Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Actualidades Biológicas
versión impresa ISSN 0304-3584
Actu Biol vol.36 no.100 Medellín ene./jun. 2014
RESEARCH PAPERS
Viability of Basidiomycete fungal strains under different conservation methods: cryopreservation vs. freeze-drying processes
Viabilidad de cepas de hongos Basidiomycetes bajo diferentes técnicas de conservación: procesos de crioconservación vs. liofilización
Ana Palacio1,3, Yessica Gutiérrez1,4, Diego Rojas1,5, Lucía Atehortúa1,2,6, Paola Zapata1,2,7
1 Grupo de Investigación de Biotecnología. Sede de Investigación Universitaria (SIU), Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia.
2 Docente, Instituto de Biología, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia.
Correos electrónicos: 3 anapa30@gmail.com; 4 ygl0317@gmail.com; 5 diego.rojas@udea.edu.co; 6 latehor@gmail.com; 7 paola.zapata@udea.edu.co.
Recibido: octubre 2009; aceptado: abril 2010.
Abstract
Four basidiomycete fungi, Agaricus blazei Murrill (Agaricomycetideae), Ganoderma lucidum (W.Curt.: Fr.) P. Karst., Grifola frondosa (Dicks.: Fr.) S.F. Gray (Higher Basidiomycetes), and Pleurotus pulmonarius (Fr.) Quél. (Agaricomycetideae) were evaluated using three conservation methods for 12 months, recording their viability in order to establish the best conservation method. Growth kinetics, biomass, and polysaccharide production were studied. The conservation methods implemented included: distilled water at 24 °C; sawdust and rice bran with 10% glycerol at -20 °C; sawdust and rice bran with 10% glycerol at -80 °C; and freeze-drying of biomass with trehalose or skimmed milk. After conducting the analysis of the results after 12 months of conservation, we determined that the distilled water treatment at 24 °C was the best conservation method with the highest percentage of recoverability, at 83.3% during the 12th month, followed by the cryoconservation treatment at 80 °C, where 75% were recovered with no negative effects on biomass and polysaccharide production. The -20 °C and freeze-drying treatments were not effective; with cryoconservation at -20 °C treatment, strain recovery only occurred during the first month and with freeze-drying it was not possible to recover any strains during the entire 12-month period evaluated.
Key words: Cryoconservation, freeze-drying, basidiomycete, biomass, exopolysaccharides, intrapolysaccharides.
Resumen
Cuatro hongos basidiomycetes, Agaricus blazei Murrill (Agaricomycetideae), Ganoderma lucidum (W.Curt.: Fr.) P. Karst. y Grifola frondosa (Dicks.: Fr.) S.F. Gray (Higher Basidiomycetes), y Pleurotus pulmonarius (Fr.) Quél. (Agaricomycetideae) fueron evaluados bajo tres métodos de conservación durante 12 meses, observando su viabilidad con el fin de establecer el mejor método de conservación. La cinética de crecimiento, producción de biomasa y polisacáridos fueron estudiados. Los métodos de conservación implementados incluyeron: agua destilada a 24 °C; aserrín y salvado de arroz con glicerol 10% a -20 °C; aserrín y salvado de arroz con glicerol 10% a -80 °C y; liofilización de biomasa con trehalosa o leche desnatada. Luego de realizar el análisis de los resultados de 12 meses de conservación, se determinó que el tratamiento de agua destilada a 24 °C fue el mejor método de conservación con el porcentaje de recuperabilidad más alto un 83,3% en el mes 12, seguido por el tratamiento de crioconservación a - 80 °C donde se recuperó el 75%, sin afectar negativamente la producción de biomasa y polisacáridos. El tratamiento a -20 °C y la liofilización no fueron efectivos; con la crioconservación a -20 °C solo se recuperaron cepas en el primer mes y con la liofilización no fue posible recuperar cepas en el período de 12 meses evaluado.
Palabras clave: Crioconservación, liofilización, basidiomycetes, biomasa, exopolisacáridos, intrapolisacáridos.
INTRODUCTION
Recently, the use of microorganisms for the production of the commodity service industry and environmental control has become an alternative for the sustainable development of technologies. In this field the use of basidiomycetes is of great importance due to its wide range of applications including bioremediation, enzyme production, degradation of Lignocellulosic residues (Cohen et al. 2002, Sánchez 2009, Seto et al. 1999), pharmaceutical products development and functional foods design (Ghorai et al. 2009).
The increase in industrial applications that involves these types of basidiomycete species implies the establishment of techniques for the optimization in the use of resources and yield for industrial products such as biomass, polysaccharides, proteins, enzymes and others; therefore is necessary the application of ex-situ conservation protocols in order to preserve for a long time, not only the viability but also the morphological, physiological and genetic characteristics (Day and Stacey 2007). The factors involved in the maintenance process often cause loss yield or poor quality of the product, as it has been the case for the production of these fungi over the last few years. Hence, there is a market pressure to improve the yield and quality of the edible fungi currently produced, and to increase studies for the formulation of appropriate substrates, culture conditions and conservation process for these species (Homolka et al. 2007).
