Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Actualidades Biológicas
versión impresa ISSN 0304-3584
Actu Biol vol.38 no.105 Medellín jul./dic. 2016
https://doi.org/10.17533/udea.acbi.v37n105a08
RESEARCH PAPERS
doi: 10.17533/udea.acbi.v37n105a08
Assessment of toxicity in industrial wastewater treated by biological processes using luminescent bacteria
Evaluación de la toxicidad en aguas residuales industriales tratadas por procesos biológicos utilizando bacterias luminiscentes
Diana C. Rodríguez-Loaiza1,2, Omaira Ramírez-Henao1,3, Gustavo A. Peñuela-Mesa1,4
1 Grupo de Diagnóstico y Control de la Contaminación (GDCON; SIU-UdeA), Escuela Ambiental, Facultad de Ingeniería, Sede de Investigaciones Universitarias (SIU), Universidad de Antioquia A. A. 1226, Medellín (Antioquia), Colombia.
Emails: 2 diana.rodriguez@udea.edu.co; 3 omairarh@gmail.com; 4 gustavo.penuela@udea.edu.co.
Received: September 2015; accepted: April 2016 (Recibido: septiembre 2015; aceptado: abril 2016).
Abstract
The toxicity of wastewater from a meat by-products processing company was evaluated before and after treatment using the Sequencing Batch Reactor (SBR). Toxicity tests were carried out by analyzing the inhibitory effect of samples in relation to light emission from marine bacteria of the species Vibrio fischeri. The results found that the effluents prior to treatment were highly toxic (EC50 < 60%) whereas post-treatment results showed low or no toxicity (EC50 > 82%). In some operational stages of the SBR reactor, a high correlation between the ammonia nitrogen present in each sample and the toxicity of wastewater from both the influents and the effluents was found, with correlations (R2) of 0.6141 and 0.8158, respectively. As a consequence of these results, the SBR system can be considered efficient at removing organic matter, and nitrogen, and thereby decreasing toxicity in treated water.
Key words: ammonia nitrogen, organic matter, Sequencing Batch Reactor, toxicity, Vibrio fischeri.
Resumen
La toxicidad de las aguas residuales provenientes de empresas de procesamiento de productos cárnicos fue evaluada antes y después del tratamiento usando un reactor secuencial por lotes (SBR). Las pruebas de toxicidad fueron evaluadas mediante el análisis del efecto inhibidor de muestras en relación con la emisión de luz a partir de bacterias marinas de la especie Vibrio fischeri. Los resultados demostraron que los efluentes antes del tratamiento eran altamente tóxicos (EC50 < 60%), mientras que los resultados después del tratamiento mostraron baja o nula toxicidad (EC50 > 82%). En algunas etapas operativas del reactor SBR, se encontró correlación entre el nitrógeno amoniacal presente en cada muestra y la toxicidad de las aguas residuales tanto en el afluente y los efluentes, con correlaciones (R2) de 0,6141 y 0,8158, respectivamente. Como consecuencia de estos resultados, el sistema SBR puede ser considerado eficiente en la eliminación de materia orgánica y nitrógeno, y de ese modo disminuir la toxicidad en el agua tratada.
Palabras claves: materia orgánica, nitrógeno amoniacal, reactor secuencial por lotes, toxicidad, Vibrio fischeri.
INTRODUCTION
The treatment of industrial wastewater is a highly complex process that generally involves factors associated with load fluctuations and high concentrations of organic matter. These factors are often due to inhibitors in biological processes that have not been properly introduced in the environmental or contaminants that have not been treated before being discharged into water reservoirs. The Sequencing Batch Reactor system (SBR) is a technology for wastewater treatment that combines different cycles and stages of operation depending on the quality required of the effluent water. The most common stages are filling, reaction (aeration and mixing), sedimentation, draw off, and purge, all carried out within the same tank (Rodriguez et al. 2011a, b).
In recent years, the need to implement effective systems for the treatment of industrial effluents has been established (Dalzell et al. 2002, Rigopoulos and Linke 2002) in order to reduce toxic waste (Araújo et al. 2005). Toxicity is usually determined by the capacity of a substance to have an adverse effect on an organism and depends on the chemical properties of the compound and its concentration. It is also determined by the duration and frequency of exposure to the toxin, and furthermore, by the relationship of the substance to the organism's life cycle (Morales 2004).
