Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Actualidades Biológicas
Print version ISSN 0304-3584
Actu Biol vol.35 no.99 Medellín July/Dec. 2013
RESEARCH ARTICLES
WHAT DO ANOLIS EAT?: EVALUATION OF SEXUAL DIMORPHISM AND GEOGRAPHIC VARIATION IN THE DIET OF ANOLIS VENTRIMACULATUS (SQUAMATA: DACTYLOIDAE) IN COLOMBIA
¿QUÉ COMEN LOS ANOLIS?: EVALUACIÓN DEL DIMORFISMO SEXUAL Y VARIACIÓN GEOGRÁFICA EN LA DIETA DE ANOLIS VENTRIMACULATUS (SQUAMATA: DACTYLOIDAE) EN COLOMBIA
Leidy A. Barragán-Contreras1,2; Martha L. Calderón-Espinosa1,3
1 Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Sede Bogotá. Grupo de Biodiversidad y Sistemática Molecular.
Correos electrónicos: 2 labarraganc@unal.edu.co; 3 mlcalderone@unal.edu.co.
Recibido: marzo 2013; aceptado: octubre 2013.
Abstract
Anolis lizards exhibit high morphological diversity, partially related to variation in structural resource use, that probably influences foraging behavior and prey selection of individuals and species. Anoles are largely insectivorous: most species are generalists/opportunists and few are specialists. Dietary differences between sexes and among individuals from different populations have been observed in several species. Sexual size dimorphism, spatial niche divergence between sexes and species, competition and food availability are some of the factors responsible for these differences. We characterized the diet of Anolis ventrimaculatus (Squamata: Dactyloidae), a species with sexual size and shape dimorphism, widely distributed in highland Colombian environments. Stomach and proximal intestinal content of preserved adults were analyzed. Prey items were classified to order and, when possible, to family. A. ventrimaculatus eats variable preys (mostly Hymenoptera, Coleoptera, and insect larvae) and is classified as a generalist/opportunist. Vegetal debris, shed skin, and stones presumably were ingested incidentally. Diets of males and females are similar. Sexual dimorphism and geographic variation in the diet were minimal. Males and females exhibited differences in total number and percentage of use of frequently consumed prey (Orthoptera and Hymenoptera), and these differences varied among localities. Total prey numbers consumed by females also varied among localities, whereas consumption of Coleoptera and Orthoptera varied in both sexes. Differences in prey size could explain the variation in prey number between sexes, with males probably ingesting larger items. Variation in prey availability (most likely attributable to differences in structural microhabitat use), sample sizes and dates of collecting events could explain minor geographic variation in some aspects of the foraging ecology in this species.
Key words: Anolis ventrimaculatus, Colombia, diet, geographic variation, sexual dimorphism.
Resumen
Las lagartijas Anolis presentan alta diversificación morfológica, relacionada con la variación en el uso de la vegetación, que posiblemente influencia el nicho trófico de individuos y especies. Los anoles son insectívoros, la mayoría generalistas/oportunistas y algunos especialistas. En varias especies, la dieta difiere entre sexos y entre poblaciones. El dimorfismo sexual en tamaño, las diferencias en el nicho espacial entre sexos y especies, la competencia y disponibilidad de alimento son algunos factores relacionados con estas diferencias. Caracterizamos la dieta de Anolis ventrimaculatus (Squamata: Dactyloidae), una especie con dimorfismo sexual en tamaño y forma, distribuida en ambientes de montaña en Colombia. Analizamos el contenido estomacal y porción proximal del intestino de ejemplares adultos preservados. Clasificamos taxonómicamente las presas hasta orden y familia cuando fue posible. Los especímenes de A. ventrimaculatus contenían diferentes elementos digeridos (principalmente Hymenoptera, Coleoptera y larvas de insectos) y fueron clasificados como insectívoros, generalistas/oportunistas. La ingesta de material vegetal, mudas de piel y rocas fue incidental. Machos y hembras consumen similares recursos alimenticios. El dimorfismo y variación geográfica en dieta fue mínimo. Machos y hembras consumieron diferente cantidad de presas y usaron distintamente los elementos de mayor consumo (Orthoptera e Hymenoptera); estas diferencias variaron entre localidades. Las hembras ingirieron diferente número de presas entre localidades, y el consumo de coleópteros y ortópteros presentó variación geográfica en hembras y machos, respectivamente. Diferencias en el tamaño de la presa podrían explicar las diferencias en el número de presas consumidas entre sexos, y probablemente los machos consumen presas de tamaño mayor. Diferencias en la disponibilidad de presas (debido posiblemente a diferencias en el uso del microhábitat estructural), el tamaño muestral y época de muestreo, podrían explicar las diferencias geográficas menores en algunos aspectos de la dieta de esta especie.
