Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Actualidades Biológicas
versión impresa ISSN 0304-3584
Actu Biol v.31 n.1 Medellín jul./dic. 2009
ARTÍCULOS DE INVESTIGACIÓN
MACROINVERTEBRATE ASSEMBLAGES IN GORGONA ISLAND STREAMS: SPATIAL PATTERNS DURING TWO CONTRASTING HYDROLOGIC PERIODS
ENSAMBLE DE MACROINVERTEBRADOS DE LAS QUEBRADAS DE LA ISLA GORGONA: PATRONES ESPACIALES DURANTE DOS PERIODOS HIDROLÓGICOS CONTRASTANTES
Ana M. Gómez-Aguirre1; Magnolia C. Longo-Sánchez2; Juan F. Blanco3
1 Instituto de Biología, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia, A.A. 1226, Medellín, Colombia. anago35@yahoo.com
2 Instituto de Biología, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia, A.A. 1226, Medellín, Colombia. mc_longo@hotmail.com
3 Docente. Instituto de Biología, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia, A.A. 1226, Medellín, Colombia. jfblanco73@yahoo.com, blanco@matematicas.udea.edu.co.
Abstract
Studies comparing various streams are scarce in tropical insular systems and inexistent in Colombia. In order to understand the spatial patterns of macroinvertebrates in tropical insular streams, and the environmental drivers of such patterns, we tested for patterns among streams but using a nested sampling design from streams to microhabitats in Gorgona Island (Colombia, Tropical Eastern Pacific) during two seasons (low and high precipitation). We found that benthic macroinvertebrate assemblages showed clear differences among streams despite of the variability within nested levels. Hydrologic disturbance tended to homogenize spatial patterns in most of the streams. Although ordinations of sampling units using either macroinvertebrate composition or environmental variables were not fully consistent, it was evident that water physicochemistry influenced by underlying geology was a pervasive driver of macroinvertebrate distribution. We highlight the importance of incorporating multiscale sampling designs for studying distributions of macroinvertebrates in tropical islands, and for biomonitoring.
Key words: Gorgona Island, hydrologic disturbance, macroinvertebrate assemblages, multiscale patterns, tropical insular streams, water physicochemistry
Resumen
Los estudios que comparan varias quebradas son aun escasos en los ambientes insulares tropicales e inexistentes en Colombia. Se sometió a prueba la hipótesis de que los ensambles de macroinvertebrados son diferentes entre quebradas utilizando un diseño de muestreo completamente anidado desde el nivel de quebrada hasta el nivel del microhábitat en la Isla Gorgona (Pacífico Oriental Tropical Colombiano) durante dos periodos hidrológicos contrastantes (época seca y época lluviosa). Se encontró que los ensambles de macroinvertebrados son diferentes entre quebradas a pesar de las diferencias observadas en niveles inferiores anidados. El disturbio hidrológico tendió a homogenizar los patrones espaciales. Aunque las ordenaciones basadas en la composición de macroinvertebrados de los ensambles y las variables ambientales no fueron completamente consistentes, fue evidente que las características físico- químicas del agua, influenciadas por la geología, explicaban la distribución de los macroinvertebrados. Resaltamos la importancia de incorporar diseños de muestreo multinivel para estudiar los ensambles de macroinvertebrados insulares tropicales y para el biomonitoreo.
Palabras clave: disturbio hidrológico, ensamblajes de macroinvertebrados, físico-química del agua, Isla Gorgona, patrones multi-espaciales, quebradas tropicales insulares
INTRODUCTION
Understanding ecological patterns and their drivers is a main challenge in science and it is cor nerstone for developing management principles (Levin 1992). Because these patterns and processes are linked to multiple scales (Boyero 2003, Levin 1992), their detection depends on appropriate selection of observation scales (Levin 1992, Parsons et al. 2004, Poff 1997). For this reason, the problem of scale must be considered a primary objective of ecological i nvestigation ( Wiens 1989). T his problem is particularly critical in streams that show extreme heterogeneity in their environmental conditions and biotic assemblages at multiple spatial scales, from microhabitats to landscapes and complete ecoregions (Heino et al. 2004). Streams are hierarchically organized (Frissell et al. 1986) and thus their physical and biotic properties are highly variable in time and space (Cooper et al. 1998, Hildrew and Giller 1994, Poff 1997). For this reason, the emergence of scale as an important ecological concept (Levin 1992, Wiens 1989) has stimulated multi-scale- research in streams (Parsons et al. 2004). In streams, as in any ecosystem, it is critical to make an appropriate selection of the scales of observation within the context of the hierarchical theory (Wiens 1989). Then, the selection could be derived from a parallel physical hierarchy such as the fluvial geomorphic hierarchy proposed by Frissell et al. (1986).
Despite of the current progress on multiscale research in streams, there are still few studies incorporating this hierarchical context (e.g., Parsons et al. 2003 and Thomson et al. 2004). According to the review by Parsons et al. (2004), st udies examining the distribution of lotic macroinvertebrate assemblages have focused on either end of the spatial hierarchy and have used a stratified design (macroscale: Corkum 1991, 1992, Li et al. 2001, Rabeni et al. 1999, Sheldom and Walter 1998, Townsend et al. 1997; microscale: Boyero and Bailey 2001, Downes et al. 1993, 1995, Downes et al. 2000). In addition, Robson et al. (2005) cautioned on the high universal variation at the scale of the sampling unit in riffles at perennial cobble-bed streams in Australia, and highlighted that few studies had been conducted to examine the change of this source of variation along time or if the results apply to other habitats (such as pools) or other stream type (e.g., intermittent).