Toxicity was evaluated before and after treatment to determine the operational efficiency of the SBR system (Gutierrez et al. 2002). The organisms most frequently used for toxicity testing are bacteria, fish, algae, Daphnia, and Rotifera. For trials of this nature these types of organisms have the advantage of presenting biochemical pathways similar to those of higher organisms. Furthermore, they have short life cycles and respond quickly to changes in the environment (Jennings et al. 2001). The most common application of this form of toxicity testing is related to the determination of lethal concentration (LC50) and effective concentration (EC50), where LC50 is the concentration of a compound that causes death in 50% of the organisms tested, while EC50, is the concentration causing adverse effects in 50% of the population (Onorati and Mecozzi 2004). Since 1979, the luminous bacteria Vibrio fischeri have been used for toxicity testing. This species is a Gram negative, facultative anaerobic bacteria of the Vibrionaceae with bioluminescence being its most representative characteristic (Araújo et al. 2005).
The Microtox toxicity test is based on the relationship between the reduction of light produced by these bacteria (Bennett and Cubbage 1992, Jennings et al. 2001) and the toxicity of the sample. The bioluminescence reaction of these bacteria is linked to the electron transport system in their cellular respiration and is indicative of the metabolic status of the cell. Therefore, a decrease in bioluminescence indicates a decrease in cellular respiration. Physiochemical pollutants all affect cell respiration by altering the rate of synthesis of proteins, and lipids and thus changing the level of luminescence emission (Onorati and Mecozzi 2004).
The results reported by the Microtox tests are presented as effective concentration (EC50). However, in order to standardize the results and the correlation with physicochemical parameters from the Pearson index (Zar 1996), toxicity units (TU50) were calculated using following formula (Araújo et al. 2005):
In this way, toxicity can be classified as: highly toxic if EC50 < 60%; moderately toxic if 60% < EC50 < 82%; and not toxic if EC50 > 82% or when a decrease in luminance is not exhibited (Araújo et al. 2005, Lanciotti et al. 2004, Movahedian et al. 2005).
In this study, toxicity tests were conducted using the luminescent bacteria V. fischeri, industrial wastewater generated in a meat by-products processing company and the effluents of this water that were treated using an SBR system. The SBR system operated for 252 days, in eight different stages, in order to remove organic matter and ammonia nitrogen. The results are of great importance due to their contribution to the field of wastewater treatment at industrial level.
MATERIALS AND METHODS
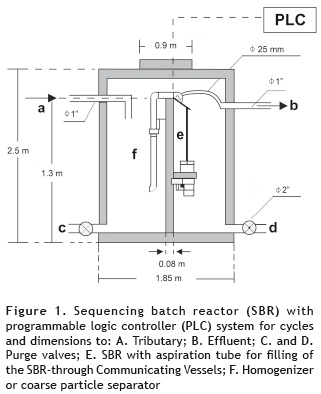
Study area. A pilot-scale SBR system with a volume of 2.96 m3 (figure 1) was installed in the Agropecuaria San Fernando S. A. (AGROSAN) meat products processing company for the removal of both organic matter and nitrogen. The company is located in the southwest of the department (administrative region) of Antioquia, Colombia. It is engaged in the production of meat and bone meal and fat for meat by-product based animal feed (using viscera, feathers, blood, bone, and fat), from slaughterhouses, farms involved in the slaughter of poultry, and swine, and from other actors in the meat industry.
Wastewater. Two types of wastewater were studied in this work. The first was water from the washing of vehicles, equipment, barrels, and packages containing meat products. The second was from the condensate water generated when transforming raw materials into meal, and fat for animal feed through cooking. The average CODT, CODF, BOD5, TSS, VSS, N-NH4+, pH values for the washing water were 8308 ± 1823 mg l-1, 3922 ± 1540 mg l-1, 2684 ± 1686 mg l-1, 1711 ± 974 mg l-1, 1242 ± 817 mg l-1, 365 ± 14 mg l-1, 6.11 ± 0.40, respectively, while for the condensate water they were 1381 ± 484 mg l-1, 822 ± 215 mg l-1, 563 ± 219 mg l-1, 7 ± 3 mg l-1, 6 ± 3 mg l-1, 616 ± 129 mg l-1, 9.64 ± 0.47, respectively.