Palabras clave: Anolis ventrimaculatus, Colombia, dieta, dimorfismo sexual, variación geográfica.
INTRODUCTION
Evaluating the diet of a species is useful in understand its position within a trophic web and provides insights into its abiotic and interspecific interactions (Simmons et al. 2005). In addition, sexual and interspecific variation in some morphological traits is related to structural resource use in lizards and might be responsible for some differences in feeding behavior and diet (Losos 2009, Stamps et al. 1997).
Anolis lizards feed mainly on arthropods (insects and arachnids) (Losos 2009), but a substantial number of anoles, especially those on West Indian islands (30% of species; Henderson and Powell 2009, Herrel et al. 2004), are known to eat nectar, seeds, flowers, and fruits, vegetative items with high nutritional content (e. g., Schoener 1968, Schoener and Gorman 1968, Simmons et al. 2005, Timmermann et al. 2008). Other items such as small stones, skin shedding, and vegetal debris (e. g., sticks and fragments of leaves) were considered incidental (e. g., Perry 1996, Rodríguez 2010). Most anoles have been characterized as dietary generalists/ opportunists (e. g., Ardila-Marín et al. 2008, Stamps et al. 1997, Vitt et al. 2008), although some species like A. gingivinus (Eaton et al. 2002) and A. longitibialis (Gifford et al. 2002) could be considered dietary specialists, at least seasonally, since those lizards appear to prefer specific preys.
Some Anolis species exhibit trophic dimorphism, which may involve item identity (Rodríguez 2010), prey quantity, frequency, and volume (Perry 1996). In these cases differences could be related to sexual size dimorphism (body size and head dimensions) (Schoener 1968), foraging behavior (related to differences in male and female sexual roles) (Butler and Losos 2002, Butler et al. 2007, Perry 1996, Steffen 2009, Vincent and Herrel 2007), variation in perch use (Perry 1996, Schoener 1967), population density (Stamps et al. 1997), or differences in energetic requirements during the reproductive season (Schoener 1968, Vitt and Zani 1998).
Diets of anoles vary among and within species, and at least some of this variation seems related to differences in structural resource use (perch type and height) and the related access, and abundance of different prey (Fleming and Hooker 1975, Vitt and Zani 1998, Vitt et al. 2002, 2003b). Within species variation in feeding ecology has been recorded in Amazonian anoles like A. trachyderma (Vitt et al. 2002), A. punctatus and A. transversalis (Vitt et al. 2003a), and A. fuscoauratus (Vitt et al. 2003b).
Geographic variation in factors such as competitive interspecific interactions, prey availability, and structural resource use has been suggested as the underlying cause of intraspecific variation in feeding habits in these species (Fleming and Hooker 1975, Gutiérrez and Rumiz 2002, Régnière 2009, Rodríguez 2010, Schoener 1968) and lizards in general (Aun et al. 1999).