These investigations have been conducted in continental-temperate zones, and they are scarce in the Tropics (Boyero 2000, Jacobsen et al. 2008). Additionally, these have been conducted mainly in disturbed systems, despite the importance of searching for patterns at pristine or protected systems to understanding the structure of natural communities and to develop management plans, especially at insular tropical systems (Dudgeon 1994, Smith et al. 2003). For this reason, we aimed to understand the spatial patterns of macroinvertebrates and their environmental drivers at a protected natural insular area off the Pacific Coast of Colombia (Gorgona Island). Past research in this small island has reported that the numerous streams are heterogeneous in terms of macroinvertebrate assemblages, despite their homogeneous geology and water chemistry (Cala 1990, Vásquez et al. 1996, Zamora et al. 1996, Zapata et al. 1991). However, the generality of this statement needs to be tested because those studies: a) did not use fully-nested sampling designs, and b) did not employ enough sampling effort. Similar limitations in sampling designs are observed in most studies on stream macroinvertebrates in Colombia (JF Blanco in preparation), as biomonitoring has been the main theme over the past thirty years (Roldán and Ruíz 2001). Therefore, the current understanding of ecological patterns and processes at insular and continental streams in Colombia, as elsewhere in the Tropics (Smith et al. 2003), is still incomplete. In addition, it is not clear if the concepts, paradigms and management plans der ived from high latitudes could be applied in these systems (Boulton et al. 2008). For this reason, tropical st rea m ecolog ists a re developi ng approximations to understand, to manage and to conserve these environments (Ramírez et al. 2008). The results show that studies with comparative focus are necessary, because insular systems differ of continentals in a huge range of geologic, climatic, biogeographic and anthropocentric characteristics. We expect to provide the baseline information for including Gorgona Island in future global comparisons.
The aim of this paper was to test if streams on Gorgona Island were homogeneous in terms of macroinvertebrate assemblages, biotic (e.g., leaflitter standing stocks, riparian forest classification) and abiotic (e.g., geology, geomorphology, hydraulics, water physico-chemistry) properties. Patterns were compared between two seasons (low and high precipitation).
MATERIALS AND METHODS
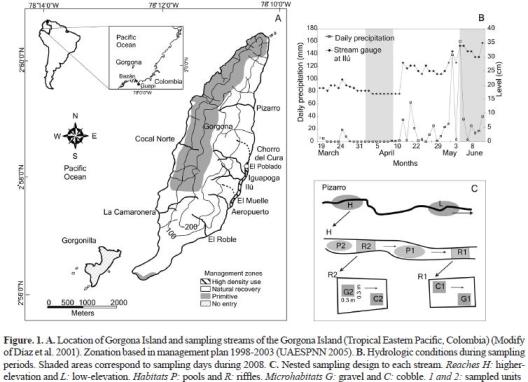
Study area. Gorgona Island is a continental volcanic massif (Kerr 2005), (9 x 2.5 km) in the Colombian Tropical Eastern Pacific (figure 1; 2° 47' - 3° 06' N, 78° 06' - 78° 18' W), 54 km off Guapi. Elevation ranges from 0 to 330 masl. Nearly 90% of the island is steep gradient (50-75%) surrounded by smooth gradient (< 12%) lowlands. Average air temperature, relative humidity and annual rainfall are 26 °C, 90%, and 6.778 mm, respectively. Two climatic seasons are differentiated in Gorgona: rainy (May- November) and low precipitation (December-April), (Díaz et al. 2001, UAESPNN 2005). High annual precipitation feeds nearly 100 streams, 25 of which are permanent. Nine of these were selected to this study (Appendix 1). A detailed account of hydrology and stream physico-chemistry is found in Blanco (2009a, b, this issue).
Gorgona was a penitentiar y island between 1960 and 1982, but it was declared National Natural Park (NNP) in 1983. Because of intervention during the penitentiar y period, nat ural and dist urbed forests occur on the island. Accordingly, the management plan (UAESPN N 2005) establishes three zones: 1) primitive, 2) natural recover y, and 3) high- density use (figure 1A). A detailed account of landuse histor y is found in Valencia-G. et al. (2009, this issue).
Sampling. It was conducted during two seasons in 2008: low (2-9 April) and high (4-10 June) precipitation (figure 1B). In both seasons, we applied the ''underlying hierarchy'' approach (Parsons et al. 2004). Sampling units (0.3 x 0.3 m plots) were distributed across the fluvial spatial hierarchy (i.e. from subregions to microhabitats) as shown in figure 1C. We sampled 9 streams (2 within the Western Slope, and 7 at the Eastern Slope). However during the rainy season, El Roble stream was not sampled due to a flashflood. Benthic organisms were collected using a Surber net (0.09 m2, 0.5 mm mesh), preserved in 70% alcohol and transported to the laboratory to be identified to the lowest possible taxonomic level (genus or family morphotype, and subfamily to Chironomidae) using the available literature and expert knowledge. Biotic and abiotic descriptors were qualitatively and quantitatively measured at each hierarchical level (Appendices 2 and 3). Thirty four descriptors and twenty five were respectively measured during low and high precipitation periods.
Data analyses. Total densities of taxa were calculated for each microhabitat (individual observation unit) pooling all individual sampling units, then we obtained 43 total density values during low precipitation season and 39 during high precipitation. Since the organisms were identif ied at different taxonomic levels (family, genus or mor ph) we call them ''operational taxonomic units'' (OTUs) for convenience. Total densities, biotic and abiotic variables were square-root transformed (Costa and Melo 2008, McCune and Mefford 1999, Von Ellenrieder 2007). R ichness (number of OTUs), Shannon's diversity index, and Pielou's diversity index were computed for describing assemblage str ucture at each stream.
