Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Actualidades Biológicas
Print version ISSN 0304-3584
Actu Biol vol.31 no.90 Medellín Jan./June 2009
ARTÍCULOS DE INVESTIGACIÓN
Micronuclei test application to wild tropical ichthyic species common in two lentic environments of the low zones in Colombia
Aplicación de la prueba de micronúcleos a especies ícticas silvestres frecuentes en dos ambientes lenticos de zonas bajas de Colombia
Isabel Palacio Betancur1; Jaime A. Palacio Baena1,3; Mauricio Camargo Guerrero2,3
1 Grupo de Investigación en Gestión y Modelación Ambiental (GAIA). Facultad de Ingeniería. Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. isabel.palacio@gmail.com
2 Grupo de Investigación Genética de Poblaciones. Instituto de Biología. Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. japalacio@udea.edu.co
3 Docente, Universidad de Antioquia. mauricio.camargo@siu.udea.edu.co
Abstract
The presence of genotoxic stress in eight ichthyic species in two tropical lentic environments of Colombia [Cachimbero (Santander) and Ayapel (Cordoba) floodplains lakes] was evaluated by the micronuclei test. After capturing and identifying each specimen, morphometric measurements were taken, and a blood sample drawn through cardiac or caudal vein puncture. Blood smears were stained with Giemsa and the frequency of micronuclei was quantified in 2000 cells per individual. A total of 11 species, 128 individuals, and 256.000 erythrocytes were evaluated. The condition factor for each captured specimen was calculated and a K-W analysis was applied in order to compare the number of MN among the different species in each lentic systems. The results showed a statistically significant relation between the condition factor and the frequency of micronucleated erythrocytes for the Ayapel flooplain lake. The less human-intervened floodplain lake (Cachimbero) showed the highest number of specimens with zero micronucleus, being Hoplias malabaricus the most sensitive species. The contrary occurs in the Ayapel floodplain lake contaminated with alluvial gold mining residues, where Ctenolucius sujeta and the native Cyphocharax magdalenae species presented the highest micronuclei averages. Our results further suggest that both sensitive and non-sensitive species should be included as sentinels in biomonitoring studies in Colombia.
Key words: micronuclei, ichthyic species, genotocixity, lentic environments, Colombia
Resumen
Se evaluó la presencia de estrés genotóxico en algunas especies ícticas en dos ambientes lénticos tropicales de Colombia [Cienaga de Cachimbero (Santander) y Cienaga de Ayapel (Cordoba)] a través de la aplicación de la prueba de micronúcleos. Después de la captura de los peces y la identificación de las especies se extrajo la muestra de sangre por medio de punción cardiaca y se tomaron las medidas morfométricas. Las muestras se tiñeron con Giemsa y se cuantificó la frecuencia de micronúcleos en 2.000 células por individuo. Se calculó el factor de condición para cada uno de los ejemplares capturados y se aplicó un análisis de K-W para comparar el número de MN entre las diferentes especies en cada una de las ciénagas y el número de MN de las especies comunes en los dos sistemas lagunares. Los resultados mostraron una relación estadísticamente significativa entre el factor de condición y la frecuencia de eritrocitos micronúcleados. Aunque no existen diferencias estadísticamente significativas entre las frecuencias de los micronúcleos de las diferentes especies en las ciénagas de Cachimbero y de Ayapel, Hoplias malabaricus presentó un promedio de micronucleos muy superior a las demás especies en la ciénaga de Cachimbero y Ctenolucius sujeta y Cyphocharax magdalenae para la ciénaga de Ayapel. Nuestros resultados sugieren que tanto especies sensitivas como no sensitivas podrían ser incluidas como especies centinelas en los estudios de biomonitoreo en Colombia.
Palabras clave: micronúcleos, especies ícticas, genotoxicidad, ambientes lénticos, Colombia
INTRODUCTION
The frequent application of agricultural chemicals for pest control and the introduction of chemical substances of industrial origin into aquatic environments, even at very low concentrations, negatively affect the biota. Therefore, the presence of xenobiotics in lentic environments demands the use of biological evaluation procedures, which should be sensitive enough and of fast and easy application in field conditions.
At the DNA level, a high percentage of spontaneous or induced alterations are translated into chromosomal ruptures and losses. In studies on quality of natural environments it is important to identify these alterations, which affect the gene pool of the species exposed to genotoxic substances. Currently, cytogenetic bio-markers are being applied and validated to study the species exposure to genotoxic agents in order to predict biological alterations and long term harmful effects on natural systems caused by these agents (De Flora et al. 1993). The most frequently used bio-markers for genotocixity testing in aquatic environments are the DNA-comet assay and the micronuclei test (MN test).