Anolis ventrimaculatus Boulenger 1911 is a highland Andean species distributed throughout Western Colombia. This species exhibits sexual-size and body-shape dimorphism, which also varies among localities (Calderón-Espinosa et al. 2013). Dimorphism in those traits is suggestive of differences in resource use between sexes and possibly, at least some variation among populations. We evaluated this hypothesis by describing the diets of males and females at different localities within the species' range.
MATERIALS AND METHODS
We analyzed the content of stomachs and proximal intestine of 118 preserved adult Anolis ventrimaculatus (Squamata: Dactyloidae), (61 females and 57 males) stored in the Reptile Collection (ICN-R), at the Instituto de Ciencias Naturales, Universidad Nacional de Colombia. Specimens came from six different localities as follows: 1) Pueblo Rico, Risaralda (thirteen females and twelve males, ICN-R 9374-9378, 9606, 9607, 9618, 9621, 9623-9625, 9627, 9629, 9633, 9641, 9642, 9647, 9648, 9651, 9663, 9665, 9678, 9683 y 9685); 2) Frontino, Antioquia (nine females and six males, ICN-R 9838-9851, 9853); 3) El Cairo, Valle del Cauca (ten females and nine males ICN-R 9350-9368); 4) Mistrató, Risaralda (seven females and fourteen males ICN-R 9707-9727); 5) Filandia, Quindío (twelve females and six males ICN-R 5790, 5792-5796, 9695-9706); 6) Urrao, Antioquia (ten females and ten males ICN-R 9244, 9246-9249, 9251, 9253-9255, 9257, 9335, 9337, 9339-9344, 9347- 9348). Elevations were 1316-1901 m above sea level. Lizards were collected from 1981 to 1992 (mostly during 1987, 1991, and 1992), although most samples from each locality came from the same year.
We measured the snout vent length (SVL) of each individual with a digital caliper to 0.1 mm and removed stomachs and proximal portions of intestines. Stomachs and intestinal content was identified to order and family when possible, following Triplehorn and Johnson (2005). We then estimated prey number by using heads, wings, legs, antennae and other identifiable body parts. Since preys were partially digested, we could not estimate prey size. Subsequently in this paper, ''item'' refers to taxonomic identity (e. g., Orthoptera, Coleoptera), whereas ''prey'' refers to individual prey ingested by lizards.
Data analyses. We evaluated dietary dimorphism at species and locality levels by comparing the number and frequency of observed items (those identified at order level) between sexes using Chi square tests. Prey number was compared by t tests, after evaluating any relationship to lizard SVL using Spearman correlation tests (Zaar 1990). We also analyzed the percentages of use of items that represented > 70% of ingesta, and used t test to compare them between sexes. Prey use was derived from the number of preys per item per individual/total number of prey ingested by each individual.
We also analyzed geographic variation using ANOVA and a posteriori Tukey test to compare diets of females and males and the percentage of use of items that represented > 70% of ingesta at the various localities.
Normality and homocedasticity were evaluated for all data and transformed to Log10 those that did not meet assumptions. Data analyses were performed in Statistica ver. 8.0 (demo).
Trophic niche breadth, niche overlap, and dominance indices. We estimated the trophic niche breadth for males and females by using the inverse Simpson index (1949) as follows: B = (Σpi2)-1,
where p represents the percentage of use of item i. We also determined if males and females used similar food resources by estimating niche overlap as proposed by Pianka (1973):
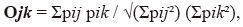
where pij pik represents the percentage of use of resource i by females (j) and males (k). These indices were calculated using Ecosim software.
Finally, we estimated the dominance Lambda index (Aun et al. 1999, Montori 1988, 1991, 1992) as follows:
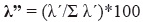
In this equation, λ' = λ/n*100 and λ = Σpi²,
where n corresponds to the number of analyzed stomachs, and pi is the probability that a prey belongs to a specific taxonomic item in each stomach (e. g., percentage of use). Four hierarchical item categories were defined from this index: primary items (λ'' > 75%), secondary items (λ'' = 50-75%), accessory items (λ'' = 25-50%) and accidental items (λ'' = 5-25%) (Montori 1992).