In order to continue originating innovative proposals based in the basidiomycete species that are currently being used, and also with other potential species as the ones reported for Colombia; 272 species recorded (Franco and Uribe 2000) with 119 species only in the Amazon region (Vasco et al. 2005); in this work different methods for conservation of four different basidiomycetes of industrial interest were evaluated (Zhong and Tang 2004).
Because of their medicinal, functional, and industrial uses, the fungi Agaricus blazei Murrill (Agaricomycetideae), Ganoderma lucidum (W.Curt.: Fr.) P. Karst. and Grifola frondosa (Dicks.: Fr.) S.F. Gray (Higher Basidiomycetes), and Pleurotus pulmonarius (Fr.) Quél. (Agaricomycetideae) have been broadly studied (Zhang et al. 2007). These species are characterized for their content of β-D glucans β(1→3), β(1→6), proteoglycans, triterpenes (ganoderic acids, ganodermanotriol, and ganoderiol), phenolic compounds, among others, that have a wide variety of activities as immunostimulating, hypoglycemic, antifungal, antiviral antibacterial, anti-inflammatory, antiallergenic, antioxidant, and cholesterol-lowering (Chen and Seviour 2007, Chen et al. 2012, Gunde-Cimerman et al. 1993a, b, Gunde-Cimerman and Cimerman 1995, Illana-Esteban 2008, Kodama et al. 2003, Lavi et al. 2010, Lindequist et al. 2005, Soares et al. 2009, Zhang et al. 2002).
In order to guarantee viability for these microorganisms, various procedures have been carried out allowing the management of strains and at the same time saving funds since there is no need to acquire new strains, by subculturing or serial transferring, which is frequently used as a routine method for preservation of fungi. However, it is not practical for storing large numbers of cultures. Also, it is timeconsuming, more susceptible of contamination and does not prevent genetic and physiological changes during long-term storage (Homolka et al. 2001, 2007, Kitamoto et al. 2002, Mata and Pérez 2003).
Different methods have been developed in order to eliminate these disadvantages like the storing of agar disks in sterile distilled water or mineral oil storing at 15 °C with paraffin, storing in liquid nitrogen and freeze-drying, commonly used in the preservation of fungi (Homolka et al. 2007, Kitamoto et al. 2002, Voyron et al. 2009); nevertheless, it is necessary to point out that although storing in liquid nitrogen can guarantee stability, it can be just as laborious as subculturing since samples have to be submitted to a specific freezing rate depending on the species until reaching a temperature of -40 °C in order to be kept at -196 °C without any collateral effect for the microorganism, it also involves higher costs and the results are variable depending on some factors like age of the mycelia, mycelia matrix, presence or absence of cryoprotector agents, type of cryoprotector, thawing temperatures, etc. (Homolka et al. 2007, Kitamoto et al. 2002).
Besides the above mentioned methods, there are others that have been applied to fungal strains but need to be more studied in order to standardize protocols that could give the required stability; methods as freeze-drying and deep freezing within the range of -70/ -85 °C, in several cases, have demonstrated to be efficient in the recovery and specific characteristics as growth rate, enzymatic activity and high genetic stability (Kitamoto et al. 2002, Voyron et al. 2009). Experiments have been undertaken already in which some basidiomycete species were frozen at -85 °C for 10 years managing to survive; even the strains evaluated were able to produce fruiting bodies after three months of strain recovery and after having intervals of freezing and thawing of six months in the conservation time (Kitamoto et al. 2002).
Because a well-established method for some basidiomycetes using freeze-drying does not exist yet, further experimentation is required in order to determine the effectiveness and feasibility since the results can be seriously affected by the variations in the protocol, like the type of suspension solution used for the process and the perfusion time prior to freezing (Homolka et al. 2001, Singh et al. 2004, Voyron et al. 2009).
Several factors affect the effectiveness of the preservation process, strain, preservation temperature, composition of the growth media, time storage, and cryoconservation/freezedrying protecting agent (like glycerol, dimethyl sulfoxide, bovine albumin serum, skimmed milk, ethylene glycol sorbitol, and trehalose or myo-inositol). Few microorganisms can survive after the preservation process without a protecting agent; these agents can provide a longer storage time, avoiding cellular injuries (Hubálek 2003).
Based on the above background, the aim of this work, was to analyze non-conventional, practical and economical methods for basidiomycete strain conservation using the fungi A. blazei, G. lucidum, G. frondosa, and P. pulmonarius as models with the purpose of establishing the best conservation method that provides an affordable procedure for later use. This project included the evaluation of the growth kinetics, biomass exopolysaccharides (EPS) and intrapolysaccharides (IPS) production of each fungi after 12 months of conservation; the treatments were distilled water at 24 °C, sawdust and rice bran with 10% glycerol at -20 °C, sawdust and rice bran with 10% glycerol at -80 °C, and freeze-drying of biomass with trehalose or skimmed milk.