Given the nucleated nature of erythrocytes in fish, the practicality of the MN test has gain high relevance in bio-monitoring of aquatic environments, also including assessment of water quality. The MN are chromatin masses in the form of small nuclei which appear within the cytoplasm and close to the main nucleus in interphase cells. They are originated spontaneously or as consequence of clastogenic and/or aneugenic effects, which generate acentric chromosomal fragments and/or lagging chromosomes during the mitotic anaphase (Fenech and Morley 1985). In the first case, the chromosomal fractures may lead to the formation of acentric fragments, which by not having centromeres can not join to the mitotic spindle and, consequently, will not be included in the daughter nucleus after cellular division. During the ana-telophase these fragments are surrounded by nuclear membrane and appear in the cytoplasm of the offspring cells as small nuclei.
In the second case, if the genotoxic effect alters chromatid adhesion, kinetocore proteins, centromere structure or the mitotic spindle, the migration and distribution of a particular chromosome(s) could be distorted, and the lagging chromosomes will be encircled by nuclear membrane during telophase. This process will produce micronuclei, usually of bigger size. In either case, cellular division is a prerequisite for the formation and visualization of MN (Fenech and Morley 1985).
The application of the MN test has been adapted to both ex situ lab conditions (Al-Sabti 1994, 1995, Al-Sabti et al. 1994, Belpaeme et al. 1996, Cavas and Ergene-Gozukara 2005) and in situ bio-monitoring (Al-Sabti 1986, Bahari et al. 1994, Campana et al. 1999, Carrasco et al. 1990, Das and Nanda 1986, Hooftman and Raat 1982, Hose et al. 1987, Manna et al. 1985, Metcalfe 1988, Minissi et al. 1996, Nepomuceno et al. 1997, Odelgah and Osanyipeju 1995, Poongothai et al. 1996, Porto et al. 2005, Sánchez-Galán et al. 1998). In the later, several biotic and abiotic factors such as type of species, age, gender, reproductive status, health condition, and water temperature may affect the genotoxic responses of the organisms (Al Sabti and Metcalfe 1995, Hayashi et al. 1998).
In consequence, the worldwide bibliographic back up, as well as our own experiences in Colombia (Henao et al. 2005, Peñalosa et al. 2003), have shown that the MN test is a practical tool in eco-toxicology, since the micronucleated piscine erythrocytes have shown to be a good indicator of environmental contamination. Furthermore, nucleated erythrocytes belong to the distributional pool of cells that vastly become in contact with all tissues and their metabolites, adding robustness to the use of sentinel freshwater vertebrates to evaluate the potential risk to human population exposed to genotoxic agents in water (Al-Sabti 1986, Al-Sabti and Metcalfe 1995, Arcand-Hoy and Metcalfe 2000, Bombai et al. 2001, Carrasco et al. 1990, Hayashi et al. 1998, Henao et al. 2005, Hose et al. 1987, Hughes and Hebert 1991, Peñalosa et al. 2003).
Among the ichthyic species used in Europe as in situ bio-indicators of water quality, are Barbus plebejus (Minissi et al. 1996), Carassius sp., Zacco platypus, Leiognathus nuchalis, Ditrena temmincki, (Hayashi et al. 1998), Oncorhynchus mykiss (De Flora et al. 1993), and Salmo trutta (Reyes-Gavilán et al. 1996, Sanchez-Galan et al. 1998).
Several authors have reported a direct relationship between the MN frequencies and heavy metal concentrations in water, although with differential species sensitivity (Al-Sabati 1994, 1995, Al-Sabati et al. 1994, Nepomuceno et al. 1997, Sanchez-Galan et al. 1999, 2001). Similarly, a significant increase in the frequency of MN in specimens from rivers contaminated with chemical substances was reported for Barbus plebejus (Minissi et al. 1996), Lepidocephalus sp. (Poongothai et al. 1996), Salmo trutta (Sanchez-Galan et al. 1998), Carassius sp., and Zacco platypus (Hayashi et al. 1998). Additionally, Grisolia and Starling (2001) showed a significant increase in the number of micronuclei in specimens of Cyprinus carpio, Oreochromis niloticus, and Tilapia rendali at the Paranoa lake (Brazil) affected by the discharge of municipal waste waters.
After exposure of Onchorhynchus mykiss, Carassius auratus, Clarias lazera, and Exos lucious to different types of industrial discharge, it was found a significant increase in the frequency of micronucleated erythrocytes and a positive relationship between the contamination degree and the micronuclei frequency (Al-Sabti et al. 1994, D`Agostini et al. 1992, Ilyinskikh et al. 1998, Marlasca and Sampera 1998, Odeigah et al. 1995, Wrisberg and Van der Gaag 1992).