RESULTS
All 118 individuals examined had some ingesta in stomachs and proximal intestines. A total of 1021 prey, (593 in females and 428 in males) were classified in 16 items at the ordinal level of insects, arachnids (Araneae), myriapods, and crustaceans (isopods were present in one female). Hymenoptera and Coleoptera, which exhibited the highest taxonomic diversity at the familial level, were classified in 24 families. Some individuals had ingested vegetal material identified as Marchantiophyta, shed and small stones. These were considered incidental, because they were observed only in three individuals (table 1) and presumably were adventitiously ingested.
These lizards feed mostly on Coleoptera, Hymenoptera (mainly ants) and insect larvae, with Coleoptera classified as a primary item, whereas Hymenoptera and larvae were classified as secondary items, and others were considered, accidental (table 1). Most stomachs from all localities contained unidentified parasitic nematodes.
Sexual dimorphism and diet. Fourteen items were observed in stomachs of each sex. Males and females had fed on the same number of items (X2 = 1.37, p > 0.05). However, some items were observed in males or females only. Isopods and isopterans (small and mostly terrestrial prey) were found only in one and two females, respectively, whereas trichopterans and neuropterans (large and mostly arboreal prey) were present only in two males from different localities. Despite these differences, male and female diets were very similar, as indicated by the very high niche overlap (0.99). Niche breadths were 6.09 for males, and 6.17 for females.
Prey number was not correlated with SVL, males (r = 0.02, p = 0.88) or females (r = 0.03, p = 0.78). Females ingested more prey than males (t = 2.59, p = 0.010), although this species level pattern (pooling data from all localities) was repeated only within localities one and six, and apparently was not related to sample size.
Males and females from locality one exhibited different percentage of use of orthopterans (t = 3.76, p = 0.002) and hymenopterans (t = 2.44, p = 0.02), and those from locality six ingested different quantities of orthopterans (t = 2.24, p = 0.04). At locality two, only females fed on orthopterans. Males and females at other localities did not differ significantly in the use of these items.
Geographic variation. Only those items that were apparently exclusive of males or female, and present in very low frequencies and low percentages of use exhibited geographic variation. Isopods were observed only at locality one, isopterans were found in two females at locality six, trichopterans at locality three, neuropterans at locality two, Blattodea at localities two and six, myriapods at localities three and six, and collembolans at localities three and four (figure 1).
Males ate similar numbers of prey at all localities, but females did not (F60, 5 = 3.56, p = 0.007) with significant differences observed among females at locality one and those from localities three, four, and five (Tukey test, p < 0.05).
Females ingested different proportion of coleopterans (F60, 5 = 3.16, p = 0.016), and the most significant differences were observed between localities four and six (Tukey test, p = 0.01); males consumed different numbers of orthopterans (F56, 5 = 4.91, p = 0.004), with those from locality two being the most different from other five localities (Tukey test, p < 0.05).
DISCUSSION
Anolis ventrimaculatus is an insectivore as are most other Anolis species including A. tolimensis (Ardila-Marín et al. 2008), A. homolechis (Rodríguez 2010), A. cybotes (Fobes et al. 1992), and A. aeneus (Stamps et al. 1997). Coleoptera, Hymenoptera, and insect larvae were the most frequently consumed items, probably because these items are most abundant (Fobes et al. 1992). Our results also suggest that this species can be considered a dietary generalists/opportunists that forages mainly in tree perches, and rarely on the ground.
Nematodes found in stomach and intestinal contents are common parasites in Anolis lizards (Fobes et al. 1992, Goldberg and Bursey 2002, Lenart et al. 1994). Parasite load might influence lizard reproductive success and behavior (Suzán-Azpiril et al. 2008), but that information is not available for A. ventrimaculatus.