MATERIALS Y METHODS
Strain maintenance. Spawn of four basidiomycete fungi, A. blazei, G. lucidum, G. frondosa, and P. pulmonarius, were provided by the Plant Biotechnology Research Group of the University of Antioquia. The strains were maintained in a standardized media for fungi called as MGL1 designed in the group which consists of (in g l-1): Barley flour-30, yeast extract-3, saccharose-5.3 and agar-8 with a pH of 5.5 ± 0.1 (Zapata et al. 2009).
Evaluation of conservation method. Conservation methods used in this research were distilled water at room temperature (24°C), cryoconservation, and freeze-drying. The treatments were evaluated during a period of 12 months.
Distilled water treatment. Mycelial agar disks of 1 cm of diameter were removed from the margin of an actively growing colony and transferred to 2 ml vials with 1 ml sterile distilled water and kept at 24 ± 1 °C, made as a triplicate for further analysis after 1, 6, and, 12 months of conservation. For recovery of strains, mycelia agar disks were directly Inoculated in MGL1 agar and incubated at 24 ± 1 °C under darkness conditions for 15 days.
Cryoconservation processes: -20 and -80 °C. Mycelial agar disks of 1 cm of diameter were removed from the margin of an actively growing colony and transferred to 2 ml vials to be cryoconserved in 0.05 g of sawdust and 0.05 g of rice bran using 1 ml of 10% glycerol as cryoprotective agent (Kitamoto et al. 2002). Vials were stored at -20 °C. Each essay was made as a triplicate for further analysis after 1, 6, and 12 months of conservation; the same methodology was employed for the vials stored at -80 °C. For recovery, vials were thawed at 24 ± 1°C, inoculated in MGL1 agar and incubated under darkness conditions for 15 days.
Freeze-drying process. Mycelial agar disks of 1 cm of diameter were removed from the margin of an actively growing colony and inoculated in 65 ml of MGL1 under submerged culture conditions, the media consisted of (g l-1): Barley flour-50, NaNO3-0.08, MgSO4.7H2O-0.02, KCl-0.01 y KH2PO4-0.03; pH 5.5 ± 0.1 (Zapata et al. 2009). After 12 days of incubation, 10 ml of media culture with biomass were disposed in conical tubes with 5 ml of 10% skimmed milk (p/v) or 10% trehalose (p/v) depending on the sample to be freezedried (Voyron et al. 2009). Each essay was made as a triplicate for further analysis after 1, 6, and 12 months of conservation.
For recovery 5 mg of freeze-dried biomass were resuspended in 5 ml of distilled water for 1 h at 24 ± 1 °C. Then, fragments extracted from each sample were inoculated in MGL1 agar at 24 ± 1 °C under darkness conditions for 15 days.
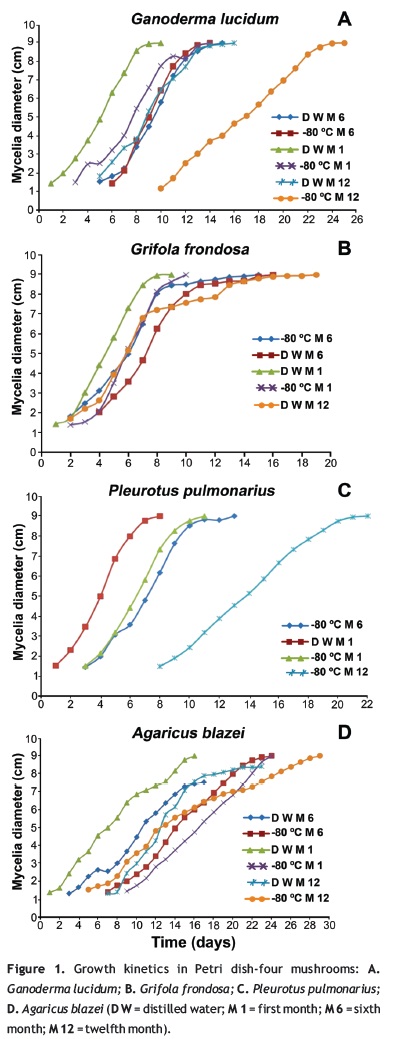
Evaluation of strain viability. Determination of the growth rate and biomass production in Petri dish. The measurement of mycelial growth was made by method described by Donini et al. 2006 modified by the authors, using a millimetrical ruler for the measurement of growth diameter in Petri dish in four directions every 24 hours during the period of incubation until the mycelia invaded all the media in the dish (Donini et al. 2006).
After the last measurement, the agar and mycelia contained in each Petri dish were placed in boiling water in order to separate the fungal biomass of the agar. The biomass was then dried in an oven at 70 °C until constant weight was reached. The results were registered in g per petri dish (Donini et al. 2006).