Colombia is a tropical country that still conserves a high richness of native fish species per basin (Leprieur et al. 2008). Therefore, the application of the MN test constitutes an optimal way to document the effect of human activity on both non-native and native species. Because of the introduction of large quantities of pesticides and chemicals of industrial origin into aquatic environments in Colombia, carrying out investigations that may contribute to increase the knowledge of the ecotoxcicologic and genotoxic effects of these substances on the species becomes a requirement.
The purpose of this work is to evaluate by the MN test the presence of genotoxic stress in eleven native ichthyic species in two tropical lentic environments that present a different degree of anthropic intervention.
Materials and methods
Study area. Cachimbero floodplain lake. Belongs to a swampy complex located in the jurisdiction of the vicinity of Puerto Olaya, municipality of Cimitarra, departament of Santander (Colombia), 6º 27' N, 74º 22' W, 200 m.a.s.l, within the geographical region called Magdalena Medio (figure. 1). The floodplain lake had its origin in the depression left by the former course of the Magdalena River. Currently isolated from the main course of the river, the Cachimbero floodplain lake mainly depends on drainage from adjacent hills and rains. These water flows carry large quantities of organic material in suspension, which is accumulated at the bottom of the floodplain lake. This phenomenon is caused due to the lentic regime of the floodplain lake and the low rates of decomposition associated with anoxia and extremely acidic pH (humic and fulvic acids), which limits the microbial activity. In general terms, the Cachimbero floodplain lake is in a good state of conservation and presents low human influence.
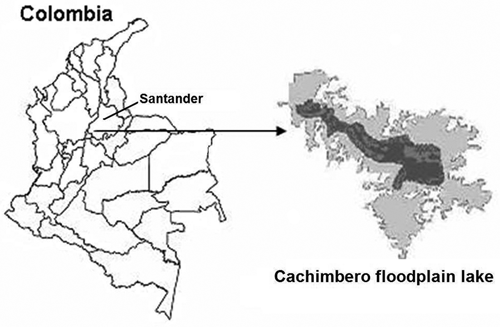
Figure 1. Location of the Cachimbero floodplain lake, Cimitarra (Santander), Colombia
Ayapel floodplain lake. In its alluvial plain, the San Jorge River has several floodplain lake systems of great importance, among them Ayapel. This is located in the jurisdiction of the municipality of Ayapel, in the departament of Córdoba (Colombia), in the region that limits with Sucre, Bolívar, and Antioquia departaments, at 8º 04' - 8º 30' N, 74º 84' - 75º 20' W, and 25 m.a.s.l. (figure 2). It has an approximate area of 45 km2 and has its own hydrological system which collects waters from several creeks at the east and south margins. Its main hydrological link establishes the exchange flow with San Jorge River through the Caño Grande creek. As regulator of the hydrological regimes of the zone, the Ayapel floodplain lake is fed by the flood of San Jorge River, and occasionally by the Cauca River. It receives the waste waters discharge of a population of about 62.200 inhabitants, and in some of its tributaries, alluvial gold mining was practiced few years ago.
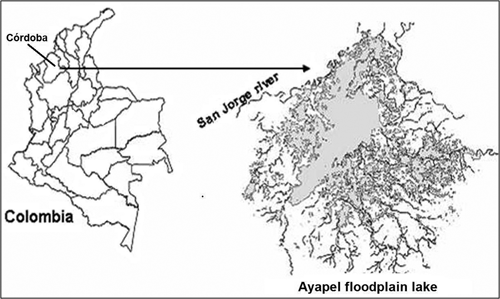
Figure 2 Location of the Ayapel floodplain lake (Córdoba), Colombia MN test. Fish captures were made using one gill net with different mesh sizes between opposite knots, for each floodplain lake. The gill nets were checked every eight hours.
The captured specimens were identified to species level, weighed and measured for total length. Blood samples were obtained through cardiac or caudal vein puncture with an insulin syringe impregnated with anticoagulant EDTA, stored in Eppendorf tubes and immediately refrigerated. Two blood smears per individual were prepared on clean microscope slides and air dried at room temperature. Afterwards at the lab, the slides were fixed with Methanol for five minutes and air dried for at least one day. After fixation, the slides were stained with Giemsa at 10% for ten minutes. The micronuclei were scored according to the criteria already described by Grisolia et al. (2002) taking into account only the rounded non-refractive structures, separated from the main nucleus. For each specimen 2000 cells were scored at 100X magnification, for a total of 256.000 nucleated erythrocytes analyzed. Statistical analysis. The condition factor (CF) for each captured specimen was calculated with the following formula: CF = (W) (1000)/L3, were W = weight in grams, and L = length in centimeters (Ricker, 1975).
