INTRODUCTION
Development is a general term that refers to the set of intrinsic processes that occur during aging. Within this, two fundamental processes are distinguished: growth and ontogeny. Growth results in a change in size or in an existing measurable or countable characteristic of an organism, while ontogeny is related to the acquisition of new characteristics and/or the reorganization or loss of existing traits (Fuiman and Higgs, 1997).
The great morphological similarity of freshwater fish species during their larval period makes morphometric analysis a valuable tool. Morphometric analysis can help resolve obstacles in the identification of different species in any given area or region, especially when monitoring the reproduction of species in the wild (Arias-Gallo et al., 2010; Sanches et al., 1999) and when more than one species is reproducing in a given area at the same time (Arias-Gallo et al., 2010; Nakatani et al., 2001).
Embryonic studies can support phylogenetic analysis by presenting evidence to determine or infer an organisms ancestral form (Aral et al., 2011). The study of embryogenesis and larval development is important not only for taxonomic purposes, but also for captive culture, especially to determine when yolk sac absorption and mouth opening occurs, indicating the need for exogenous feeding (Maria et al., 2017; Sato et al., 2003).
Brycon moorei (Steindachner, 1878) is a species of the family Bryconidae (Order Characiformes) restricted to the macrobasins of the Cauca and Magdalena rivers in Colombia (Howes 1982; Maldonado-Ocampo et al, 2005; Miles, 1947; Ortega-Lara et al., 2000). This species prefers still waters with abundant vegetation along the banks and does not actively penetrate torrential systems (Dahl, 1971; Maldonado-Ocampo et al., 2005). Brycon moorei is an omnivorous species that mainly consumes fruit, flowers, leaves, fish and insects. During the rainy season (e.g., between May and June), adults of this species migrate to small streams and creeks with clear, torrential waters in order to reproduce (Maldonado-Ocampo et al., 2005, according to fishermen from Alto Cauca). Once spawning occurs, embryos and larvae drift downstream and the river acts as an incubation area, favoring the development of new individuals (Jiménez-Segura et al., 2016). Thus, B. moorei is a frequent species within the ichthyoplankton assemblages that drift during floods in the Magdalena River (Jiménez-Segura, 2007).
Since there is no description of the initial development of B. moorei, this work aimed to analyze the ontogenetic development of embryos and larvae of this species, with the purpose of contributing to its conservation, taxonomy and the improvement of induced reproduction and larviculture techniques (Gomes et al., 2011).
MATERIALS AND METHODS
Parental specimens
The parents were weighed to calculate the amount of hormone necessary for induced reproduction and were contained in two circular plastic tanks (7 m3). Samples were collected from nine females and eighteen males (two males per female). Five females were placed in one tank with ten males and the remaining individuals in the other tank. Parents were subjected to induced reproduction by means of carp pituitary hormone extract (CPE). This exercise was conducted at Piscícola Doradal (municipality of Sonsón) during the rainy season (September-October 2018).
Spawning, fertilization and sampling
The induction process for fertilization began on September 26th, 2018 at 21:30 h by applying an intramuscular injection of hormone to females in the dorsal area (first dose) to induce egg maturation. The second female dose was applied 12 h later together; the single dose applied to males was also given at this time. All specimens underwent the same procedure. Spawning occurred 4.5 h later. Fertilization was performed following the wet method, which consists of manually extracting eggs and semen in a container by means of abdominal massage and mixing them with water to activate the process. Once the oocytes were fertilized, they were transferred to 250 L Woynarovich type incubators. The physicochemical parameters of the water were not controlled, but the temperature was recorded at the time the samples were taken. During the embryonic and larval process, the temperature fluctuated between 25.4-27.8 ºC; temperature was recorded with the a YSI Proodo probe. Embryos were kept in the incubators for 2.7 days. Upon hatching, larvae were placed in 1000 L cement tanks with constant water renewal and supply, where they remained for 5.5 days. Larvae were fed with copepods and artemia (Artemia salina).