Sexual differences in diet were minimal and were mainly related to prey number, with males probably eating larger prey, explaining the lower number of prey ingested when compared to females, and as observed in other anoles (e. g., Perry 1996, Rodríguez 2010, Schoener 1967, 1968). Although not formally determined due to partial digestion of ingesta, differences in prey size were suggested by some very large items found only in males (Montealegre 1997, Triplehorn and Johnson 2005).
Minor differences observed between sexes are similar to those seen in A. trachyderma (Vitt et al. 2002) and A. aeneus and A. richardii (Simmons et al. 2005). Some of the differences such as prey number or percentage of item use, could be related to differences in prey availability attributable to different structural microhabitat use between sexes, as suggested by variation in body size and shape dimorphism (Calderón-Espinosa et al. 2013). However, microhabitat use or prey availability in this species has not been described, and causes that underlie the minor sexual differences in diet of A. ventrimaculatus remain unknown.
Geographic variation observed in item identity within males and within females could be explained as a sample-size effect, since this variation involved only those items, found at very low frequencies at each locality. These items have been observed in the diet of other species at similarly low frequencies (Ardila- Marín et al. 2008, Rodríguez 2010).
Total number of prey varied only among females from different localities. Food quantity consumption might be related to reproductive condition of individuals, as energetic requirements of gravid females are different than those of non-gravid females (Andrews and Asato 1977, Losos 2009, Rodríguez 2010). However, most females included in this study were gravid, only three were at an early vitellogenic stage and one was a postgravid. Consequently, variation in the amount of food ingested by females was apparently not related to energetic requirements for follicle or egg development.
Also, seasonal fluctuations in prey availability could affect feeding habits of these lizards. Densities of insects and other invertebrates usually fluctuate among seasons (Régnière 2009), affecting prey availability for lizards, as described for A. homolequis (Rodríguez 2010), A. cupreus (Fleming and Hooker 1975), and other species of this group (Aun et al. 1999).
Our study represents a first effort to understand effects of body-size and shape dimorphism, and geographic variation in an Andean anole. However, additional ecological data are necessary to explain the minor dietary variation observed in this species.
AKNOWLEDGEMENTS
This study was made possible through access to preserved specimens stored in the Reptile Collection of the Instituto de Ciencias Naturales, UN. We thank C. Sarmiento, G. Amat, E. Flores, J.H. García, D. Martínez, and F. Fernández by help in identifying arthropods and J. Uribe by helping identifying vegetal content of lizard stomachs. An anonymous review improved this manuscript.
REFERENCES
Andrews RM, Asato T. 1977. Energy utilization of a tropical lizard. Comparative Biochemistry and Physiology, 58A: 57-62. [ Links ]
Ardila-Marín DA, Hernández-Ruz EJ, Gaitán-Reyes DG. 2008. Ecología de Anolis tolimensis (Sauria, Iguanidae) en la cordillera Oriental de Colombia. Herpetotropicos, 4 (2): 71-78. [ Links ]
Aun L, Martori R, Rocha C. 1999. Variación estacional de la dieta de Liolaemus wiegmannii (Squamata: Tropiduridae) en un agroecosistema del sur de Córdoba, Argentina. Cuadernos de Herpetología, 13 (1-2): 69-80. [ Links ]