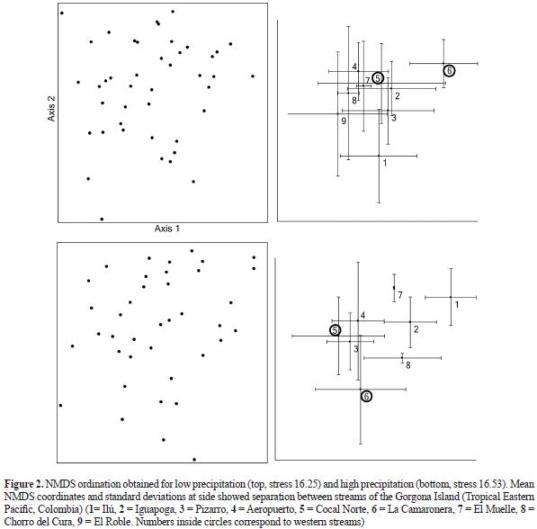
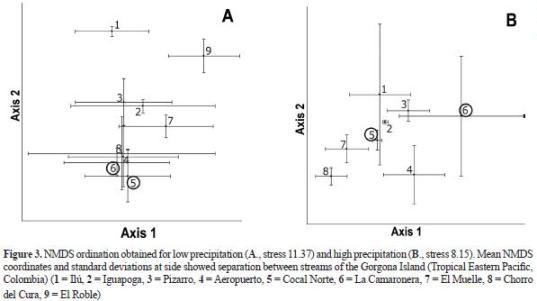
A mong-stream patterns. The similarities among streams were tested by firstly ordinating sampling units with a Non-Metric Multidimensional Scaling (NMDS) based on macroinvertebrate OTU composition (Ilmonen and Paasivirta 2005, Legendre and Legendre 1998, McCu ne a nd Mef ford 1999). Mea n NMDS coordinates and standard deviations were computed by pooling all sampling units within a single stream and plotted using PAST (Palaeontological statistics, version 1.82b, Hammer et al. 2001). Differences among streams were then tested using the Multiple Response Per mut ation Procedu re (MRPP) usi ng Sorensen ( Bray- Curtis) measu re of distance (Dinger et al. 2006, Heino et al. 2005, McCune and Mefford 1999, Soldner et al. 2004, Von Ellenrieder 2007). The above procedure was repeated using a matrix of abiotic and biotic descriptors (qualitative and quantitative) to test for differences among st reams based in the ''parallel hierarchy''. Seasonal consistency of spatial patterns was tested by separately running the analyses for both low and high precipitation periods. NMDS and MRPP analyses were perfor med w it h PCOR D (version 4.25, McCune and Mefford 1999, MjM Software, Gleneden Beach, Oregon).
RESULTS
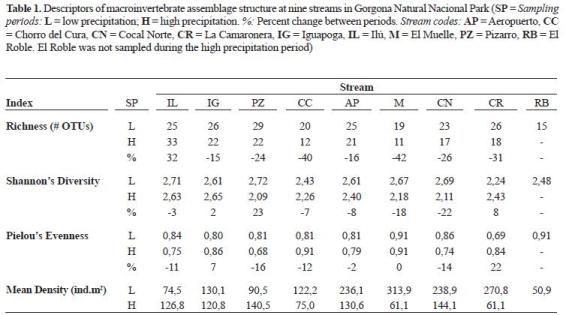
Assemblage structure. Number of OTUs, Shannon's diversity index, Pielou's evenness index and mean density changed across streams and between sampling periods (table 1). Pizarro and Ilú showed the largest number of OT Us a nd d iversit y values. El Chor ro del Cura, El Roble, El Muelle showed low richness, particularly during the rainy season. These streams also exhibited low diversity. Regarding temporal patter ns, richness was reduced at all streams (except Ilú) during the rainy period. Diversity did not show a clear temporal patter n because it was reduced at some streams and increased at some others. In addition the degree of reduction of diversity during the rainy season was highly variable among streams, as high as > 20% at Pizar ro, Cocal Nor te and El Muelle. Evenness was relatively high (> 70%) in all streams. Percent of cha nge bet ween sampli ng per iods was variable among streams. Mean density was reduced at all streams (except Ilú and Pizarro) during the rainy period.
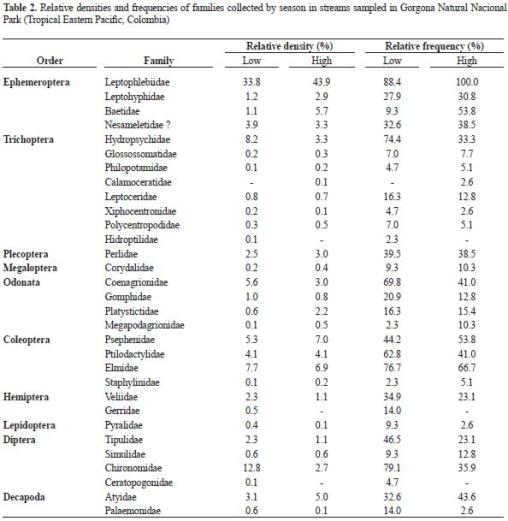
Taxonomic composition. We found 10 arthropod orders (9 Insecta and 1 Crustacea), and 31 families (table 2). Trichoptera was the richest order, followed by Ephemeroptera, Odonata, Coleoptera, and Diptera. Leptophlebiidae (Ephemeroptera) was the most abundant and frequent family islandwide, particularly during the rainy period. The Chironomidae were also abundant and frequent but mainly during the low precipitation per iod. Hyd ropsychidae (Trichoptera) and Coenagrionidae (Odonata) were also frequent, but not abundant, during the low precipitation. The Psephenidae, Ptilodactylidae, and Elmidae (Coleoptera) were frequent during both periods but not abundant. Decapod shrimps (Atyidae: Potimirim, Palaemonidae: Macrobrachium) were observed but not abundant in our collections. The gastropod Neritina latissima was also observed, but it was not collected in our samplings because it was restricted to the first hundred meters upstream from the confluence with the ocean and it was not abundant.