For the total length, weight and condition factor of each species, the mean, the standard deviation (SD) and ranges, were calculated. On the other hand, the frequency, the standard deviation, and the range of MN for each species, were also calculated.
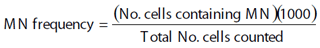
Additionally, a simple regression analysis was applied between the condition factor and the number of MN for each species in each floodplain lake.
To compare the number of MN among the different species in each floodplain lake, a Kruskal-Wallis analysis was performed. The Mann-Whitney analysis was used to compare the number of MN for the shared species of both floodplain lake systems.
RESULTS
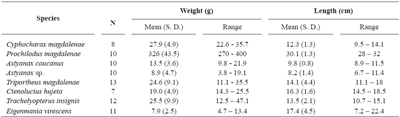
Cachimbero floodplain lake. Table 1 shows the number of species and specimens analyzed, their mean weights and lengths with their respective standard deviations and ranges.
Important variation was observed in the weight and length of the individuals of the species analyzed, basically due to the low selectivity of the fishing nets used.
Table 2 shows the number of individuals positive and negative for micronucleated erythrocytes for each species. It also shows the standard deviation and the frequency range of MN/1000 erythrocytes for each species, which fluctuated between 0.46 for Caquetaia krausii and 2.50 for Hoplias malabaricus.
Table 1. Sampled species at Cachimbero floodplain lake (Santander), Colombia
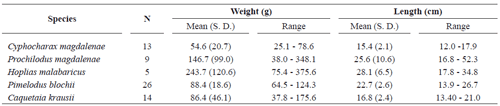
Table 2. Mean frequency cases of micronuclei for ichthyic species captured at Cachimbero floodplain lake (Santander), Colombia

A stricking finding at the Cachimbero floodplain lake is the high number of individual with zero micronucletaed erythrocytes in all species captured (except for H. malabaricus), reaching near 50% in three of them: Cyphocharax magdalenae, Pimelodus blochii, and Caquetaia krausii.
The frequency values for MN were similar for Caquetaia krausii, Prochilodus magdalenae, and Pimelodus blochii as shown in figure 3. Hoplias malabaricus presents a higher average to the other species and an important variation in the number of micronuclei among the individuals bled (figure 3.). Nevertheless, the Kruskall and Wallis test, with a value P = 0.723, indicates that no statistical significant differences exist among the micronuclei frequencies of the different species at Cachimbero floodplain lake.
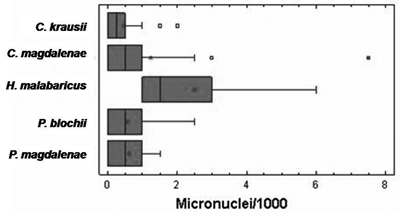
Figure 3. Box diagram of micronuclei frequency for the species bled at Cachimbero floodplain lake, Cimitarra (Santander), Colombia
Ayapel floodplain lake. Table 3 shows the number of species and specimens analyzed, together with their average weight, length, and their respective standard deviations and ranges.
Table 3. Species sampled at Ayapel floodplain lake (Córdoba), Colombia
In all the cases there was a wide fluctuation in size and weight of the individuals bled, as a result of the use of non selective fishing methods, as noted above for Cachimbero. Contrary to the Cachimbero floodplain lake, the Ayapel floodplain lake sampling showed a lower percentage of individual with zero micronucleated erythrocytes, with the exception of Cyphocharax magdalenae (table 4).
Table 4. Mean frequency cases of micronuclei for ichthyic species captured at Ayapel floodplain lake (Córdoba), Colombia (S. D. = standard desviation)

The micronuclei mean frequency varied between 1.41 for T. insignis and 3.75 for C. magdalenae (figure 4.). The maximum numbers of micronuclei found were for Ctenolucius sujeta (7 NM) and Cyphocharax magdalenae (10.5). According to the Kruskall and Wallis test (P = 0.169) no significant differences were observed among the micronuclei mean frequency in the species considered at Ayapel (figure 4).