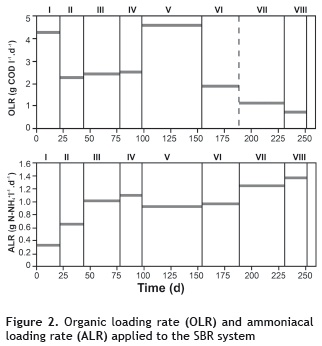
SBR operation. The steps undertaken by the SBR in each cycle were filling, reaction, sedimentation and emptying. Each full 8 hour cycle was divided into 6 hours for the reaction, which included, intermittent aeration (for a duration of 8 minutes), anoxia-mixture, (for a duration of 15 minutes), and 2 hours for the sedimentation phase. The experiments were divided into eight different stages that varied the mix of washing water, and condensate ratios (figure 2). The sludge retention time (θc) was 30 days, in order to enhance oxidizing bacterial growth.
Inocula. The reactor was inoculated with 1.0 g l-1 of VSS from a UASB reactor located in the same company, which was acclimated to high organic loads. The VSS had a Sludge Volume Index (SVI) of 16.8 ml g-1 indicating that it was a sludge with good sedimentability. It was also inoculated with 1.075 g l-1 of mixed liquor from the VSS of an activated sludge system from a municipal wastewater treatment plant, with a SVI of 142.97 ml g-1, therefore being sludge with acceptable sedimentability (Rodriguez et al. 2011a).
Chemical analysis. In the experiments the following physicochemical parameters were determined: chemical oxygen demand (COD), complying with the established protocols of Standard Methods (APHA 2005); ammonium, (N-NH4+) using a Kjeldahl instrument (Bϋchi), and pH, employing a Shott handylab pH 11/SET.
Toxicity test. The toxicity was determined with a BioFix Lumi-10 (Macherey-Nagel) apparatus, using the marine bacteria V. fischeri. The procedure was performed in accordance with that established by DIN ISO 11348-3 (Araújo et al. 2005). Readings were taken by measuring the emission of light for 15 minutes with different dilutions of wastewater and a suspension of luminescent bacteria prepared from a strain of lyophilized V. fischeri. The tests were carried out at 15 °C using a thermostated Lumis therm block. For controlling bacteria V. fischeri, luminescence was measured in NaCl solution. Samples were taken from the influent and effluent of the SBR system in each of the eight stages, and were transferred to the laboratory at 4 °C. The pH was maintained within the range of 6.0-7.0 and samples high in turbidity were centrifuged for 10 min at 5000 rpm.
Statistical analysis. Multidimensional analysis was performed to find correlations between the different parameters analyzed. Using the Statgraphics plus 5.1 software, and ANOVA analysis, the Pearson product moment correlations were obtained.
RESULTS AND DISCUSSION
The washing water showed an EC50 of 18.1% while the condensate water revealed an EC50 of 5.9%. These results indicated that both the washing water and the condensate are highly toxic (EC50 < 60%), with the condensate being more so. These toxicity results are consistent with the characterization carried out on the two types of water, which revealed that they had an average concentration of 365.14, and 615.54 mg l-1 of N-NH4+ and a pH of 6.11, and 9.64, respectively. According to the pKa, the ratio of NH3 to NH4+ depends mainly on pH, meaning that for washing water and condensate water the N-NH3 concentrations were 0.324, and 532.67 mg l-1. When free ammonia is greater than 0.2 mg l-1 it causes mortality in several fish species (Anthonisen 1976), due to physiological damage associated with high concentrations of ammonium. It poses an environmental risk and because of this, the National Academy of Sciences (NAS) in the United States does not allow values higher than 0.02 mg l-1. Therefore, the toxicity percentages found for the two types of water studied can be considered extremely high.
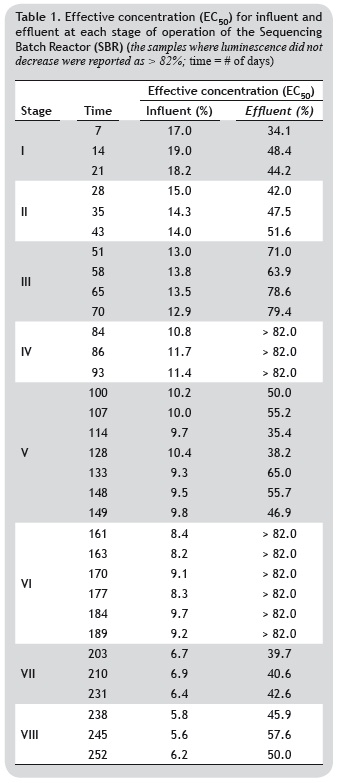
For the effluent in phases I, II, V, VII, and VIII (table 1), EC50 values were of high toxicity, whereas phases IV and VI were not found to be toxic. In contrast, all influents showed high toxicity (stages I to VIII), with EC50 percentages below 60% for all cases. The increase in EC50 in the effluent at each stage indicates that the treatment system used is capable of removing substances regarded as being toxic to the environment, such as ammonium (Campos et al. 2008, Carrera et al. 2004, Pynaert et al. 2003).