Specimens were sampled every 15 min for the first three hours, then every 30 min until hatching. After hatching, specimens were sampled every hour until post-flexion and then every 12 h until juvenile. In each sample, 30 specimens were extracted and water temperature was recorded, observing the moments when structures emerged or were lost.
Samples were preserved in vials with transeau solution (6 parts water: 3 parts 90% alcohol: 1 part 40% formalin) and labeled appropriately for subsequent laboratory analysis. Photographic documentation was performed using a Leica S8 APO stereoscope with a Leica MC 120 HD digital camera. Each photograph was subjected to morphometric image analysis using the Las Ez program (image acquisition software for Windows operating systems for documentation and annotation “EZ” Leica LAS EZ, n. d.).
The times at which each structure was formed and the organs important for their vital functions were plotted, where the x-axis corresponds to the time in min and the y-axis to the time at which a given structure appears. A description was made of the body proportions in each period and stage of development. Table 1 shows the variables considered.
Table 1 Morphological variables measured in the specimens
| Variable | Type | Type of measure | Unit of measure | |
|---|---|---|---|---|
| 1 | Vitellus diameter | Continua | Distance | mm |
| 2 | Yolk space diameter | Continua | Distance | mm |
| 3 | Standard length | Continua | Distance | mm |
| 4 | Total length | Continua | Distance | mm |
| 5 | Head length | Continua | Distance | mm |
| 6 | Mouth length | Continua | Distance | mm |
| 7 | Preanal length | Continua | Distance | mm |
| 8 | Body height | Continua | Distance | mm |
| 9 | Head height | Continua | Distance | mm |
| 10 | Eye diameter | Continua | Distance | mm |
| 11 | Pre-anal myomeres | Discreet | Counting | Number |
| 12 | Post-anal myomeres | Discreet | Counting | Number |
| 13 | Eye pigmentation | Discreet | Binary | Yes, no |
| 14 | Mouth opening | Discreet | Binary | Yes, no |
| 15 | Intestine | Discreet | Binary | Yes, no |
| 16 | Open Anus | Discreet | Binary | Yes, no |
| 17 | Otoliths | Discreet | Counting | Number |
| 18 | Skull | Discreet | Binary | Yes, no |
| 19 | Upper jaw teeth | Discreet | Binary | Yes, no |
| 20 | Lower jaw teeth | Discreet | Binary | Yes, no |
| 21 | Swim bladder | Discreet | Binary | Yes, no |
| 22 | Pectoral fin (PF) | Discreet | Binary | Start, end |
| 23 | Dorsal fin (DF) | Discreet | Binary | Start, end |
| 24 | Adipose fin (AF) | Discreet | Binary | Start, end |
| 25 | Pelvic fin (PvF) | Discreet | Binary | Start, end |
| 26 | Anal fin (AnF) | Discreet | Binary | Start, end |
| 27 | Caudal fin (CF) | Discreet | Binary | Start, end |
