frequency of positive cases of isolation of B. sphaericus OT4b25 from organs, and instead of the spleen the liver was the most frequently implied in a possible clearance route.
All these findings and the fact that the microorganism was not found in the same organs in all cases, support the hypothesis of various alternative routes of elimination that could be influenced by bacteria strain, conditions of culture, doses and routes of administration. The absence of the microorganism in the brain and peripheral blood, suggest an alternative way of dispersion inside the animal body different to blood, that could be the lymphoid system based on the findings of Mancebo et al. (2003).
The absence of the microorganism in feces and organs inside the other experimental groups (inoculum inactivated by autoclave and controls) show that the microorganism components and the culture media have no toxic effects on model animals, and that the viable microorganism from the treated group was not transmissible to the other experimental groups or this transmission was not effective.
In concordance with the results obtained by Mancebo et al. (2003) the results of our dermal tests (directed on albino rabbits in both studies) show that both the microorganism and the components associated with the culture media are not related to alterations on the treated animals, supported by the absence of effects after dermal administration.
Taken together, our results show that there are no adverse effects occurring from the dermal, oral, inhalatory or intraperitoneal administration of a single high dose (109–108 CFU) of the strain of B. sphaericus OT4b25 under our conditions of culture; and there is no evidence that the microorganism by itself, a microbially produced substance, or other ingredients associated to the culture media, alter the mammal models used in this study.
The tests presented here are considered initial tests for evaluating the toxic or pathogenic characteristics of a microbial pest control agent preparation (US EPA, 1996). As there was no evidence of toxicity or pathology it is unnecessary to identify toxic components on the dosing material that could affect mammals by dermal, oral, inhalatory and intraperitoneal routes of administration. The recovery data shows that strain B. sphaericus OT4b25 cultured as described previously does not represent a hazard for mammalian health, due to the absence of toxic and pathogenic effects after a single high dose by dermal, oral, inhalatory or intraperitoneal routes of administration.
ACKNOWLEDGEMENTS
We thank our sponsors Research committee of science Faculty (universidad de los andes), m. a. bautista and c. Rozo for their help and all who encouraged this work.
REFERENCES
1. DeBach P. 1987. El uso de los microorganismos en el control biológico. Pp. 715–737. En: P. DeBach (ed.). Control biológico de plagas de insectos y malas hierbas. Cia. Editorial Continental S. A. Mexico DF, Mexico. [ Links ]
2. Dussan J, Andrade D, Lozano L. 1996. Caracterización de ADN plasmídico de bacterias nativas útiles en control biológico de mosquitos transmisores de malaria. Revista Colombiana de Entomología, 22:131–135. [ Links ]
3. Dussan J, Andrade D, Lozano L. 1997. Relación de ADN plasmídico y actividad larvicida en cepas nativas de Bacillus sphaericus. Revista Colombiana de Entomología, 23:103–106. [ Links ]
4. Dussan J, Andrade D, Lozano L, Vanegas S. 2002. Caracterización fisiológica y genética de cepas nativas de Bacillus sphaericus. Revista Colombiana de Biotecnología, 4:89–99. [ Links ]
5. Mancebo A, González B, Riera L, Lugo S, González Y, Arteaga ME, Fuentes D. 2003. Ausencia de toxicidad/patogenicidad de una formulación de Bacillus sphaericus 2362 (Griselesf). Revista de Toxicología, 20:210–215. [ Links ]
6. Ortega G. 2004. Evaluación de la producción piloto de la cepa nativa Bacillus sphaericus OT4b25 utilizando sustratos comerciales. Tesis de maestría. Departamento de Ciencias Biológicas, Universidad de los Andes. Bogotá, Colombia. [ Links ]
7. Shadduck J, Singer S, Lause S. 1980. Lack of mammalian pathogenicity of entomocidal isolates of Bacillus sphaericus. Environmental Entomology, 9:403–406. [ Links ]
8. Siegel J, Shadduck J. 1990. Clearance of Bacillus sphaericus and Bacillus thuringiensis ssp. isrraelensis from mammals. Journal of Economic Entomology, 83(2):347–355. [ Links ]
9. Singer J. 1990. Introduction to the study of Bacillus sphaericus as a mosquito control agent. Pp. 221–227. In: De Barjac H, Sutherland D (eds.). Bacterial control of mosquitoes and black flies, biochemistry, genetics, and applications of Bacillus thuringensis isrraeliensis and Bacillus sphaericus. Rutgers University Press. New Brunswick (NJ), United States of America. [ Links ]
10. US EPA. 1996. Environmental Protection Agency. Washington D.C., United States of America. Federal Register: September 11, 1998 (Volume 63, Number 176).<http://www.epa.gov/EPA–PEST/1998/september/Day–11/p24469.htm>. Consulted on: June 13, 2007. [ Links ]
11. US EPA. 1998. Environmental Protection Agency. Washington D.C., United States of America. <http://www.epa.gov/opptsfs/publications/oppts_Harmonized/885_Microbial_Pesticide_Test_Guidelines/Series/>. Consulted on: June 13, 2007. [ Links ]
12. US EPA. 2006. Environmental Protection Agency. Washington D.C., United States of America. <http://www.epa.gov/>. Consulted on: June 13, 2007. [ Links ]
13. US EPA. 2007. Office of Prevention, Pesticides and Toxic Substances. US EPA. Washington D. C.: United States of America. <http://www.epa.gov/pesticides/science/guidelines.htm>. Consulted on: September 7, 2006. [ Links ]
14. Zamora J. 2006. Evaluación toxicológica de la cepa nativa de Bacillus sphaericus OT4b25 cultivada en batch usando acetato de sodio como sustrato para su producción industrial. Departamento de Ciencias Biológicas, Universidad de los Andes. Bogotá, Colombia. [ Links ]
Recibido: julio 2008
Aceptado para publicación: noviembre de 2008