Boulenger GA. 1911. Descriptions of new reptiles from the Andes of South America, preserved in the British Museum. The Annals and Magazine of Natural History, 8th series, 7 (37): 19-25. [ Links ]
Butler MA, Losos JB. 2002. Multivariate sexual dimorphism, sexual selection, and adaptation in Greater Antillean Anolis lizards. Ecological Monographs, 72 (4): 541- 559. [ Links ]
Butler MA, Sawyer SA, Losos JB. 2007. Sexual dimorphism and adaptive radiation in Anolis lizards. Nature, 447: 202-205. [ Links ]
Calderón-Espinosa ML, Ortega-León A, Zamora-Abrego JG. 2013. Intraspecific variation in body size dimensions in an Andean highland anole species (Anolis ventrimaculatus: Squamata, Dactyloidae). Revista de Biología Tropical, 61 (1): 255-262. [ Links ]
Eaton JM, Larimer SC, Howard KG, Powell R Parmerlee JS Jr. 2002. Population densities and ecological release of the solitary lizard Anolis gingivinus in Anguilla, West Indies. Caribbean Journal of Science, 38 (1): 27-36. [ Links ]
Fleming TH, Hooker RS. 1975. Anolis cupreus: The response of a lizard to tropical seasonality. Ecology, 56 (6): 1243-1261. [ Links ]
Fobes TR, Powell R, Parmerlee JS Jr, Lathrop A, Smith DD. 1992. Natural history of Anolis cybotes (Sauria: Polychrotidae) from an altered habitat in Barahona, Dominican Republic. Caribbean Journal of Science, 28 (3-4): 200-207. [ Links ]
Gifford ME, Ramos YA, Powell R, Parmerlee JS Jr. 2002. Natural history of a xeric-adapted anole, Anolis longitibialis (Squamata: Polychrotidae), from Hispaniola. Herpetological Natural History, 8 (1): 42-44. [ Links ]
Goldberg SR, Bursey CR. 2002. Seasonal variation in the helminth community of the Brown Anole, Anolis sagrei (Sauria: Polychrotidae), from Oahu, Hawaii. American Midland Naturalist, 148 (2): 409-415. [ Links ]
Gutiérrez T, Rumiz D. 2002. Patrones de diversidad de grupos selectos de insectos en el bosque chiquitano y pampas del cerrado de Santiago y Tucavaca, Santa Cruz, Bolivia. Revista Boliviana de Ecología, 11 (1): 37-46. [ Links ]
Henderson EW, Powell R. 2009. Natural history of West Indian reptiles and amphibians. Gainesville (Florida): University of Florida Press. p. 495. [ Links ]
Herrel A, Vanhooydonck B, Joachim R, Irschick DJ. 2004. Frugivory in polychrotid lizards: effects of body size. Oecologia, 140 (1): 160-168. [ Links ]
Lenart LA, Powell R, Parmelee JS Jr, Smith DD, Lathrop A. 1994. Diet and a gastric parasite of Anolis armouri, a cybotoid anole from montane pine forests in southern Hispaniola. Herpetological Natural History, 2 (1): 97-100. [ Links ]
Losos JB. 2009. Lizards in an evolutionary tree. Ecology and adaptative radiation of anoles. Los Angeles (CA, U. S. A.): University of California Press. p. 507. [ Links ]
Montealegre F. 1997. Estudio de fauna de Tetigoniidade (Orthoptera: Ensifera) del Valle del Cauca) [Undergraduate thesis]. [Cali, Colombia]: Universidad del Valle, Facultad de Ciencias, departamento de Biología. p. 268. [ Links ]
Montori A. 1988. Estudio sobre la biología y ecología del tritón pirenaico Euproctus asper (Dugès, 185) en la Cerdanya [Ph. D. thesis]. [Barcelona, España]: Universidad de Barcelona. p. 487. [ Links ]
Montori A. 1991. Alimentación de los adultos de Euproctus asper (Dugès, 1852) en la montaña media del prepirineo Catalán (España). Revista Española de Herpetología, 5 (1): 23-36. [ Links ]
Montori A. 1992. Alimentación de las larvas de tritón pirenaico, Euproctus asper, en el prepirineo de la Cerdaña, España. Amphibia-Reptilia, 13 (1): 157-167. [ Links ]