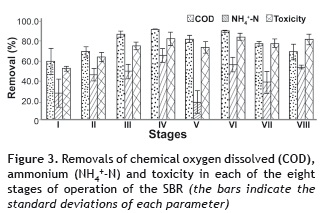
The removal results are shown in figure 3, where the maximum removal of COD and N-NH4+ occurred during phases IV and VI, with 98.7 and 97.6% for COD, and 70.5 and 60.9% for N-NH4+, respectively. Furthermore, when the removal of toxicity was correlated with the results of the removal of COD and N-NH4+, toxicity was reduced by as much as 88.7% in stage IV and in by 91.2% in stage I. Therefore, it is evident that the SBR system is efficient at treating industrial wastewater from the processes involved in the transformation of meat by-products, operating under different organic (OLR) and ammonia (ALR) loading rates.
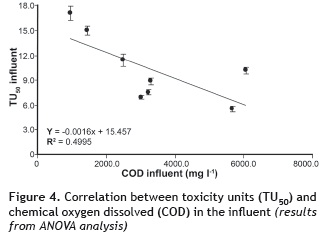
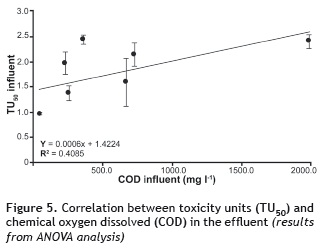
Toxicity unit (TU50) values were correlated with the concentration of COD and N-NH4+ for both influents and effluents. In the case of COD (figure 4), ANOVA analysis shows an R2 of 0.4995 with a P-value of 0.0507 and a confidence level of 90% for the influent of each stage. As for the effluents, the correlation was also low, with an R2 of 0.4085, a P-value of 0.079, and a confidence level of 90% (figure 5).
According to Araújo et al. (2005), wastewater generally has a broad mix of different components, which implies that the resulting toxic properties may vary depending on the interaction between each of them. This high variability may be responsible for the zero or poor correlation of COD and toxicity values. Furthermore, Boluda et al. (2002) note that in complex samples such as in the case of wastewater, COD cannot provide a good correlation with toxicity.
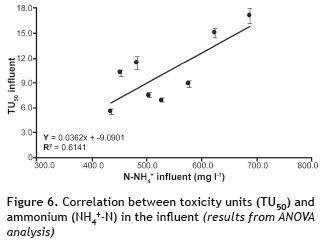
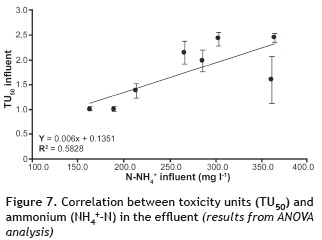
On the other hand, looking at the N-NH4+ in the influent (stage I to VIII), an R2 of 0.6141 can be seen with a P-value of 0.0215 and a confidence level of 95% (figure 6). In the effluents (figure 7) the relationship was directly proportional (R2 = 0.8158 and P-value = 0.0019 with a confidence level of 99%). Thus, high concentrations of N-NH4+ caused increases in toxicity, while for low concentrations toxicities were low. Although the result is expressed as N-NH4+, in reality it is the ammonia that produces the toxicity. At pH > pKa, ammonia strongly influences the toxicity of the water. As the pH decreases below the pKa (9.1 at 28 °C), ammonia no longer influences toxicity. While the effect of temperature was not evaluated, it is possible to infer that an increase in this variable would have a positive effect on microorganisms as these better metabolize compounds as temperature increases.
CONCLUSIONS
The results indicated that both the washing water, and the condensate were highly toxic EC50 < 60%, with the condensate being more so. However, in stages IV and VI where greatest ammonium removal occurred (70.5% and 60.9%, respectively), the EC50 in the effluents reached values greater than 82%, indicating effluents that were non-toxic.
ACKNOWLEDGEMENTS
The authors wish to thank the company AGROSAN for their cooperation and the GDCON group of the Universidad de Antioquia for financing the Project.