Determination of the biomass production, EPS, and IPS in submerged culture. Biomass production. Mycelial agar disks from the recovered strains were subcultured in MGL1 agar; from the subcultured strains 250 ml flasks containing 63 ml of MGL1 liquid media were inoculated with mycelial agar disks of 1 cm of diameter, these cultures were incubated at 100 rpm, 24 ± 1 °C and light periods of 12 h for 12 days (Zapata et al. 2009).
The biomass obtained from each flask was filtered with a mesh sieve # 35 (Grand Test®), then, washed with abundant distilled water and finally the biomass was dried in an oven at 70 °C until constant weight was obtained (Tang and Zhong 2002).
Extraction of polysaccharides (EPS and IPS). IPS extraction was made from 1 g of dry biomass, polysaccharides were extracted with boiling water for 20 min, the solution was immediately filtered with filter paper of Ø 125 mm (Whatman®), 4 volumes of 96% ethanol were added to this solution and this mix was kept at 4 °C for at least 1 h; finally this mix was centrifuged at 4.000 rpm at -4 °C for 20 min, the supernatant was discarded and the pellet was diluted in 5 ml of distilled water (Tang and Zhong 2002).
For EPS extraction the filtered media was centrifuged at 2.500 rpm at 25 °C to eliminate suspended solids, 10 ml of the supernatant were taken and 4 volumes of 96% ethanol were added. From here, the same protocol for IPS was performed in order to get the polysaccharide extract.
Quantification of polysaccharides. Colorimetric method of phenol-sulfuric acid reported by Dubois et al. (1956) and modified by Masuko et al. (2005), was followed for IPS and EPS quantification. The measurement of the samples was made in microplate format (Dubois et al. 1956, Masuko et al. 2005).
Experimental design. The experimental design applied was a multifactor ANOVA, completely randomized with 3 replicates per treatment; two factors were evaluated, preservation method, and species with the purpose of determining if there was any difference in the mean of biomass and polysaccharides production after 12 days of culture. The design was evaluated with the software StatGraphics Centurion XV®.
RESULTS
Each strain submitted to evaluation was recovered after 1, 6, and 12 months of conservation, -20 °C method only shown recovery of 3 from 4 fungi in the first month, with no representative data (data not shown). For the freeze-dried samples, loss of the strain viability was observed. According to these facts only distilled water method and cryoconservation method at -80 °C were suitable for analysis.
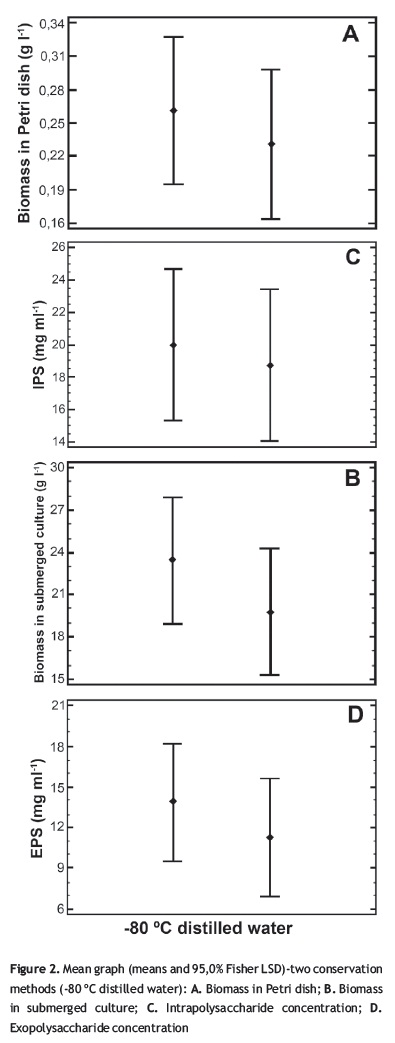
The different conservation methods as well as the conservation time have an influence in the growth kinetics. This could be observed because the mycelia development had a retarded growth in the -80 °C method compared with the distilled water method (figure 1). However, for the present work this does not imply a negative effect since the number of days in which the petri dish was invaded was similar and equal in some cases for both methods. This type of results could be confirmed with the values of biomass and polysaccharide production that, in some cases, were bigger for the -80 °C method, and there were few cases in which lower concentration was obtained without major divergence (figure 2).