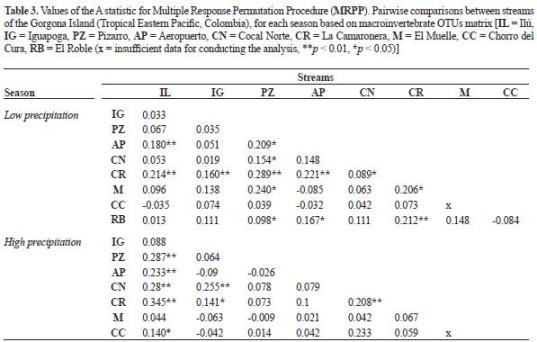
Patterns among streams. Macroinvertebrate assemblages.TheNMDSandMRPPshowedseparation of sampling units among stream assemblages during both seasons (figure 2). During the low precipitation season (table 3), the streams at the Western Slope (Cocal Norte and La Camaronera) exhibited distinctive macroinvertebrate assemblages. In contrast, Cocal Norte showed more similarities with several streams at the central part of the Eastern Slope. Macroinvertebrate assemblages at the two most isolated streams at the Eastern Slope (El Roble and Pizarro) were similar to neighbouring streams but not between them. During the high precipitation period, the differences between the two streams at the Western Slope were maintained.
However, both streams showed similarities with the smallest streams at the opposite slope. Contrary to the previous season, they showed similarities with Pizarro, but not with Ilú and Iguapoga. During this season, Pizarro was more similar to most streams at the Eastern Slope.
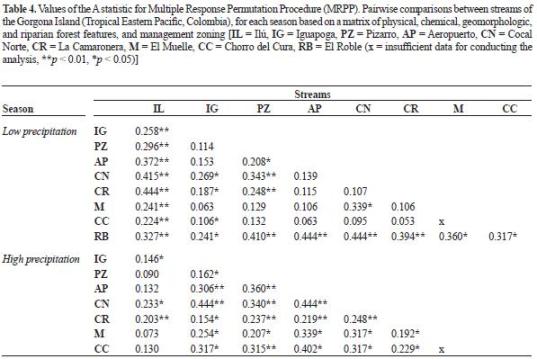
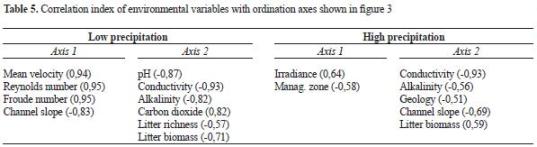
Environmental characteristics. The ordinations based on environmental variables were not consistent with those based on macroinvertebrate assemblages (figure 3, table 4). During the low precipitation period, despite the differences in geology between Cocal Norte and La Camaronera (volcanic and sedimentary, respectively), they were similar in terms of the descriptors used. In addition, La Camaronera was similar to several streams at the Eastern Slope (Aeropuerto, El Muelle, and El Chorro del Cura). Cocal Norte was not similar to El Muelle, Ilú, and Iguapoga as obser ved using macroinver tebrate assemblages. Ilú and El Roble (1 and 9, respectively in figure 3) were different from all streams. During the rainy period, Cocal Norte and La Camaronera (5 and 6, respectively in figure 3) differed among them and among the streams at the Easter n Slope. In addition, while Iguapoga (2 in figure 3) differed from all streams at the Eastern Slope, Ilú was similar to all streams. During the low precipitation period, hydraulic (mean water velocity, Reynolds and Froude numbers), and geomorphic (channel slope) variables correlated with Axis 1, while water hysic-chemistry (pH, conductivity, alkalinity, and CO2 concentration) and leaflitter richness and biomass correlated with Axis 2 (table 5). During the rainy period, management zones cor related with Axis 1, while water physic-chemistry (conductivity and alkalinity), geology and geomorphology (channel slope), and leaflitter biomass correlated with Axis 2.
DISCUSSION
Taxonomic composition. In Gorgona Island's streams, insects dominated over gastropods and decapods, groups otherwise dominant in insular tropical streams (Bass 2003). Such dominance in species richness and abundance may result from the continental origin of the island, as observed elsewhere (Bass 2003, March et al. 2003, Smith et al. 2003). The short distance between Gorgona Island and the continent (< 54 km) may explain the presence of all insect orders commonly found in tropical islands (Bass 2003). The most abundant insect orders were Ephemeroptera, Diptera, Coleoptera and Trichoptera, especially the families Leptophlebiidae, Chironomidae, Elmidae and Hydropsychidae, respectively. This pattern has been reported, in general, for tropical stream assemblages (Jacobsen et al. 2008) and is similar to the reported by Boyero and Bailey (2001) at Juncal stream (Coiba Island, Panamá). The authors indicated that the above-mentioned families (except Hydropsychidae, and including Psephenidae) were the most abundant and common.
Contrary to our findings, previous studies at the island (Cala 1990, Zamora et al. 1996, Zapata et al. 1991) reported the presence of gastropods and a broad distribution and dominance of stream shrimps. Such differences may reflect the different sampling nets used in those studies and our study. The Surber method used in our study is more efficient for collecting insects than shrimps. This method sub-estimates shrimp abundance because they are highly mobile as postlarvae and adults, and they drift as larvae (Ramírez and Pringle 1998a, b). Field observations and leaf litter samples for studies in progress in Gorgona Island (Longo, data not published) indicate, higher shrimps abundances than the reported by our study. The inclusion of other sampling methods and variables would have let us obtaining higher abundances and testing for marked distribution patterns as reported for shrimps in other tropical islands (Fièvet et al. 2001, Fossati et al. 2002, Pyron et al. 1999).
Gastropods were not found in this study. However, Neritina latissima was obser ved at the lowest elevations in some streams (not included in sampling) and with low abundances. According to Blanco and Scatena (2006) this limited distribution of neritid gastropods could be related to barriers (cascades and dams), steep slopes, absence of connectivity with ocean (thus restricting upstream migration) and water chemistry (high conductivity and concentrations of dissolvedions).