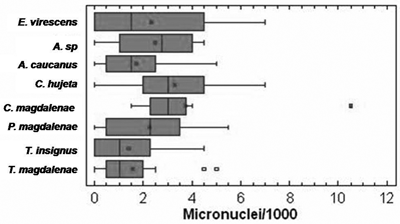
Figure 4. Box diagrams of the micronuclei frequency for the species bled at Ayapel floodplain lake (Cordoba), Colombia
Through Mann-Whitney test, statistically significant differences were evident in the mean frequencies for micronuclei between the individuals of P. magdalanae (P = 0.042) and Cyphocarax magdalenae (P = 0.0018) for the floodplains lakes considered in this study.
DISCUSSION
The in situ quantification of micronuclei in piscine erythrocytes has demonstrated to be an adequate bio-marker in the evaluation of aquatic ecosystems quality (Al-Sabti and Metcalfe 1995, Ali et al. 2008, Arkhipchuk and Garanco 2005, Bahari et al. 1994, Campana et al. 1999, Cavasand Könen 2008, Cavas 2008, De Flora et al. 1993, Hayashi et al. 1998, Hoshina et al. 2008, Porto et al. 2005, Sanchez-Galan et al. 1999, Udroi, 2006,). However, some authors have pointed out the importance of selecting adequate species (Rodríguez-Cea et al. 2003).
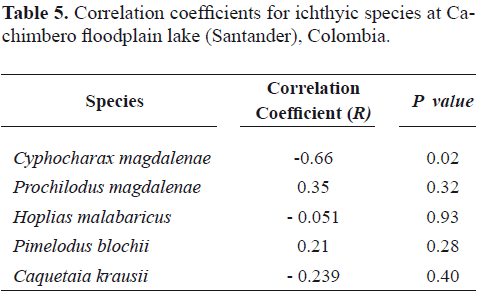
According to the results in table 5, for all the species studied at Cachimbero floodplain lake, no statistically significant relationship was found between the condition factor and the micronucleted erythrocytes frequency. For all the cases, the correlation coefficient value was very low, which explains why the relationship between them is very weak.
Table 5. Correlation coefficients for ichthyic species at Cachimbero floodplain lake (Santander), Colombia.
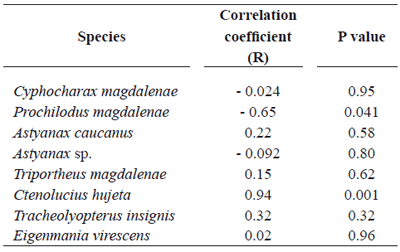
The correlation analysis for frequencies of micronucleated erythrocytes in the fish at Ayapel floodplain lake, a system subjected to mercury contamination as a result of the mining activity at its tributaries, showed a moderately strong relationship between the micronuclei frequency and the condition factor in Prochilodus magdalenae, Ctenolucius hujeta, and Eigenmania virescens.
However, for Prochilodus magdalenae the relation is inversed (table 6).
Table 6. Correlation coefficients for ichthyic species at Ayapel floodplain lake (Córdoba), Colombia
These results indicate that for a wide range of sizes of the individuals of the species at Cachimbero floodplain lake, non important differences were detected in the number of micronuclei related to the size of the individuals. A similar situation was reported by Bombail et al. (2001) for closer condition range factors in the marine fish Pholis gunnellus.
The spontaneous formation of micronuclei in erythrocytes of fish living in ecosystems with lower levels of human intervention could not be affected by their size or condition factor. In contrast, in environments contaminated with genotoxic substances, intra-specific differences and important variations in the micronucleated erythrocytes frequencies can appear as a result of individual sensitivity differences associated with the stage of the life cycle and to the physiologicalOnly for Hoplias malabaricus at the Cachimbero floodplain lake, and Cyphocharax magdalenae in Ayapel, there were no negative results for micronucleated erythrocytes. Percentages for the negatives at Cachimbero floodplain lake were higher than in Ayapel, and even for the two shared species of both floodplains lakes (P. magdalenae and C. magdalenae), the percentages for the negatives were significantly higher in Cachimbero. These results could be indicating differences in the water quality between both floodplain lake systems.
When considering the micronucleated erythrocytes mean frequency in all the species included in the study, in general a higher value for the individuals captured at Ayapel floodplain lake is observed. Nevertheless, H. malabaricus presented an average frequency similar to the one calculated for the species of Ayapel. H. malabaricus is a carnivorous species, benthonic associated with sediment and relatively sedentary. These characteristics undoubtedly favor the exposure and bio-accumulation of persistent substances.
According to Porto et al. (2005), the erythrocyte micronuclei frequency in H. malabaricus (1.76/1000 cells) detected in Madeira River (Brazil), was a response of the ichthyic community to this river's contamination with mercury. The nucleated erythrocytes mean frequency in the individuals of H. malabaricus at Cachimbero floodplain lake (2.12/1000 cells), a system apparently undisturbed, was higher than the value reported for Madeira River, and largely surpassed the mean frequency for individuals of this species coming from Solimoes River (0.06/1000 cells), considered as an environment with little disturbance (Porto et al. 2005).
