INTRODUCTION
In December of 2019, China informed the World Health Organization (WHO) about the spread of a new pathogen in the Chinese city of Wuhan, this pathogen was named Severe Acute Respiratory Syndrome Coronavirus 2, (SARS-CoV-2) (Abd El-Aziz and Stockand, 2020) causing the Covid-19 disease. The Covid-19 disease has been categorized as a global pandemic. The SARS-CoV-2 virus infects by little droplets (Rothan and Byrareddy, 2020) of mucus and saliva. Once a person is infected with the SARS-CoV-2 virus, the spike homotrimer glycoprotein (Lan et al., 2020) of the virus connects to the integral membrane protein ACE2 of human cells (Lan et al., 2020; Rothan and Byrareddy, 2020) many of these receptors are found in the lung epithelium layer, making them one of the main sites of infection of the SARS-CoV-2 virus (Connors and Levy, 2020).
In the SARS-CoV-2 infection, most patients do not develop symptoms (Risitano et al., 2020). However, for patients who develop severe symptoms, it’s due to the abnormal production of cytokines (Conti et al., 2020; Hirano and Murakami, 2020; Rothan and Byrareddy, 2020) by infected cells and deregulated activation of the complement system especially the C3 and C5 proteins (Fletcher-Sandersjöö and Bellander, 2020; Java et al., 2020; Jodele and Köhl, 2020; Mastaglio et al., 2020; Mastellos et al., 2020; Ramlall et al., 2020; Risitano et al., 2020; Stahel and Barnum, 2020). This deregulated activation of the complement system and cytokines induces the frenzy of the immune cells, which will generate injuries in the body causing serious symptoms in the infection by SARS-CoV-2 virus (Risitano et al., 2020). Such as a pneumonia (Jiang et al., 2020; Lescure et al., 2020; Naicker et al., 2020; Rothan and Byrareddy, 2020), acute lung injury (ALI) (Fu et al., 2020; Polycarpou et al., 2020), extreme inflammation (Carvelli et al., 2020; Didangelos, 2020; Fu et al., 2020; Noris et al., 2020) thrombotic microangiopathy, (Fletcher-Sandersjöö and Bellander, 2020; Middleton et al., 2020; Ramlall et al., 2020; Skendros et al., 2020), coagulopathies (Connors and Levy, 2020), acute kidney injury (Fanelli et al., 2020), multi-organ failure, (Devaux et al., 2020; Mastellos et al., 2020; Noris et al., 2020), heart failure (Devaux et al., 2020), immunothrombinosis (Mastaglio et al., 2020b; Mastellos et al., 2020), acute stroke (Conway and Pryzdial, 2020; Yeboah et al., 2020), and dysfunctional immune responses in the attempt to control the disease (Tay et al., 2020).
The partial inhibition of the complement system is a possible treatment to regulate the uncontrolled immune reactions (Java et al., 2020; Polycarpou et al., 2020) of the host and the severe symptoms generated during it, reducing the number of patients with severe symptoms and therefore the number of patients in intensive care.
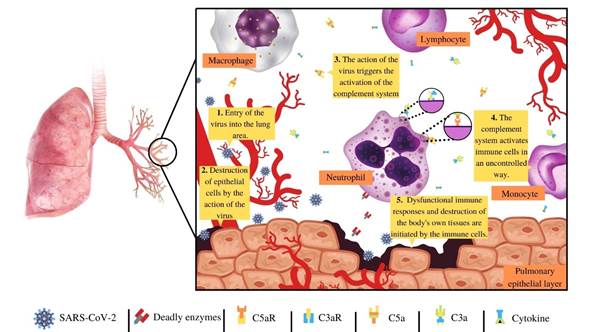
Figure 1 Once a person is infected with the SARS-CoV-2, in stage one (1) the virus arrives in the lung area, get into the cells of the epithelial layer of the lung with the purpose of reproducing, and produces mores viruses, (2) in this process many cells of the epithelial layer of lung died, which triggers the immune response. In step three and four (3, 4) the complement system and other immune complexes like cytokines are activated, which leads to a cascade of immunological reactions, activating and polarizing a multitude of immune cells in an uncontrolled way by the complement system and cytokines, especially neutrophils. Involving that in step five (5), the uncontrolled activation and overwhelming of the complement system and other immune complexes, triggers them in a frenzy of the immune cells producing a multitude of the collateral damage of the epithelial tissue of the lung, and other tissues of many organs leading to many of the worst symptoms producing during SARS-CoV-2 infection.
MATERIALS AND METHODS
In this manuscript, sixty articles were reviewed. Where thirty articles correspond to the role and treatments for the complement system in covid-19, as well symptoms associated, eight articles correspond to pathologies related to the complement system (out Covid-19), seven articles correspond to the characteristics, origin, and role of the complement system in human health, six articles correspond to the treatments designed for the complement system during Covid-19, five articles correspond to the immunological factors associated, and four articles correspond to the epidemiology, and etiology of the virus.