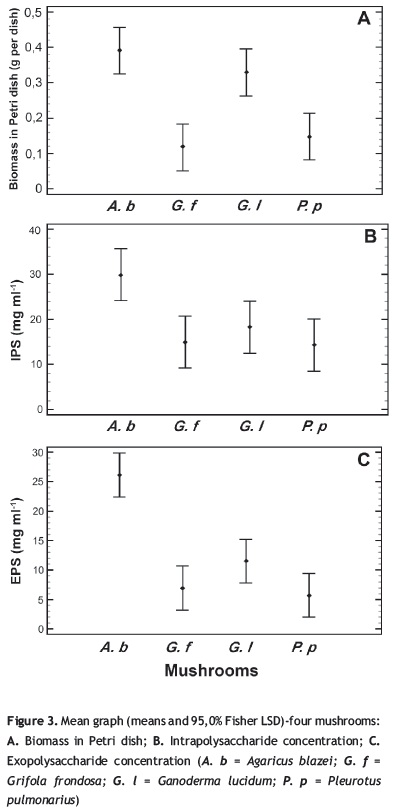
In order to verify which was the best method, a multifactor ANOVA was performed with a p value < 0.05 employing the results obtained for the 12th month. The analysis shows that there is no significant statistical difference for the preservation method factor for all response variables: biomass in Petri dish, IPS, EPS, and biomass in submerged culture with p values of 0.4961; 0.7502; 0.3128, and 0.3631, respectively. However, for the species factor there are significant statistical differences, except for biomass in submerged culture, with pvalues for biomass in Petri dish, IPS, and EPS of 0.0006, 0.0377, and 0.0001 respectively; this is clear when observing the behavior of the fungi A. blazei whose mean values were major than those shown for the rest of the fungi in three of the four variables (figure 3) and also in the cases of P. pulmonarius.
DISCUSSION
Initially it is important to mention, that the ability of fungi cells to resist different conservation methods like cryoconservation and freeze-drying may be affected by factors such as the age and physiological state of the hyphae, as well as the nature of cytoplasmic contents (Mata and Rodríguez 2005).
In the analysis of results it was observed that the -80 °C temperature showed improved results when compared with the -20 °C temperature; a feasible reason for these results, is that at -80 °C cells can be better preserved, since for some fungi a high freezing rate might not lead to ice crystal formation (Smith 1983). In the work published by Morris et al. (1988), the fungi Lentinus edodes, Serpula lacrymans, Sporobolomyces roseu, and Volvariella volvacea did not formed crystals at a freezing rate of -100 °C min-1; in the case of Serpula lacrymans a rate of 0.5 °C min-1 to -40 °C and then rapid cooling to -196 °C gave much higher recoveries than freezing to -196 °C at a cooling rate of 1 °C min-l (Morris et al. 1988), the growth of these ice crystals formed during cooling can be a key factor promoting cell damage during freezing and thawing procedures (Morris et al. 1988). These are other reasons for the lack of good results at -20 °C temperature; the quantity of formed crystals in this method could have been higher since the freezing rates were lower.
In general, the -80 °C method was a good treatment and allowed the recovery of the fungi; only in the last month a single experimental unit was recovered for G. frondosa, then it is feasible that, in the cases of non-recovered samples, the bond formed between matrix and mycelia was not strong enough, as several authors have reported about the use of immobilization techniques for cryoconservation using different matrixes like alginate beads, silica gel, minerals as perlite and sorghum seed invaded with mycelia ''spawn'' (Homolka et al. 2006, 2007, Mata and Pérez 2003, Ryan 2001, Sharma and Smith 1999); in data reported by Mata and Pérez (2003); it is also shown that even better results were obtained when cryoconservation of spawn was made without a cryoprotectant or water and hyphae could grow from the seed hilium or from fissures on the surface of the seeds. These immobilization techniques can represent an alternative in the protection of fungal hyphae from mechanical damage like crystal formation; at the same time a matrix can serve as substrate before and after freezing. In the present work fungal hyphae growth in the Petri dish occurred not only from the agar disk but also from pieces of sawdust inoculated in the media; this confirms that having only agar as a matrix might not be suitable for attachment of the mycelia in the preservation process, which was the case of this work, since it could be observed that after the freezing this material disintegrated, which made the recovery of the mycelia for a new inoculation process very difficult.
Based on the statistical analysis, it could be inferred that the preservation in distilled water was the best method for A. blazei, G. lucidum, and G. frondosa, which implies that this method is reliable, trustworthy, simple, and very economic; these conclusions can be supported by the work made by Burdsall et al. (1994) that evaluated 151 basidiomycete species, where 94% of the isolated strains were viable after being kept in water during seven years (Burdsall et al. 1994). In our case 83.3% of recovery in the 12th month was accomplished in the distilled water method; a higher percentage compared with the obtained by cryoconservation at -80 °C in which 75% was recovered; however, it is essential to highlight that for P. pulmonarius the last one mentioned, was the most proper method and it further becomes an alternative when there is no access to cryoconservation in liquid nitrogen since this one implies more costs and it is labor intensive because a freezing rate needs to be established; moreover a feasible reason for the absence of growth of this strain when conserved in distilled water is that the moisture requirements probably were higher and combined with possible evaporation resulted in depletion of water which was not enough for this conservation period.