Temporal variation. In addition to the observed reduction in macroinvertebrate abundances in Gorgona Island (Gómez-Aguirre 2009), we here reported a reduction in richness and an homogenization of taxonomic composition of the assemblage during the rainy period (although differences among several streams were still evident). It is well known that flash flooding is an important disturbance in temperate and tropical streams, temporally reducing benthic invertebrate abundances because of the catastrophic drift and the alteration of potential flow refugia (Flecker and Feifarek 1994, Hildrew and Giller 1994, Matthaei et al. 2000, Ramírez and Pringle 1998b, Ramírez et al. 2006). In Gorgona Island assemblage homogenization occurred at intermediate spatial scales (reach and habitat) during the rainy season, but differences were observed between cobble and gravel patches (Gómez-Aguirre 2009).
Spatial patterns. The differences observed a mong macroinvertebrate a ssemblages in Gorgona Island streams may partially reflect physical, chemical and biological properties. The assemblages at El Roble may reflect the highly turbulent nature of the streamflow, and the steep slope. These properties also correlated with leaflitter poverty and scarcity. Assemblages at Cocal Norte, although located on the Western Slope, were more similar to streams at the central part of the Eastern Slope probably because the watershed divide is not high enough to isolate them. On the contrary, El Roble and Pizarro located at opposite ends of the Eastern Slope are ver y far from each other and therefore their assemblages are very distinct. Therefore, adult insect migration is a seemly important homogenizing process in Gorgona Island. It is, however, sur prising that marked spatial differences occurred along the main axis of the island given that it is only 9 km long. During the rainy period, although macroinvertebrate assemblages were homogenized across streams, water physic-chemical proper ties and litter standing stocks were signif icantly different among streams and slopes.
Watershed geology and water properties (particularly conductivity) explained spatial patterns among streams in Gorgona Island (see Blanco 2009a in this issue). In addition, habitat and microhabitat-scale hydraulics were coupled with channel geomorphology, particularly with slope. Leaflitter standing stock may reflect both channel and riparian processes. For instance, the reduced leaflitter biomass observed at El Roble may result from the high gradient and the flashy hydrograph. On the other hand, reduced leaflitter richness and biomass in several streams may reflect past deforestation (Ilú, Iguapoga, El Muelle, El Chorro del Cura, Aeropuerto) or tree falling and landsliding disturbances (La Camaronera, Pizarro) (see Valencia-G. et al. 2009 in this volume for detailed analysis). Therefore, future studies should look for impacts of land conversion during the penitentiary period (1960-1982) on macroinvertebrate assemblages, because legacies of past land uses have been reported elsewhere (Harding et al. 1998, Iwata et al. 2003).
The lack of complete agreement between stream classifications based either on macroinvertebrate assemblages or independent variables suggest that assemblages could have occurred by chance and that the populations not necessarily were in equilibrium, thus controlled by particular env i ron ment al factor (Crowl and Schnell 1990, Evans and Norris 1997). Nonetheless, the sedimentary geology at La Camaronera seems to be a per vasive feature separating macroinvertebrate assemblages from those in volcanic streams elsewhere in the island (Gómez- Aguirre 2009). Overall, pH, conductivity, and alkalinity exerted a stronger influence on macroinvertebrates. The influence of geology on physicochemical factors such as conductivity and pH, which are positively related with the densities of benthic insects, has been previously reported by Ramírez and Pringle (1998b) and Ramírez et al. (2006) at volcanic landscapes in Costa Rica. In addition to geology, stream physicochemistry is affected by atmospheric inputs such as sea salt aerosols (Blanco 2009a in this issue).
In conclusion, Gorgona Island showed a rich macroinvertebrate assemblage typical of continental insular streams, where nondiadromous insects coexist with several species of diad romous decapods and a gast ropod. Macroinvertebrate assemblages differed among study streams but spatial patterns were inconsistent with differences in environmental differences other than pH and conductivity seemly influenced by watershed geology. We are confident that differences in macroinvertebrate assemblages and environmental factors among streams are robust because our hierarchical sampling design covered several reaches, habitats and microhabitats. Therefore among stream patterns were unbiased due to variability at finer scales. We recommend that future studies explore additional biotic and abiotic variables for explaining differences of macroinvertebrate assemblages among streams.
ACKNOWLEDGEMENTS
This research was funded by the Comité para el Desar rollo de la I nvestigación (CODI), Universidad de Antioquia though the project ''Gorgona Island Stream Bio-Assessment''. We thank to Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales de Colombia (UAESPNN-Gorgona) for issuing the research permit DTSO-G-03/08. Assistance in the field by Juan Carlos Arias, Justino Bonilla, and Héctor Javier Montaño is greatly acknowledged. Lilian Barreto and Héctor Montaño provided laboratory space at the Henry von Prahl Research Station at Gorgona Natural National Park. Nancy Murillo and Corazón Aguiño at the Park and other officers at the Dirección Territorial Suroccidente in Cali provided additional data. Hydrologic data were also provided by the Instituto de Estudios Ambientales (IDEAM). Thanks are also due to Teo, Armando, Alonso, Goyo, Colacho, and Guasa for logistical support and sharing their knowledge about the island. Laboratory space and equipment at Universidad de Antioquia was provided by the Limnology Group (LimnoBasE). We are grateful with comments provided by the anonymous reviewers.