REFERENCES
Anthonisen RC, Loehr AC, Prakasam T, Srinath EG. 1976. Inhibition of nitrification by ammonia and nitrous acid. Journal Water Pollution Control Federation, 48 (5): 835-852. [ Links ]
Araújo C, Nascimento R, Oliveira C, Strotmann U, Da Silva E. 2005. The use of Microtox to assess toxicity removal of industrial effluents from the industrial district of Camaçari (BA, Brazil). Chemosphere, 58 (9): 1277-1281. [ Links ]
Bennett J, Cubbage J. 1992. Review and evaluation of Microtox® test for freshwater sediments. Olympia (Washington State, U. S. A.): Sediment Management Unit, Washington State Department of Ecology, Environmental Investigations and Laboratory Services Program, Toxics, Compliance, and Ground Water Investigations Section. 92-e04. p. 1-28. [ Links ]
Boluda R, Quintanilla JF, Bonilla JA, Saez E, Gamon M. 2002. Application of the Microtox test and pollution indices to the study of water toxicity in the Albufera Natural Park (Valencia, Spain). Chemosphere, 46 (2): 355-369. [ Links ]
Campos JL, Carvalho S, Portela R, Mosquera A, Mendez R. 2008. Kinetics of denitrification using sulphur compounds: effects of S/N ratio, endogenous and exogenous compounds. Bioresource Technology, 99 (5): 1293-1299. [ Links ]
Carrera J, Vicent T, Lafuente J. 2004. Effect of influent COD/N ratio on biological nitrogen removal (BNR) from high-strength ammonium industrial wastewater. Process Biochemistry, 39 (12): 2035-2041. [ Links ]
Dalzell DJ, Alte S, Aspichueta E, Etxebarria J, Gutierrez M, Hoffmann CC, Sales D, Obst U, Christofi N. 2002. A comparison of five rapid direct toxicity assessment methods to determine toxicity of pollutants to activated sludge. Chemosphere, 47 (5): 535-545. [ Links ]
Gutierrez M, Etxebarria J, de las Fuentes L. 2002. Evaluation of wastewater toxicity: comparative study between Microtox and activated sludge oxygen uptake inhibition. Water Research, 36 (4): 919-924. [ Links ]
Jennings VL, Rayner-Brandes MH, Bird DJ. 2001. Assessing chemical toxicity with the bioluminescent photobacterium (Vibrio fischeri): a comparison of three comercial systems. Water Research, 35 (14): 3448-3456. [ Links ]
Lanciotti E, Galli S, Limberti A, Givannelli L. 2004. Ecotoxicological evaluation of wastewater treatment plant effluent discharge: a case study in Parto (Italy). Annali di Igiene, 16 (4): 549-558. [ Links ]
Morales C. 2004. Ensayos tóxicológicos y métodos de evaluación de calidad de aguas. 1ª. ed. México: Centro Internacional de Investigaciones para el Desarrollo. p. 190. [ Links ]
Movahedian H, Bina B, Asghari GH. 2005. Toxicity evaluation of wastewater treatment plant effluents using Daphnia magna. Environmental Health Science & Engineering, 2 (2): 1-4. [ Links ]
Onorati F, Mecozzi M. 2004. Effects of two diluents in the Microtox toxicity bioassay with marine sediments. Chemosphere, 54 (5): 679-687. [ Links ]
Pynaert K, Smets B, Wyffels S, Beheydt D, Siciliano SD, Verstraete W. 2003. Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Applied and Environmental Microbiology, 69 (6): 3626-3635. [ Links ]
Rice EW, Baird RB, Eaton AD, Clesceri LS. 2005. Standard methods for examination of water and wastewater. 16th ed. Washington: American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). p. 1496. [ Links ]
Rigopoulos S, Linke P. 2002. Systematic development of optimal activated sludge process designs. Computers & Chemical Engineering, 26 (4-5): 585-597. [ Links ]
Rodriguez DC, Pino N, Peñuela G. 2011a. Monitoring the removal of nitrogen by applying a nitrification-denitrification process in a Sequencing Batch Reactor (SBR). Bioresource Technology, 102 (3): 2316-2321. [ Links ]
Rodriguez DC, Ramirez O, Peñuela G. 2011b. Behavior of nitrifying and denitrifying bacteria in a sequencing batch reactor for the removal of ammoniacal nitrogen and organic matter. Desalination, 273 (2-3): 447-452. [ Links ]
Zar JH. 1996. Biostatistical analysis, 5th. ed. New York: Prentice-Hall. p. 751. [ Links ]