The effect of the different conservation methods on polysaccharide production is a new report, since the majority of the research that has been done at the moment focuses in recovery rate, growth rate and enzymatic activity like response variables (Voyron et al. 2009, Homolka et al. 2010). In this work was possible to establish that the polysaccharide production was not negatively affected in a significant way for all the species and treatments, except for P. pulmonarius in distilled water; contrary, a rise was observed with values of (mg/ml) 18.29; 14.94; 29.94 of IPS and 11.50; 6.977; 26.11 of EPS for G. lucidum, G. frondosa, A. blazei, respectively (figure 3), compared with data reported by other authors with values of (mg/ml) 4.74; 1.3; 1.40 of IPS and 10.5; 7.2; 5.08 of EPS for G. lucidum, G. frondosa, A. blazei, respectively (Bae et al. 2005, Cao et al. 2010, Papinutti 2010, Tang et al. 2011, Zou 2005), no reports to date for comparison of IPS and EPS production of P. pulmonarius were found. Most of the higher values presented were obtained in IPS production, this could have occurred as a response after the treatments to adverse conditions of low temperature, lack of nutrients or water activity for proper metabolism and development; which could be reflected in subsequent cultures in solid and liquid media of the recovered strains; therefore it could be inferred that this can be a mechanism to protect mycelium from any injury accumulating IPS and secreting EPS with values above the normal yields obtained when growing actively. Although there are hardly few reports of effect of preservation methods in fungi polysaccharide production, these results can be compared with the behavior of another fungal organism like yeast, in which polysaccharide accumulation has been reported in stress conditions, and it has been reported as well that polysaccharides of these kinds of organisms have cryoprotective effects (Breierová 1992, Papinutti 2010).
Another possible reasons for this rise could be first of all related with the production of antifreeze substances, among which proteins and glycoproteins can be found; in some basidiomycete fungi as Coprinus psychromorbidus, Flammulina populicola, Lentinus edodes, and Typhula ishikariensis, it has already been reported the presence of antifreeze proteins, in the case of the fungi studied it would be interesting to analyze if there are any glycoproteins with this function, specially for A. blazei that presented the highest production of polysaccharides, and can be considerated like a way to obtain an overproducing polysaccharides strain (Hoshino et al. 2003, Raymond and Janech 2009).
Regarding the Freeze-drying method, it demonstrated to be inappropriate for all the strains under the applied conditions, since they were not viable; this could be confirmed in the first recovery, in which after one month of incubation the wet mycelia was unable to multiply, confirming thereby importance of water activity in the stability of vegetative mycelia, this was the case of the developed assays, where the whole process of freeze-drying was carried out by starting from biomass obtained from submerged culture to develop the assay; in this state the fungi that were evaluated do not form spores; and, only these structures, in the case of most of basidiomycete fungi, are capable of supporting these extreme and adverse dehydration conditions; However, there are few cases, like the mycorrhizal forming fungi that have managed to resist the hard conditions and survive successfully (Tan and Stalpers 1991).
Although freeze-drying could be used as one of many methods for the conservation of filamentous fungi, it is important to consider that in order to obtain an optimal viability without collateral damage, a strict protocol is needed; not only in terms of selecting and using the right protective agent at the moment of freeze-drying, but also a proper selection of an intracellular accumulation agent during the incubation time for diminishing the crystal formation process; moreover, it is relevant to establish a freezing rate prior to the process and also to control the drying rate. Therefore, several specific conditions and large processes of standardization of the methodology are required to achieve a useful technique without secondary effects (Croan 2001, Smith 1993).
According with all results, we suggest using distilled water as a conservation method since it gave the highest percentage of recoverability (83.3%), for its practicality and the possibility to diminish costs related with chemicals and equipment; however for specific cases as the one presented for the fungi P. pulmonarius cryoconservation at -80 °C is suggested.
ACKNOWLEDGMENTS
Special acknowledgement to the Committee for Research Development of the University of Antioquia (CODI) responsible for the financial support of the project and to the PREMEX S. A. and Productora y Comercializadora de Alimentos S. A. (PCA) companies as well as the governmental entity, COLCIENCIAS for funding the project 1115-489-25308, Contract 629-2009, and to the Universidad de Antioquia and the Division of Sustainability of the Committee for Research Development of the Universidad de Antioquia (CODI) (Estrategia de Sostenibilidad 2013-2014).