Perry G. 1996. The evolution of sexual dimorphism in the lizard Anolis polylepis (Iguania): evidence from intraspecific variation in foraging behavior and diet. Canadian Journal of Zoology, 74 (7): 1238-1245. [ Links ]
Pianka ER. 1973. The structure of lizard communities. Annual Review of Ecology and Systematics, 4: 53-74. [ Links ]
Régnière J. 2009. Predicción de la distribución continental de insectos a partir de la fisiología de las especies. Unasylva, 60 (1): 231-232. [ Links ]
Rodríguez L. 2010. Dieta de Anolis homolechis (Cope, 1864) en el Jardín Botánico Nacional de Cuba. Revista Colombiana de Ciencia Animal, 2 (1): 147-152. [ Links ]
Schoener TW. 1967. The ecological significance of sexual dimorphism in size in the lizard Anolis conspersus. Science, 155 (3761): 474-477. [ Links ]
Schoener TW. 1968. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology, 49 (4): 704-726. [ Links ]
Schoener TW, Gorman GC. 1968. Some niche differences among three species of Lesser Antillean anoles. Ecology, 49 (5): 420-428. [ Links ]
Simmons PM, Greene BT, Williamson KE, Powell R, Parmerlee JS Jr. 2005. Ecological interactions within a lizard community on Grenada. Herpetologica, 61 (2): 124-134. [ Links ]
Simpson EH. 1949. Measurement of diversity. Nature, 163: 688. [ Links ]
Stamps JA, Losos JB, Andrews RM. 1997. A comparative study of population density and sexual size dimorphism in lizards. The American Naturalist, 149 (1): 126-135. [ Links ]
Steffen JE. 2009. An assessment of allometry for sexual size dimorphism in mainland anoles. South American Journal of Herpetology, 4 (3): 245-252. [ Links ]
Suzán-Azpiril G, Galindo-Maldonado F, Ceballos-González G. 2008. La importancia del estudio de enfermedades en la conservación de la fauna silvestre. Veterinaria México, 31 (3): 223-230. [ Links ]
Timmermann A, Dalsgaard B, Olesen JM, Andersen LH, González M. 2008. Anolis aeneus (Grenada Bush Anole), Anolis richardii (Grenada Tree Anole). Nectarivory/pollination. Herpetological Review, 39 (1): 84-85. [ Links ]
Triplehorn C, Johnson NF. 2005. Borror's introduction to the study of insects. 7th ed. Pacific Grove (California): Thomson Brooks/Cole Press. p. 864. [ Links ]
Vincent SE, Herrel A. 2007. Functional and ecological correlates of ecologically-based dimorphisms in squamate reptiles. Integrative and Comparative Biology, 47 (2): 172-188. [ Links ]
Vitt LJ, Zani PA. 1998. Prey use among sympatric lizard species in lowland rain forest of Nicaragua. Journal of Tropical Ecology, 14 (4): 537-559. [ Links ]
Vitt LJ, Avila T, Zani PA, Espósito C. 2002. Life in shade: The ecology of Anolis trachyderma (Squamata: Polychrotidae) in Amazonian Ecuador and Brazil, with comparisons to ecologically similar anoles. Copeia, 2002 (2): 275-286. [ Links ]
Vitt LJ, Avila-Pires T, Esposito M, Sartorius S, Zani PA. 2003a. Sharing Amazon rainforest trees: ecology of Anolis punctatus and A. transversalis (Squamata: Polychrotidae). Journal of Herpetology, 37 (2): 276-285. [ Links ]
Vitt LJ, Avila T, Zani PA, Sartorius S, Esposito C. 2003b. Life above ground: Ecology of Anolis fuscoauratus in the Amazon rain forest, and comparisons with its nearest relatives. Canadian Journal of Zoology, 81 (1): 142-156. [ Links ]
Vitt LJ, Shepard DB, Vieira GH, Caldwell JP, Colli GR, Mesquita DO. 2008. Ecology of Anolis nitens brasiliensis in Cerrado Woodlands of Cantao. Copeia, 2008 (1): 144-153. [ Links ]
Zaar JH. 1990. Biostatistical analysis. 4th ed. Upper Saddle River, Nueva Jersey: Prentice-Hall Press. p. 663. [ Links ]