These geographical differences in the micronucleated erythrocytes frequency could be showing important intra-specific variations in the formation of spontaneous micronuclei and bring into relief the need to advance in the knowledge of the normal basal levels for the most important ecological ichthyic species due to their ample geographic distribution and abundance in Colombia.
The micronucleated erythrocytes mean frequencies for the species from Cachimbero floodplain lake surpass the maximum values noted by Minissi et al. (1996) for Barbus plebejus individuals (0.38/1000 cells) captured in contaminated waters of Tiber River (Italy) and are significantly higher to the ones reported for Prochilodus nigricans (0.1/1000 cells) and Mylossoma duriventris (0.1/1000 cells) in Solimoés River (Porto et al. 2005).
The micronucleated erythrocytes frequency in Pimelodus blochii and Caquetaia krausi at Cachimbero floodplain lake were slightly higher to the ones found by Grisolia et al. (2001) in fish erythrocytes at Paranoa Lake (Brazil). It could be stated that at Cachimbero floodplain lake the micronuclei frequencies were low in P. blochii, C. krausi and P. magdalenae and similar to the spontaneous levels in situ reported by Al-Sabti and Metcalfe (1995) for Genyonemus lineatus (0.8/1000 cells), Paralabrax clathratus (0.8/1000 cells), and Oncorhynchus mykiss (0.33/1000 cells).
The increases in the frequency of micronuclei and especially for C. magdalenae indicate less favorable environmental conditions at Ayapel floodplain lake as a result of the presence of mercury and probably other persistent chemical substances (Palacio et al. 2007).
In conclusion, our findings suggest that MN test is an effective way to monitor the presence of xenobiotics in aquatic environments and that both sensitive and non-sensitive species should be included as sentinels in biomonitoring studies in Colombia.
REFERENCES
1. Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B. 2008.Genotoxicity assessment of acute exposure of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere, 71: 1823-1831. [ Links ]
2. Al-Sabti K. 1986. Comparative micronucleated erythrocyte cell induction in three cyprinids by five carcinogenic-mutagenic chemicals. Cytobios, 47 (190-191): 147-154. [ Links ]
3. Al-Sabti K. 1994. Micronuclei induced by selenium, mercury, methylmercury and their mixtures in binucleated blocked fish erythrocyte cells. Mutation Research, 320 (1-2): 157-163. [ Links ]
4. Al-Sabti K. 1995. An in vitro binucleated blocked hepatic cell technique for genotoxicity testing in fish. Mutation Research, 335 (2): 109-120. [ Links ]
5. Al-Sabti K, Franko M, Andrijanic B, Stegnar S. 1994. Chromium induced micronuclei in fish. Journal of Applied Toxicology, 14: 33-36. [ Links ]
6. Al-Sabti k, Metcalfe CD. 1995. Fish micronuclei for assessing genotoxicity in water. Mutation Research, 343 (2-3): 121-135. [ Links ]
7. Arcand-Hoy, Metcalfe CD. 2000. Hepatic micronuclei in brown bulheads (Ameiurus nebulosus) as a biomarker for exposure to genotoxic chemicals. Journal of Great Lakes Research, 26 (4): 408-415. [ Links ]
8. Arkhipchuk VV, Garanko NN. 2005. Using the nucleolar biomarker and the micronuclei test on in vivo fish fin cells. Ecotoxicology and Environmental Safety, 62: 42-52. [ Links ]
9. Bahari IB, Noor F, Daud NM. 1994. Micronucelated erythrocytes as an assay to assess actions by physical and chemical genotoxic agents in Clarias gariepinus. Mutation Research, 313: 1-5. [ Links ]
10. Belpaeme K, Delbeke K, Zhu L, Kirsch-VM. 1996. Cytogenetic studies of PC B77 on brown trout (Salmo trutta fario) using the micronuclei test and the alkaline comet assay. Mutagenesis, 11: 485-495. [ Links ]
11. Bombail V, Aw D, Gordon E, Batty J. 2001. Application of the comet and micronuclei assays to butterfish (Pholis gunnellus) erythrocytes from the Firth of Forth, Scotland. Chemosphere, 44: 383-392. [ Links ]