REFERENCES
Breierová E, Kocková-Kratochilová A. 1992. Cryoprotective effects of yeast extracellular polysaccharides and glycoproteins. Cryobiology, 29 (3): 385-390. [ Links ]
Burdsall H, Dorworthl E. 1994. Preserving cultures of wood-decaying Basidiomycotina using sterile distilled water in cryovials. Mycologia, 86 (2): 275-280. [ Links ]
Cao XH, Yang QW, Lu MF, Hou LH, Jin YY, Yuan J, Wang CL. 2010. Preparation and anticoagulation activity of a chemically sulfated polysaccharide (S-Gfb) obtained from Grifola frondosa. Journal of Food Biochemistry, 34 (5): 1049-1060. [ Links ]
Chen J, Seviour R. 2007. Medicinal importance of fungal β (13), β (16) glucans. Mycological Research, 111 (6): 635-652. [ Links ]
Chen SY, Ho KJ, Hsieh YJ, Wang LT, Mau JL. 2012. Contents of lovastatin, γ- aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT - Food Science and Technology, 47 (2): 1-5. [ Links ]
Cohen R, Persky L, Hadar Y. 2002. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Applied Microbiology and Biotechnology, 58 (5): 582-594. [ Links ]
Croan SC. 2001. Preservation of hyphal-forming brown- and white-rot woodinhabiting Basidiomycetes. Stockholm (SW): International Research Group on Wood Protection. Conference: 01-05-20/25 Nara, Japan. IRG/WP 01-10397. [ Links ]
Day JG, Stacey GN. 2007. Cryopreservation and freeze-drying protocols. 2nd ed. Totowa, NJ (U. S. A.): Humana Press. p. 347. [ Links ]
Donini LP, Bernardi E, Soares J. 2006. Desenvolvimiento in vitro de Agaricus blasiliensis em meios suplementados com diferentes farelos. Pesquisa Agropecuária Brasileira, 41 (6): 995-999. [ Links ]
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. 1956. Colorimetric method for determination of sugar and related substances. Analytical Chemistry, 28 (3): 350-356. [ Links ]
Franco A, Uribe E. 2000. Hongos Agaricales y Boletales de Colombia. Biota Colombiana, 1 (1): 25-43. [ Links ]
Ghorai S, Banik SP, Verma D, Chowdhury S, Mukherjee S, Khowala S. 2009. Fungal biotechnology in food and feed processing. Food Research International, 42 (5-6): 577-587. [ Links ]
Gunde-Cimerman N, Friedrich J, Cimerman A, Benicki N. 1993a. Screening fungi for the production of an inhibitor of HMGCoA reductase: Production of mevinolin by the fungi of the genus Pleurotus. FEMS Microbiology Letters, 111 (2-3): 203-206. [ Links ]
Gunde-Cimerman N, Plemenitas A, Cimerman A. 1993b. Pleurotus fungi produce mevinolin, an inhibitor of HMGCoA reductase. FEMS Microbiology Letters, 113 (3): 333-338. [ Links ]
Gunde-Cimerman N, Cimerman A. 1995. Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase - lovastatin. Experimental Mycology, 19 (1): 1-6. [ Links ]
Homolka L, Lisá L, Eichlerová I, Nerud F. 2001. Cryopreservation of basidiomycete strains using perlite. Journal of Microbiological Methods, 47 (3): 307-313. [ Links ]
Homolka L, Lisá L, Nerud F. 2006. Basidiomycete cryopreservation on perlite: Evaluation of a new method. Cryobiology, 52 (3): 446-453. [ Links ]
Homolka L, Lisá L, Nerud F. 2007. Basidiomycete cultures on perlite survive successfully repeated freezing and thawing in cryovials without subculturing. Journal of Microbiological Methods, 69 (3): 529-532. [ Links ]
Homolka L, Lisá L, Eichlerová I, Valásková V, Baldrian P. 2010. Effect of long-term preservation of basidiomycetes on perlite in liquid nitrogen on their growth, morphological, enzymatic and genetic characteristics. Fungal Biololgy, 114 (11-12): 929-935. [ Links ]
Hoshino T, Kiriaki M, Ohgiya S, Fujiwara M, Kondo H, Nishimiya Y, Yumoto I, Tsuda S. 2003. Antifreeze proteins from snow mold fungi. Canadian Journal of Botany, 81 (12): 1175-1181. [ Links ]
Hubálek Z. 2003. Protectants used in the cryopreservation of microorganisms. Cryobiology, 46 (3): 205-229. [ Links ]
Ilana-Esteban, C. 2008. El hongo Maitake (Grifola frondosa) y su potencial terapéutico. Revista Iberoamericana de Micología, 25 (3): 141-144. [ Links ]
KitamotoY, Suzuki A, Shimada S, Yamanaka K. 2002. A new preservation method of fungus stock cultures by deep freezing. Mycoscience, 43 (2): 143-149. [ Links ]
Kodama N, Asakawa A, Inui A, Masuda Y, Nanba Y. 2003 Effect of Maitake (Grifola frondosa) D-Fraction on the Activation of NK Cells in Cancer Patients. Journal of Medicinal Food, 6 (4): 371-377. [ Links ]
Lavi I, Levinson D, Peri I, Tekoah Y, Hadar Y, Schwartz B. 2010. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Applied Microbiology and Biotechnology, 85 (6): 1977-1990. [ Links ]