REFERENCES
1. Allan JD, Johnson LB. 1997. Catchment-scale analysis of aquatic ecosystem. Freshwater Biology, 37: 107-111. [ Links ]
2. Allan JD, Erickson DL, Fay J. 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Ecology, 37:149-162. [ Links ]
3. Bass D. 2003. A comparison of freshwater macroinvertebrate communities on small Caribbean islands. BioScience, 53: 1094-1100. [ Links ]
4. Blanco JF. 2009a. The hydroclimatology of Gorgona Island: seasonal and ENSO-related patterns. Actualidades Biológicas, 31(91): 111-121. [ Links ]
5. Blanco JF. 2009b. Características físico-químicas de las quebradas del Parque Nacional Nat ural Gorgona. Actualidades Biológicas, 31(91): 123-140. [ Links ]
6. Blanco JF, Scatena FN. 2006. Hierarchical contribution of river-ocean connectivity, water chemistry, hydraulics, and substrate to the distribution of diadromous snails in Puerto Rican streams. Journal of the North American Benthological Society, 25: 82-98. [ Links ]
7. Boulton AJ, Boyero L, Covich AP, Dobson MK, Lake PS, Pearson RG. 2008. Are tropical streams ecologically different from temperate streams? In: Dudgeon D, editor. Tropical stream ecology. London: Academic Press (Aquatic Ecology Series). p. 257-284. [ Links ]
8. Boyero L. 2000. Towards a global stream ecology. Trends in Ecology and Evolution, 15: 390-391. [ Links ]
9. Boyero L. 2003. Multiscale patterns of spatial variation of stream macroinvertebrate communities. Ecological Research, 18: 365-379. [ Links ]
10. Boyero L, Bailey RC. 2001. Organization of macroinvertebrate communities at a hierarchy of spatial scales in a tropical stream. Hydrobiologia, 464: 219-225. [ Links ]
11. Cala P. 1990. Biodiversidad en aguas dulces de la isla. In: Aguirre J, Rangel O, editors. Biota y ecosistemas de Gorgona. Bogotá (Colombia): Fondo FEN. p. 261-274. [ Links ]
12. Cooper SD, Diehl S, Kratz K, Sarnelle O. 1998. Implications of scale for patterns and processes in stream ecology. Australian Journal of Ecology, 23: 27-40. [ Links ]
13. Corkum LD. 1991. Spatial patterns of macroinvertebrate distributions along rivers in eastern deciduous forest and grassland biomes. Journal of the North American Benthological, 10: 358-371. [ Links ]
14. Corkum LD. 1992. Spatial distributional patterns of macroinvertebrates along rivers within and among biomes. Hydrobiologia, 239: 101-111. [ Links ]
15. Costa SS, Melo AS. 2008. Beta diversity in stream macroinvertebrate assemblages: among-site and among-microhabitat components. Hydrobiologia, 598 (1): 131-138. [ Links ]
16. Crowl TA, Schnell GD. 1990. Factors determining population density and size distribution of a freshwater snail in streams: effects of spatial scale. Oikos, 59: 359-367. [ Links ]
17. Díaz JM, López M, Barrios LM. 2001. Introducción. In: Barrios LM, López M, editors. Gorgona marina: contribución al conocimiento de una isla única. Serie Publicaciones Especiales No. 7. Santa Marta (Colombia): INVEMAR. p. 13-16. [ Links ]
18. Dinger EC, Hendrickson DA, Winsborough BM, Marks JC. 2006. Role of fish in structuring invertebrates on stromatolites in Cuatro Ciénegas, México. Hidrobiologia, 563 (1): 407-420. [ Links ]
19. Downes BJ, Lake PS, Schreiber ESG. 1993. Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization. Freshwater Biology, 30: 119-132. [ Links ]
20. Downes BJ, Lake PS, Schreiber ESG. 1995. Habitat structure and invertebrate assemblages on stream stones: a multivariate view from the riffle. Australian Journal of Ecology, 20: 502-514. [ Links ]
21. Downes BJ, Hindell JS, Bond NR. 2000. What's in a site? Variation in lotic macroinvertebrate density and diversity in a spatially replicated experiment. Austral Ecology, 25: 128-139. [ Links ]
22. Dudgeon D. 1994. The need for multiscale approaches to the conservation and management of tropical inland waters. Mitteilungen Internationale Vereinigung für theoretische und angewandte Limnologie, 24: 11-16. [ Links ]
23. Eklund TJ, McDowell W, Pringle CM. 1997. Seasonal patterns in tropical precipitation chemistry: La Selva, Costa Rica. Atmospheric Environment, 31: 3903-3910. [ Links ]
24. Conductivity [Internet]. 2006. United States Environmental Protection Agency. Access date: 2008 Dec 28. Available in: http://www.epa.gov/volunteer/stream/vms59.html [ Links ]
25. Evans LJ, Norris RH. 1997. Prediction of benthic macroinvertebrate composition using microhabitat characteristics derived from stereo photography. Freshwater Biology, 37: 621-634. [ Links ]
26. Fièvet E, Doledec S, Lim P. 2001. Distribution of migratory fishes and shrimps along multivariate gradients in tropical island streams. Journal of Fish Biology, 59:390-402. [ Links ]
27. Flecker AS, Feifarek BP. 1994. Disturbance and the temporal variability of invertebrate assemblages in two Andean streams. Freshwater Biology, 31: 131-142. [ Links ]
28. Fossati O, Mosseron M, Keith P. 2002. Distribution and habitat utilization in two atyid shrimps (Crustacea: Decapoda) in rivers of Nuku-Hiva Island (French Polynesia). Hydrobiologia, 47 (2): 197-206. [ Links ]