An overview of the complement system
The complement system is a group of more than 40 proteins (Merle et al., 2015) that evolved more than 700 million years ago (Meng et al., 2012) which are closely related to the immune system since these are key in the opsonization of pathogens. The activation of the complement system is vital to the control of viruses, fungi, bacteria, and other pathogens, this activation triggers the opsonization of the pathogens and their elimination of them (Merle et al., 2015; Sarma and Ward, 2011). The activation complement system is sequential, through the generation of complex enzymes and proteins, which produce a catalytic cascade in which an enzyme or protein in one-step can generate many active molecules of the next step activation of the complement system (Merle et al., 2015).
The activation of the complement system can be divided into three pathways, the classical pathway, the lectin pathway, and the alternative pathway (Merle et al., 2015a; Merle et al., 2015b; Sarma and Ward, 2011). The classical pathway involves the activation of the complement system by an antibody IgG, IgM or pentraxins (PTX) (Haapasalo and Meri, 2019) which can recognize pathogens and eliminate them by binding directly to C1q complement protein (Merle et al., 2015; Sarma and Ward, 2011). The lectin activation pathway involves the activation of the complement system by a lectin molecule MASP-2, which recognizes mannose present in pathogenic organisms that enter the body (Merle et al., 2015; Sarma and Ward, 2011). For another part, the alternative pathway is highly volatile; it is activated in many ways either through the activation of carbohydrates, protein, or lipids from foreign bodies, which will mainly be triggered by the hydrolysis of the thioester bond present in the C3 protein dividing it into the protein subdomains, C3a and C3b (Merle et al., 2015; Sarma and Ward, 2011).
The complement system, C3, C5, and the SARS-CoV-2
In Covid-19, many parts of the activation of the complement system interact during infection (Java et al., 2020). For example, studies have shown that the natural antibodies IgM anti-A, and anti-B activate the complement system early by the classical pathway (Java et al., 2020), reducing the viral load, and hence avoiding the generation of serious symptoms, such as pneumonia (Java et al., 2020).
It is also evidenced that the nucleoprotein dimer synthesized by the SARS-CoV-2, activates the mannan-binding lectin serine protease 2 (MASP-2) (Gao et al., 2020; Java et al., 2020), the trigger molecule of the lectin pathwayThe deregulated activation is one of the causes of the frenzy of immune cells generated by the excessive activation of the complement system (Fletcher-Sandersjöö and Bellander, 2020; Java et al., 2020; Mastellos et al., 2020).Cytokines (Conti et al., 2020; Hirano and Murakami, 2020) produce the worst injuries and symptoms in the SARS-CoV-2 infection (Ramlall et al., 2020; Rothan and Byrareddy, 2020).
Worth noting that frenzy of the immune cells generated by excessive activation of the complement system is not an isolated factor during Covid-19 (Didangelos, 2020; Java et al., 2020; Mastaglio et al., 2020a). Since many diseases, especially autoimmune diseases (Sadik et al., 2018; Ricklin et al., 2016) have this same triggering factor, for example amyotrophic lateral sclerosis (Carpanini et al., 2019), correlation of insulin resistance in individuals with obesity and insulin resistant (Moreno-Navarrete and Fernández- Real, 2019), immune disorders and complications in the placenta which cause problems in pregnancy in women with antiphospholipid syndrome (Chighizola et al., 2020).
The overwhelming presence of neutrophils and immune cells in the lung area is due to several factors; notably, the chemoattractant action carried out by cytokines (Java et al., 2020; Rothan and Byrareddy, 2020), chemokines that attract neutrophils, leukocytes, and others immune cells (Allegra et al., 2020; Didangelos, 2020).
In this overwhelming alarm, the complement system plays a critical role in the chemotaxis, especially C3, C5. These proteins break into two protein subdomains the important effector proteins C3a, C5a (Jodele and Köhl, 2020) C3b and C5b (Merle, Church, et al., 2015; Merle et al., 2015; Sarma and Ward, 2011). The C3a and C5a, are anaphylatoxins where their job is to attract immune cells to the site of infection, inducing pro-inflammatory reactions (Ajona et al., 2019), activating neutrophils, monocytes, (Carvelli et al., 2020), B‐lymphoblasts, and the activation and polarization of lymphocytes (Shivshankar et al., 2020).
Specifically, the C3a is a chemotactic protein, vital to innate immune responses (Kwak et al., 2018) and adaptive responses (Chen et al., 2018). The interactions of C3a with its C3aR receptor, measured by associated G proteins (Chen et al., 2018; Laumonnier et al., 2020), can generate various biological reactions, ranging from the opsonization of pathogens to the formation of tumors and pathogenesis of various diseases such as Covid-19 (Chauhan et al., 2020; Kwak et al., 2018).