12. Campana MA, Panzeri AM, Moreno VJ, Dulout FN. 1999. Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using the micronuclei test in erythrocytes of the fish Cheirodon interuptus interuptus. Mutation Research, 438: 155-161. [ Links ]
13. Carrasco K, Tibury L, Myers M. 1990. Assessment of the piscine micronuclei test as an in situ biological indicator of chemical effects. Canadian Journal of Fisheries and Aquatic Science, 27 (11): 2123-2136. [ Links ]
14. Cavas T. 2008. In vivo genotoxicity of mercury chloride and lead acetate: Micronucleus test on acridine orange stained fish cells. Food and Chemical Toxicology, 46: 352-358. [ Links ]
15. Cavas T, Ergene-Gözükara S.2005. Induction of micronuclei and nuclear abnormalities in Oreochomis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquatic Toxicology, 74 (3): 264-271. [ Links ]
16. Cavas T, Könen S. 2008. In vivo genotoxicity testing of the amnesic shellfish poison (domoic acid) in piscine erythrocytes using the micronucleus test and the comet assay. Aquatic Toxicology, 90: 154-159. [ Links ]
17. Das RK, Nanda NK. 1986. Induction of micronuclei in peripheral erythrocytes of fish Heteropneustes fossilis by mitomycin C and paper mill effluent. Mutation Research letters, 175 (2): 67-71. [ Links ]
18. D'Agostini F, Di Marco C, Melodia F, Vigano L. 1992. Citogenetic damage in fish (Oncorhynchus mykiss) exposed in situ to water from a polluted river. Bollettino-Societa Italiana Biologia Sperimentale, 68: 549-553. [ Links ]
19. De Flora S, Vigano L, D'Agostini F, Camoriano A, Bagnasco M, Bennicelli C, Melodia F, Arillo A. 1993. Multiple genotoxicity biomarkers in fish exposed in situ to polluted river water. Mutation Research, 319: 167-177. [ Links ]
20. Fenech M, Morley AA. 1985. Measurement of micronuclei in lymphocytes. Mutation Reseach, 285: 35-44. [ Links ]
21. Grisolia KC and Koppe C. 2002. A comparison between mouse and fish micronuclei test using cyclophophamide, mitomicina C and various pesticides. Mutation Research, 518: 145-150. [ Links ]
22. Grisolia CK, Starling FLRM. 2001. Micronuclei monitoring of fishes from lake Paranoá, under influence of sewage treatment plant discharges. Mutation Research, 491: 39-44. [ Links ]
23. Hayashi MT, Ueda T, Uyeno K, Wada K, Kinae N, Saotome K, Tanaka N, Takai A, Sasaki YF, Asano N, Sofuni T, Ogima Y. 1998. Development of genotoxicity assay systems that use aquatic organisms. Mutation Research, 399 (2): 217-225. [ Links ]
24. Henao B, Palacio JA, Camargo M. 2005. Evaluación genotóxica de los plaguicidas cipermetrina y diacinón n tilapia roja (Oreochromis sp.). Actualidades Biológicas, 27 (82): 43-55. [ Links ]
25. Hooftman RN, Raat WK. 1982. Induction of nuclear anomalies (micronuclei) in the peripheral blood erythrocytes of eastern mudminnow Umbra pygmaea by ethylmethane sulphonate. Mutation Research, 104: 147-152. [ Links ]
26. Hose JE, Cross JN, Smith SG, Diehl D. 1987. Elevated circulating erythrocyte micronuclei in fishes from contaminated sites of Southern California. Marine Environmental Research, 22 (3): 167-176. [ Links ]
27. Hoshina MM, Angelis DF, Marin-Morales MA. 2008. Induction of micronucleus and nuclear alterations in fish (Oreochromis niloticus) by a petroleum refinery effluent. Mutation Research/ Genetic Toxicology and Environmental Mutagenesis, 656 (1-2): 19-26. [ Links ]
28. Hughes JB, Hebert AT. 1991. Erythrocyte micronuclei in winter flounder (Pseudopleuronectes americanus) result of field survey during 1980-1988 from Virginia to Nova Scotia and in Long Island Sound. Archives of Environmental Contamination and Toxicology, 20: 167-177. [ Links ]
29. Ilyinskikh N, Ilyinskikh EN, Ilyinskikh IN. 1998. Micronucleated erythocytes frecuency and radiosium bioconcentration in pikes (Esox lucius) caught in the Tom River near the nuclear facilities of the Siberian Chemical Complex (Tomsk-7). Mutation Research, 421 (2): 197-203. [ Links ]
30. Kirsch-Volders M, Elhajouji A, Cundari E, Van Hummelem P. 1997. The in vitro micronuclei test: a multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 392 (1-2): 19-30. [ Links ]
31. Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. 2008. Fish invasions in the world's river systems: When natural processes are blurred by human activities. PLoS Biology 6(2): e28. [ Links ]
32. Manna GK, Banerjee G, Gupta S. 1985. Micronuclei test in the peripheral erythrocytes of the exotic fish, Oreochromis massambica. Nucleus, 28: 176-179. [ Links ]
33. Majone F, Brunetti R, Gola I, Levis A. 1987. Persistence of micronuclei in the marine mussel, Mytilus galloprovincialis, after treatment with mitomycin C. Mutation Research, 191:157-161. [ Links ]
34. Marlasca MJ, Sampera C. 1998. Hepatic alterations and induction of micronuclei in rainbow trout (Oncorhynchus mykiss) exposed to a textile industry effluent. Histology and Histopathology, 13 (3): 703-712. [ Links ]
35. Metcalfe CD. 1988. Induction of micronuclei and nuclear abnormalities in the erythrocytes of Mudminnow (Umbra limi) and brown bulheads (Ictalurus nebulosus). Bull. Environ. Contam. Toxicology, 40: 489-495. [ Links ]
36. Minissi S, Ciccotti E, Rizzoni M.1996. Micronuclei test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: a bioassay for the in situ detection of mutagens in freshwater. Mutation Research, 367 (4): 245-251. [ Links ]
37. Nepomuceno J, Ferrari I, Spaño M, Centeno A. 1997. Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environmental and Molecular Mutagenesis, 30 (3): 293-297. [ Links ]
38. Odelgah PGC, Osanyipeju AO.1995. Genotoxic effects of two industrial effluents and ethyl methane sulfonate in Clarias lasera [published erratum appears in Food Chemical Toxicology, 1996, Feb: 34 (2)]. Food and Chemical Toxicology, 33 (6): 501-505. [ Links ]
39. Palacio JA, Aguirre NJ, Flores MT, Wills A, Gallo LJ, Hernández E. 2007. Plan de Manejo Ambiental del Complejo de Humedales de Ayapel. Informe final del proyecto CVS. Facultad de Ingeniería. Medellín (Antioquia), Colombia: Universidad de Antioquia. [ Links ]
40. Pastor S. 2002. Biomonitorización citogenética de cuatro poblaciones agrícolas europeas expuestas a plaguicidas, mediante el ensayo de micronúcleos [Tesis de Doctorado]. [España]: Universidad Autónoma de Barcelona. [ Links ]
41. Peñalosa M, Camargo M, Palacio J. 2003. Genotoxicidad del cloruro de mercurio en dos especies ícticas (Prochilodus magdalenae y Oreochromis sp.) Actualidades Biológicas, 25 (79): 105-111. [ Links ]
42. Poongothai K, Shayin S, Usharani MV. 1996. Induction of micronuclei in fish by polluted water and heavy metals. Cytobios, 86 (344): 17-22. [ Links ]
43. Porto JIR, Araujo CSO, Feldberg E. 2005. Mutagenic effects of mercury pollution as revealed by micronuclei test on three Amazonian fish species. Environmental Research, 97: 287-292. [ Links ]
44. Reyes-Gavilan F, Garrido R, Nicieza AG, Toledo MM, Braña F. 1996. Fish community variation along physical gradients in short streams of northern Spain and the disruptive effect of dams. Hydrobiology, 321: 155-163. [ Links ]
45. Ricker WE. 1975. Computation and interpretation of biological statistics of fish population. Bulletin Fisheries Research Board of Canada, 191: 1-138. [ Links ]
46. Rodriguez-Cea A, Ayllon F, Garcia-VE. 2003. Micronuclei test in freshwater fish species: an evaluation of its sensitivity for application in field surveys. Ecotoxicology and Environmental Safety, 56: 442-448. [ Links ]
47. Sanchez-Galan S, Linde AR, Izquierdo JI, Garcia-VE. 1998. Micronuclei and fluctuating asymmetry in brown trout (Salmo trutta): complementary methods of biomonitor freshwater ecosystems. Mutation Research, 412 (3): 219-225. [ Links ]
48. Sanchez-Galan S, Linde AR, Izquierdo JI, Garcia-VE. 1999. Brown trout and European minnow as target species for genotoxicity test: differential sensitivity to heavy metals. Ecotoxicology and Environment Safety, 43: 301-304. [ Links ]
49. Wrisberg MN, Van der Gaag MA. 1992. In vivo detection of genotoxicity in waste water from a wheat and straw paper pulp factory. Science of the Total Environment, 121: 95-108. [ Links ]
Recibido:enero 2009
Aceptado: junio 2009