29. Frissel CA, Liss WJ, Warren CE, Hurley MD. 1986. A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environmental Management, 10: 199-214. [ Links ]
30. Gansser A. 1950. Geological and petrological notes on Gorgona island in relation to north-west S America. Schweizerische Mineralogische und Petrographische Mitteilungen, 30: 219-237. [ Links ]
31. Gómez-Aguirre AM. 2009. Variación de la estructura y composición de los ensamblajes de macroinvertebrados bénticos en la jerarquía espacial de los sistemas lóticos del Parque Nacional Natural Gorgona. [Tesis de pregrado]. [Medellín (Colombia)]: Universidad de Antioquia. p.75. [ Links ]
32. Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Palaeontological Statistics software package for education and data analysis. Palaeontologia Electronica, 4 (1): 9. [ Links ]
33. Harding HE, Benfield JS, Bolstad EF, Helfman PV, Jones EBD III. 1998. Stream biodiversity: the ghost of land use past. Proceedings of the National Academy of Sciences of the United Status of America, 95: 14843-14847. [ Links ]
34. Heino J, Louhi P, Muotka T. 2004. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology, 49 (9): 1230-1239. [ Links ]
35. Heino J, Parviainen J, Paavola R, Jehle M, Louhi P, Muotka T. 2005. Characterizing macroinvertebrate assemblage structure in relation to stream size and tributary position. Hydrobiologia, 539: 121-130. [ Links ]
36. Hildrew AG, Giller PS. 1994. Patchiness, species interactions and disturbance in the stream benthos. In: Giller PS, Hildrew AG, Rafaelli DG, editors. Aquatic Ecology: scale pattern and process. 1992 Symposium at University College. Oxford, Cork: Blackwell. p. 21-62. [ Links ]
37. Ilmonen J, Paasivirta L. 2005. Benthic macrocrustacean and insect assemblages in relation to spring habitat characteristics: patterns in abundance and diversity. Hydrobiologia, 533: 99-113. [ Links ]
38. Iwata T, Nakano S, Inoue M. 2003. Impacts of past riparian deforestation on stream communities in a tropical rain forest in Borneo. Ecological Applications, 13 (2): 461-473. [ Links ]
39. Jacobsen D, Cressa C, Mathooko JM, Dudgeon D. 2008. Macroinvertebrates: Composition, life histories and production. In: Dudgeon D, editor. Tropical stream ecology. London: Academic Press (Aquatic Ecology Series). p. 66-106. [ Links ]
40. Kerr AC. 2005. La Isla de Gorgona, Colombia: A petrological enigma? Lithos, 84: 77-101. [ Links ]
41. Legendre P, Legendre L. 1998. Numerical ecology. Second edition. Amsterdam: Elsevier. p. 853. [ Links ]
42. Levin SA. 1992. The problem of pattern and scale in ecology. Ecology, 73:1943-1983. [ Links ]
43. Li J, Herlihy A, Gerth W, Kaufmann P, Gregory S, Urquhart S, Larsen DP. 2001. Variability in stream macroinvertebrates at multiple spatial scales. Freshwater Biology, 48: 768-785. [ Links ]
44. Llinás R, Pinto J, Peña F, Caro F. 1990. Geología. In: Aguirre J, Rangel O, editors. Biota y ecosistemas de Gorgona. Bogotá (Colombia): Fondo FEN. p. 244-251. [ Links ]
45. March JG, Benstead JP, Pringle CM, Luckymis M. 2003. Benthic community structure and invertebrate drift in a Pacific island stream, Kosrae, Micronesia. Biotropica, 35: 125-130. [ Links ]
46. Matthaei CD, Arbuckle CJ, Townsend CR. 2000. Stable surface stones as refugia for invertebrates during disturbance in a New Zealand stream. Journal of the North American Benthological Society, 19: 82-93. [ Links ]
47. McCune B, Mefford MJ. 1999. PC-ORD for Windows. Multivariate analysis of ecological data. Version 4.0. MjM Software, Gleneden Beach, Oregon, USA. [ Links ]
48. Montgomery DR, Buffington JM. 1998. Channel processes, classification, and response. In: Naiman RJ, Bilby RE, editors. River ecology and management: Lessons from the Pacific Coastal Ecoregion. New York: Springer- Verlag. p. 13-42. [ Links ]
49. Palmer MA, Arensburger P, Silver Botts P, Hackencamp CC, Reid JW. 1995. Disturbance and the community structure of stream invertebrates: patch-specific effects and the role of refugia. Freshwater Biology, 34: 343-356. [ Links ]
50. Parsons M, Thoms MC, Norris RH. 2003. Scales of macroinvertebrate distribution in relation to the hierarchical organization of river systems. Journal of the North American Benthological Society, 22: 105-122. [ Links ]
51. Parsons M, Thoms MC, Norris RH. 2004. Using hierarchy to select scales of measurement in multiscale studies of stream macroinvertebrate assemblages. Journal of the North American Benthological Society, 23: 157-170. [ Links ]
52. Poff NL. 1997. Landscape filters and species traits: toward mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society, 16: 391-409. [ Links ]
53. Pyron M, Covich AP, Black RW. 1999. On the relative importance of pool morphology and woody debris to distributions of shrimp in a Puerto Rican headwater stream. Hydrobiologia, 405: 207-215. [ Links ]