| 28 | PF Rays | Discreet | Counting | Number |
| 29 | DF Rays | Discreet | Counting | Number |
| 30 | AF Rays | Discreet | Counting | Number |
| 31 | PvF Rays | Discreet | Counting | Number |
| 32 | AnF Rays | Discreet | Counting | Number |
| 33 | CF Rays | Discreet | Counting | Number |
| 34 | Heart | Discreet | Binary | Yes, no |
| 35 | Gills | Discreet | Binary | Yes, no |
| 36 | Stomach contents | Discreet | Binary | Yes, no |
| 37 | Head pigmentation | Continua | Proportion | Percentage |
| 38 | Trunk pigmentation | Continua | Proportion | Percentage |
| 39 | Cauda pigmentation | Continua | Proportion | Percentage |
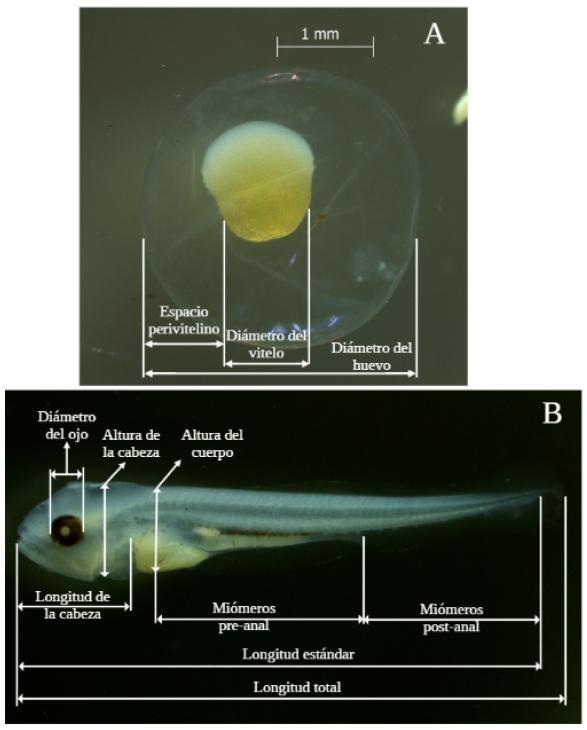
For each individual, the information extracted for the period and stage of development followed the proposal of Nakatani (2001) and Bagenal and Braum (1978) (Figure 1). The following characteristics were recorded:
Embryos: diameter (D)-Structure and diameter of the yolk (SD)-Presence and distribution of oil droplets (PD)-Size of the perivitelline space-Shape and color of unfixed embryos. The volume of the yolk was calculated using the formula V = ⁴⁄₃πr³; where: V: volume and r: radius. Using the total volume of the embryo, the perivitelline space was estimated and defined according to in categories (limited or restricted: 0-9.9%, moderate: 10-19.9%, wide: 20-29.9% and very wide >30.0%).
Larvae: total length (TL)-Standard length (SL)-Snout length (SNL)-Head length (HL)-Body height (BH)-Distance snout pectoral fin (DSPF)-Distance snout pelvic fin (DSPvF)-Distance snout anal fin (DSAnF)-Number of pre-anal myomeres-Number of post-anal myomeres-Number of total myomeres-Number of pectoral (P), pelvic (Pv), dorsal (D) and anal (An) fin rays.
RESULTS
Embryonic period
According to Alexandre et al. (2009) and Shardo (1995), the embryonic period spans from fertilization to hatching, including cleavage, gastrulation, neurulation and the onset of organogenesis.
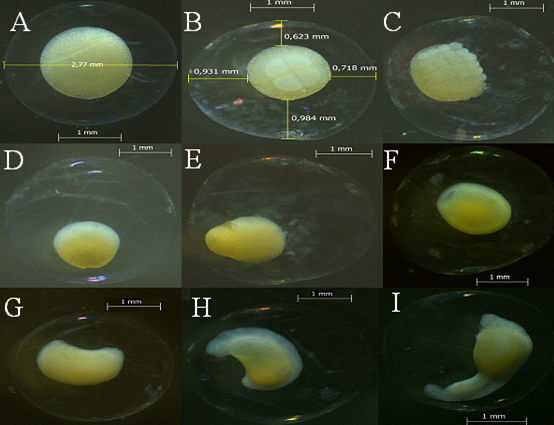
Figure 2 Phases during the embryonic period: A. Fertilization. B. Cleavage, showing the first 4 blastomeres. C. Morula, showing 32 blastomeres. D. Blastula, blastomeres arranged in a crescent above the yolk. E. Epiboly, blastomeres wraps 50% of the yolk. F. Invagination, blastomeres completely cover the yolk and the blastomere closes. G. Initial embryo. H. Embryo, the head is differentiated from the tail. I. Final embryo, detachment of the tail.
Fertilization: fertilized oocytes had an average diameter of 2.932 mm ± S.D 0.119, with a large yolk (1.703 mm ± S.D 0.165) that appeared yellow due to the fixative, a colorless chorion without ornamentation, and a reduced perivitelline space (0.603 mm ± S.D 0.061) (Figure 2).