During Covid-19, the excessive presence of C3a generates the activation and polarization of lymphocytes through the vascular endothelium during an immune response (Shivshankar et al., 2020), and an excessive inflammatory responses (Ajona et al., 2019; Fletcher-Sandersjöö and Bellander , 2020) the deregulated action of neutrophils and immune cells, aggravating the conditions of acute lung injury (Carvelli et al., 2020; Didangelos, 2020; Mastaglio et al., 2020; Mastellos et al., 2020b; Risitano et al., 2020; Shivshankar et al., 2020). In mice infected with SARS-CoV, the complement system, especially the C3a protein, shows a high expression one day after virus infection aggravating the pathogenesis of the SARS-CoV virus (Gralinski et al., 2018).
On the other hand, the C5a anaphylatoxin is a neutrophil-attracting chemokine (Didangelos, 2020). This protein has a critical role in the generation of thrombosis in the Covid-19, (Fletcher-Sandersjöö and Bellander, 2020; Mastellos et al., 2020; Middleton et al., 2020; Ramlall et al., 2020; Skendros et al., 2020), due to the interactions of these proteins with neutrophils, Alsothis proteininduces them to fight with the pathogen, producing collateral damage (Bardoel et al., 2014). In addition, the interactions of C5a and its receptor C5aR1 have a role in the initiation and maintenance of the inflammatory responses of neutrophils and monocytes (Carvelli et al., 2020).
C3b, a promoter of the formation of C3bBb (van den Bos et al., 2019) has the job of amplification of catalytic reaction of the complement system (van den Bos et al., 2019), producing more components of the complement system and increasing the autoimmune reactions (Mastaglio et al., 2020a;Risitano et al., 2020).
The C5b a first part of the membrane attack complex (MAC) (Paredes et al., 2018), in the SARS-CoV-2 infection, has been found and related to purpuric skin lesions, thrombogenic vasculopathy, with deposits in the epithelial tissue of the skin, (Java et al., 2020; Magro et al., 2020). In addition, the virus increases the number of proteins C4d and C5b-9, in the interalveolar septa and the cutaneous microvasculature (Java et al., 2020; Magro et al., 2020). There is also evidence of the presence of C5b-9 in tubular epithelial cells of the kidney (Java et al., 2020).
These molecules that interact with neutrophils and other immune cells (Carvelli et al., 2020;Didangelos, 2020;Mastaglio et al., 2020a;Risitano et al., 2020; Shivshankar et al., 2020) produce the worst cases, with acute injuries in the lung area (ALI), pneumonia, extreme inflammation, thrombotic microangiopathy, acute kidney injury, multi-organ failure, heart failure, immunothrombinosis, and dysfunctional immune responses.
The C3 and C5 target in treatment for Covid-19
Following the knowledge of the deregulated activation of the complement system, and the activity of C3 and C5 proteins in SARS-CoV-2 virus infection, new treatments destined to the complement system emerged as part of a possible first line of defense against the worst symptoms caused by Covid-19 disease, with anti-C5a treatments being promising (Campbell and Kahwash, 2020; Java et al., 2020;Risitano et al., 2020). Anti-C5 treatment shows an immediate improvement through the reaction of inflation and an increase in pulmonary oxygenation (Risitano et al., 2020) Anti-C5a inhibitors have already been previously used in various pathologies (Risitano et al., 2020), showing positive results in the recovery of patients. Such is the case of the delayed hemolytic transfusion reactions in sickle cell anemia (Floch et al., 2020), fulminant hemolytic anemia by cryoglutinins (Makishima et al., 2019), paroxysmal nocturnal hemoglobinuria (Stern and Connell, 2019) and ischemia caused by acute renal injury (Zilberman-Itskovich et al., 2019).
In SARS-CoV-2 infection, the treatment commonly used as Anti-C5a is the monoclonal antibody Eculizumab (Java et al., 2020; Laurence et al., 2020; Stahel and Barnum, 2020). This monoclonal antibody has been accepted by most patients with Covid-19 (Java et al., 2020) showing good results and noticeable improvement (Laurence et al., 2020;Mastellos et al., 2020; Risitano et al., 2020). However, the Eculizumab antibody is not the only anti-C5a antibody. New anti-C5a drugs such as the IFX-1 antibody (Vlaar et al., 2020), and Ruxolitinib in combination with Eculizumab (Giudice et al., 2020) show a favorable in addition to promising results in inhibition of the complement system and anaphylatoxin C5a (Vlaar et al., 2020).
Anti-C5a treatments are not the only ones in the first line of defense against Covid-19. Anti-C3 treatments such as the AMY-101 compstatin inhibitor (Mastaglio et al., 2020b; Mastellos et al., 2020; Risitano et al., 2020) present favorable results in the control of hyper inflammation, thrombotic microangiopathy, immunothrombinosis caused by SARS-CoV-2 infection, (Mastaglio et al., 2020b; Mastellos et al., 2020; Risitano et al., 2020). However, the search for new treatments targeting the complement system, such as the inactivation of the MASP-2 molecule (Java et al., 2020), vialectins, are promising options for the control of the serious symptoms caused by SARS-CoV-2 infection which leads to the Covid-19 disease.