Lindequist U, Niedermeyer T, Julich WD. 2005. The Pharmacological Potential of Mushrooms. Ecam, 2 (3): 285-299. [ Links ]
Masuko T, Minamib A, Iwasakib N, Majimab T, Nishimura SI, Lee YC. 2005. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format Analytical Biochemistry, 339 (1): 69-72. [ Links ]
Mata G, Pérez-Merlo R. 2003. Spawn viability in edible mushrooms after freezing in liquid nitrogen without a cryoprotectant. Cryobiology, 47 (1): 104-20. [ Links ]
Mata G, Rodríguez AE. 2005. Viability in spawn stocks of the white button mushroom, Agaricus bisporus, after freezing in liquid nitrogen without a cryoprotectant. Journal of Agricultural Technology, 1 (1): 153-162. [ Links ]
Morris GJ, Smith D, Coulson GE. 1988. A Comparative Study of the Changes in the Morphology of Hyphae during Freezing and Viability upon Thawing for Twenty Species of Fungi. Journal of General Microbiology, 134 (11): 2897-2906. [ Links ]
Papinutti L. 2010. Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresource technology, 101 (6): 1941- 1946. [ Links ]
Raymond J, Janech M. 2009. Ice-binding proteins from enoki and shiitake mushrooms. Cryobiology, 58 (2): 151-6. [ Links ]
Ryan JM. 2001. The use of inmobilisation for the preservation of Serpula lacrymans. Mycologist, 15 (2): 65-67. [ Links ]
Sánchez C. 2009. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnology Advances, 27 (2): 185-194. [ Links ]
Seto M, Nishibori K, Masai E, Fukuda M, Ohdaira Y. 1999. Degradation of polychlorinated biphenyls by a ''Maitake'' mushroom, Grifola frondosa. Biotechnology Letters 21 (1): 27-31. [ Links ]
Sharma B, Smith D. 1999. Recovery of fungi after storage for over a quarter of a century. World Journal of Microbiology and Biotechnology, 15 (4): 517-519. [ Links ]
Singh SK, Upadhyay RC, Yadav MC, Tiwari M. 2004. Development of a novel lyophilization protocol for preservation of mushroom mycelial cultures. Current Sciences, 87 (5): 568-570. [ Links ]
Smith D. 1983. Cryoprotectants and the cryopreservation of fungi. Transactions of the British Mycological Society, 80 (2): 360-363. [ Links ]
Smith D. 1993. Long-term Preservation of Test Strains (Fungus). International Biodeterioration & Biodegradation, 31 (3): 227-230. [ Links ]
Soares A, Marques de Souza C, Daniel F, Pezente Ferrari G, Gomes da Costa S, Peralta R. 2009. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chemistry, 112 (4): 775-781. [ Links ]
Tan CS, Stalpers JA. 1991. Freeze-Drying of fungal hyphae. Mycologia, 83 (5): 654-657. [ Links ]
Tang Y, Zhong J. 2002. Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acid. Enzyme and Microbial Technology, 31 (1-2): 20-28. [ Links ]
Tang YJ, Zhang W, Liu RS, Zhu LW, Zhong JJ. 2011. Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides. Process Biochemistry, 46 (1): 404-408. [ Links ]
Vasco A, Franco A, López C, Boekhout T. 2005. Macromicetes (ascomycota, basidiomycota) de la región del medio Caquetá, departamentos de Caquetá y Amazonas. Biota Colombiana, 6 (1): 127-140. [ Links ]
Voyron S, Roussel S, Munaut F, Varese GC, Ginepro, M, Declerck S, Marchisio Filipello V. 2009. Vitality and genetic fidelity of white-rot fungi mycelia following different methods of preservation. Mycological Research, 113 (10): 1027-1038. [ Links ]
Zapata P, Rojas D, Ramirez D, Atehortúa L, Fernández C. 2009. Effect of different Light-Emitting Diodes on micelial biomass production of Lingh Zhi or Reishi medicinal mushroom Ganoderma lucidum (W.Curt.: F) P. Karst (Aphyllophoromycetidaceae). International Journal of Medicinal Mushrooms, 11 (1): 93-99. [ Links ]
Zhang J, Tang Q, Zimmerman-Kordmann M, Reutter W, Fan H. 2002. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sciences, 71 (6): 623-638. [ Links ]
Zhang M, Cui SW, Cheung PCK, Wang Q. 2007. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Food Science and Technology, 18 (1): 4-19. [ Links ]
Zhong JJ, Tang YJ. 2004. Submerged Cultivation of Medicinal Mushrooms for Production of Valuable Bioactive Metabolites. Advances in Biochemical Engineering. 87, 25-59. [ Links ]
Zou X. 2005. Effects of Zn supplementation on the growth, amino acid composition, polysaccharide yields and anti-tumour activity of Agaricus brasiliensis. World Journal of Microbiology and Biotechnology, 21 (3): 261-264. [ Links ]