Cleavage and morula: the animal pole presented its first mitotic division at 0.2 hours post-fertilization (HPF), this period includes the first division until 64 blastomeres were observed. The morula was formed at 1.3 HPF, and a number of cells were observed on the vegetal pole (yolk); these cells are the blastomeres. Over time the counting of blastomeres becomes difficult due to successive divisions. The pattern of cells in the cleavage was 1x1, 2x2, 8x4 cells and 8x8 in morula; as these divisions occur the cells decrease in size (Figure 2).
Blastula: this phase started at 2.6 HPF, where a layer of cells is formed; these cells cannot be observed as individualized, thus ending mitotic division and the start cell movement. This accumulation of cells is called the blastodisc (Figure 2).
Gastrulation: at 2.9 HPF the blastomere begins to advance over what will become the yolk. At 4.4 HPF epiboly was observed (i.e., 50% of the vitellus or vegetal pole is covered by the blastomere). Invagination leads to closure of the blastopore at 6.4 HPF, marking the end of gastrulation (Figure 2). The diameter of the yolk underwent a reduction during the first 36 HPF to give way to embryo formation and subsequently to exogenous feeding. The perivitelline space in the embryonic stages is considered moderate with respect to the egg diameter since it represents a percentage between 10-19.9%. Egg diameter remained constant throughout the phases (Table 2).
Table 2 Morphological measurements for the embryo period in mm (Mean ± standard deviation)
| Period | Phase | n | Vitellus diameter | Perivitelline space | Egg diameter |
|---|---|---|---|---|---|
| Embryo | Fertilization | 30 | 1.730 ± 0.165 | 0.603 ± 0.061 | 2.932 ± 0.119 |
| Embryo | Cleavage | 30 | 1.437 ± 0.070 | 0.878 ± 0.122 | 3.256 ± 0.257 |
| Embryo | Morula | 30 | 1.219 ± 0.091 | 1.024 ± 0.115 | 3.364 ± 0.244 |
| Embryo | Blastula | 30 | 1.236 ± 0.104 | 0.981 ± 0.117 | 3.322 ± 0.219 |
| Embryo | Epiboly | 30 | 1.316 ± 0.049 | 1.026 ± 0.076 | 3.466 ± 0.145 |
| Embryo | Invagination | 30 | 1.244 ± 0.133 | 0.943 ± 0.088 | 3.317 ± 0.223 |
| Embryo | Initial embryo | 30 | 1.511 ± 0.148 | 0.972 ± 0.090 | 3.214 ± 0.151 |
| Embryo | Embryo | 30 | 1.517 ± 0.154 | 0.970 ± 0.089 | 3.079 ± 0.196 |
| Embryo | Final embryo | 30 | 1.276 ± 0.157 | 1.079 ± 0.047 | 3.296 ± 0.266 |
Organogenesis and hatching: embryo differentiation (early embryo) begins at 7 HPF. Neurulation, formation of the first myomeres and the optic vesicle were evident at 9 HPF. The final embryo was observed at 11.4 HPF, presenting detachment of the caudal region of the yolk sac and movement. Differentiation of the primordial fin was observed at 12 HPF. Repeated movement of the caudal region ruptured the chorion, giving way to hatching at 13.4 HPF. By 14.4 HPF all embryos had hatched.
Larval period
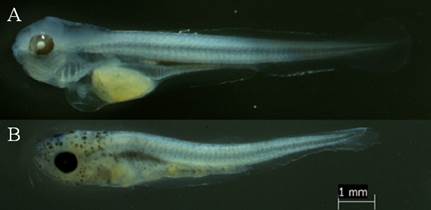
Larval yolk: once hatched, the otic vesicle becomes visible (Figure 3A). The larva presented an average standard length of 3.69 mm ± S.D. 0.096 (Table 3), the number of preanal myomeres was 23 ± S.D. 1, and total of 33 ± S.D. 2 (Table 4). They have an unpigmented body, rudimentary digestive tract and a colorless vitellus before fixation and yellow after fixation.