54. Rabeni CF, Wang N, Sarver RJ. 1999. Evaluating adequacy of the representative stream reach used in invertebrate monitoring programs. Journal of the North American Benthological Society, 18: 284-291. [ Links ]
55. Ramírez A, Pringle CM. 1998a. Invertebrate drift and benthic community dynamics in a lowland neotropical stream, Costa Rica. Hydrobiologia, 386: 19-26. [ Links ]
56. Ramírez A, Pringle CM. 1998b. Structure and production of a benthic insect assemblage in a neotropical stream. Journal of the North American Benthological Society, 17: 443-463. [ Links ]
57. Ramírez A, Pringle CM, Douglas M. 2006. Temporal and spatial patterns in stream physicochemistry and insect assemblages in tropical lowland streams. Journal of the North American Benthological Society, 25: 108-125. [ Links ]
58. Ramírez A, Pringle CM, Wantzen KM. 2008. Tropical stream conservation. In: Dudgeon D, editor. Tropical stream ecology. London: Academic Press (Aquatic Ecology Series). p. 285-304. [ Links ]
59. Ríos SL, Bailey RC. 2006. Relationship between riparian vegetation and stream benthic communities at three spatial scales. Hydrobiologia, 553: 153-160. [ Links ]
60. Robson BJ, Hogan M, Forrester T. 2005. Hierarchical patterns of invertebrate assemblage structure in stony upland streams change with time and flow permanence. Freshwater Biology, 50: 944-953. [ Links ]
61. Roldán G, Ruiz E. 2001. Development of limnology in Colombia. In: Wetzel RG, Gopal B, editors. Limnology in developing countries. Vol. 3. International Association of Theoretical and Applied Limnology. New Delhi (India): International Scientific Publications. p. 69-119. [ Links ]
62. Sheldom F, Walter KF. 1998. Spatial distribution of litoral invertebrates in the lower Murray-Darling River system, Australia. Marine and Freshwater Research, 49: 171-182. [ Links ]
63. Smith GC, Covich AP, Brasher AMD. 2003. An ecological perspective on the biodiversity of tropical island streams. Bioscience, 53: 1048-1051. [ Links ]
64. Soldner M, Stephen I, Ramos L, Angus R, Wells NC, Grosso A, Crane M. 2004. Relationship between macroinvertebrate fauna and environmental variables in small streams of the Dominican Republic. Water Research, 38: 864-874. [ Links ]
65. Thomson JR, Taylor MP, Brierley GJ. 2004. Are River Styles ecologically meaningful? A test of the ecological significance of a geomorphic river characterization scheme. Aquatic Conservation: Marine and Freshwater Ecosystems, 14: 25-48. [ Links ]
66. Townsend CR, Arbuckle CJ, Crowl TA, Scarsbrook MR. 1997. The relationship between landuse and physico- chemistry, food resources, and macroinvertebrate communities in tributaries of the Taieri River, New Zealand: a hierarchy scaled approach. Freshwater Biology, 37: 177-191. [ Links ]
67. UAESPNN. 2005. Plan de Manejo Parque Nacional Natural Gorgona. Resumen Ejecutivo. Cali: Unidad Administrativa Especial del Sistema de Parques Nacionales Naturales de Colombia Dirección Territorial Suroccidental. SUT 021105. [ Links ]
68. Vásquez GL, Naundorf GI, Zamora H. 1996. Caracterización físico-química de ecosistemas dulceacuícolas del Parque Natural Nacional Isla Gorgona, Cauca. Unicauca Ciencia [Universidad del Cauca (Popayán), Colombia], 1: 19-24. [ Links ]
69. Valencia-G. SM, Pérez-Z. GA, Lizarazo-M. PX, Blanco JF. 2009. Patrones espacio-temporales de la estructura y composición de la hojarasca en las quebradas del Parque Nacional Natural Gorgona. Actualidades Biológicas, 31(91): 197-211. [ Links ]
70. Von Ellenrieder N. 2007. Composition and structure of aquatic insect assemblages of Yungas mountain cloud forest streams in NW Argentina. Revista de la Sociedad Entomológica Argentina, 66 (3-4): 57-76. [ Links ]
71. Wiens JA. 1989. Spatial scaling in ecology. Functional Ecology, 3: 385-397. [ Links ]
72. Zamora H, Vásquez GL, Naundorf GI. 1996. Macroinvertebrados dulceacuícolas del Parque Natural Nacional Isla Gorgona, Cauca. Unicauca Ciencia [Universidad del Cauca (Popayán), Colombia], 1: 12-18. [ Links ]
73. Zapata LA, Beltrán BS, Collazos A, Prahl H. 1991. Estudio de la macrofauna asociada a la quebrada La Camaronera, isla Gorgona, Pacífico colombiano. Cespedesia, 18 (61): 23-51. [ Links ]
Recibido: mayo 2009;
Aceptado: noviembre 2009.
Appendix
Appendix 1. Study streams at Gorgona Island NNP (Tropical Eastern Pacific, Colombia). In parenthesis other names found in previous literature and cartography. Coordinates at the confluence with the ocean, and altitude of sampled reaches. A general description is given.


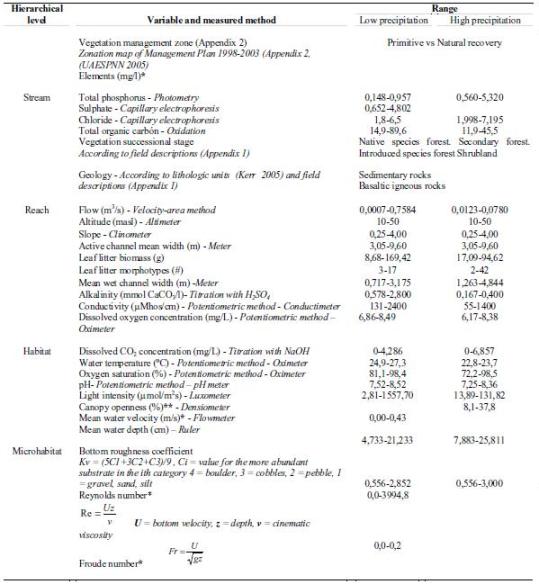
Appendix 2. Qualitative variables included in this study.
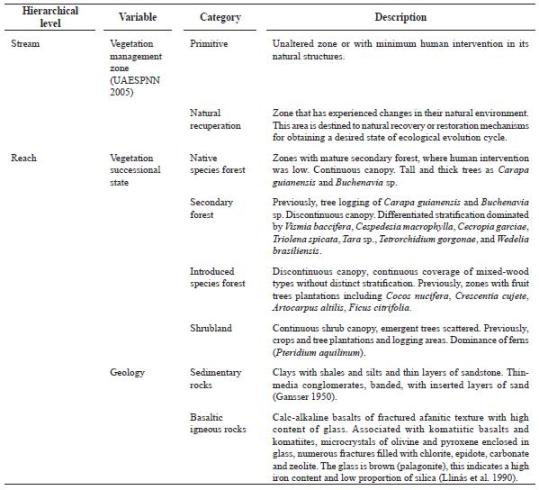
Appendix 3. Qualitative and quantitative variables measured at each hierarchical level and the range of variation within each season of the Gorgona Island (Tropical Eastern Pacific, Colombia) (*variables included only for analyses of low precipitation season. **only for high precipitation period)