The olfactory bulb, the heart, some otoliths, the optic capsule and a vertical swim are observed. Opening of the mouth occurred at 23.4 HPF, and opening of the anus at 24.4 HPF. Gill arches were observed at 25.4 HPF. Eye pigmentation was observed at 29.4 HPF, starting from the iris rims; blood plasma pigmentation was evident at 31.4 HPF. Teeth in the mandible and maxilla were evident at 30 HPF, and at 39 HPF the mouth is wide open and showing movement. Dendritic pigmentation of the trunk located above the intestine was observed at 34.4 HPF.
Preflexion: started at 43.4 HPF with exogenous feeding and cannibalistic behavior of the larvae. The larvae present pigmented eyes; the yolk is maintained; the intestine is green before fixation and colorless after fixation. The average standard length was 6.754 mm ± S.D. 0.243 (Table 3). The mandible and maxilla are well developed and have teeth. The pectoral fins are observed without rays and the base membranes for the formation of the caudal and anal fins are evident. Observation of the otoliths was difficult. At this stage the insufflation of the swim bladder began (48.4 HPF) and melanophores appear on the head (78.4 HPF).
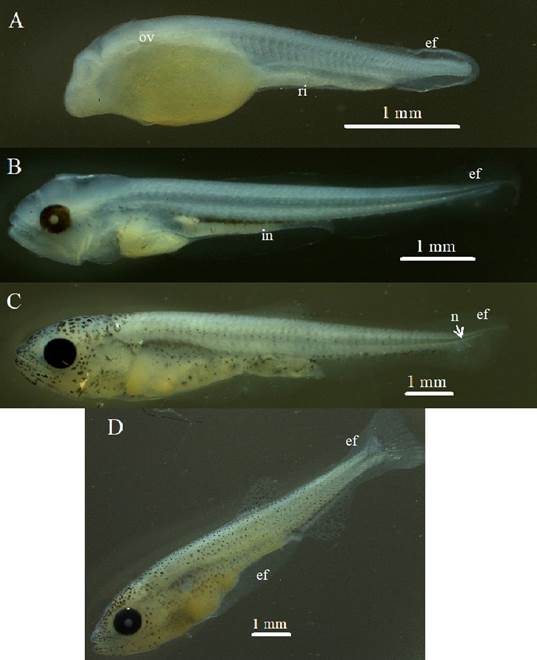
Figure 3 Larval period phases: A. Larval yolk, otic vesicle (ov) and rudimentary intestine (ri), embryonic fin (ef) are observed. B. Preflexion, reduction of the vitellus, formation of the intestine (in), C. Flexion, initiation of notochord flexion (n). D. Postflexion, observed increase of pigmentation.
Table 3 Morphological measurements of the larval period (mm). SN-AnF: snout-anal fin, SN-PF: snout-pectoral fin, SN-DF: snout-dorsal fin
| Period | Phase | n | Standard Length | Total length |
|---|---|---|---|---|
| Larva | Yolk larvae | 30 | 3.692 ± 0.096 | 3.759 ± 0.095 |
| Larva | Preflexion | 30 | 6.754 ± 0.243 | 6.833 ± 1.120 |
| Larva | Flexion | 30 | 8.824 ± 0.748 | 9.202 ± 0.775 |
| Larva | Postflexion | 30 | 11.916 ± 0.469 | 13.212 ± 0.596 |
Table 4 Morphological measurements of the larval period (mm). DF: dorsal fin; AnF: anal fin; CF: caudal fin
| Period | Phase | n | Eye diameter | Pre-anal myomeres | Post-anal myomeres | Total myomeres | DF Radios | AnF Radios | CF Radios |
|---|---|---|---|---|---|---|---|---|---|
| Larva | Yolk larvae | 30 | 0.192 ± 0.015 | 23 ± 1 | 10 ± 1 | 33 ± 2 | 0 | 0 | 0 |
| Larva | Preflexion | 30 | 0.434 ± 0.017 | 25 ± 1 | 19 ± 1 | 45 ± 1 | 0 | 0 | 0 |
| Larva | Flexion | 30 | 0.615 ± 0.056 | 25 ± 2 | 20 ± 2 | 45 ± 2 | 1 ± 2 | 2 ± 3 | 3 ± 1 |
| Larva | Postflexion | 30 | 0.986 ± 0.046 | 23 ± 2 | 20 ± 1 | 43 ± 2 | 10 ± 0 | 22 ± 1 | 20 ± 0 |
Flexion: the onset of notochord flexion was observed at 105.4 HPF, along with the presence of caudal fin support elements (Figure 3). The average standard length was 8.824 mm ± S.D. 0.748. The swim bladder is full, rays are beginning to emerge in the dorsal, anal and caudal fins. Stomach contents are present. The pigmentation of the head covers about 50% of its area, the rest of the body has incipient pigmentation. At the end of this phase, absorption of the primordial fin begins.
Postflexion: begins with the formation of the pelvic fin button, this occurred at 183.4 HPF (7.6 days). The average standard length is 11.916 mm ± S.D. 0.469. Adipose fin development becomes evident. Pelvic fin differentiation occurs at 228.7 HPF. The individuals of the last sample obtained at 348.7 HPF (14.5 days) did not reach the juvenile period since neither the formation of scales nor the total development of the fin rays was evident.
Body lengths (body height, head length and eye diameter, among others) in larvae had an increase that is associated with the number of HPF (Figure 4).
The preanal myomeres remained relatively constant (23-25) during the larval period and the caudal (3 ± 1 to 20 ± 0), anal (2 ± 3 to 22 ± 1) and dorsal (1 ± 2 to 10 ± 0) fin rays increased dramatically when changing from flexion to postflexion. Some individuals in preflexion did not present radio in the caudal, anal and dorsal fins (Table 3 and 4), probably due to the difficulty of observation in this phase; for this reason, their standard deviation is greater than the mean of the data.
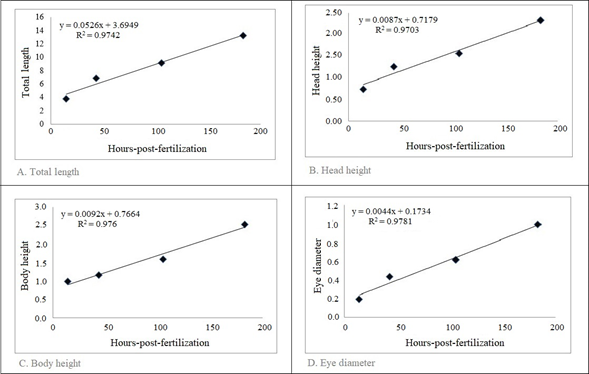
Figure 4 Relationship between the number of HPF and body lengths (means) of Brycon moorei during the larval period. Each point represents the average value of the variable measured within the same stage and obtained at the same time after fertilization.
Some embryos suffered anatomical deformities (curvature of the spine, underdeveloped or skewed jaws) (Figure 5), which would prevent them from feeding and eventually cause their death (Baras and Lucas, 2010).
DISCUSSION
Fertilized eggs of B. moorei, like most teleost fish, are classified as telolithic because they contain a large amount of evenly distributed yolk (Gomes et al., 2011; Marques et al., 2008; Perini et al., 2010). The size of hydrated eggs of B. moorei is 2.932 mm ± S.D 0.119 and of B. orthotaenia is 3.094 ± 0.080 mm (Gomes et al., 2011). These measurements are usually similar among species of the same genus and depend on various factors or environmental variables. Oocyte cleavage of B. moorei like B. orthotaeniaGomes et al., 2011) and B. cephalus (Alexandre et al., 2009) followed the meroblastic pattern restricted to the animal pole, as commonly observed in teleosts (Amorim et al., 2009; Gomes et al., 2007; Gomes et al., 2011; Meijide and Guerrero et al., 2000; Ninhaus-Silveira et al., 2010). Decreasing blastomere size as their number increases is also reported for B. orbignyanus (Ganeco, 2003 cited in Alexandre et al., 2009), Prochilodus lineatus (Ninhaus-Silveira et al., 2006 cited in Alexandre et al., 2009), B. cephalus (Alexandre et al., 2009) and B. moorei in the current study.
The period between egg fertilization and hatching is called the incubation period (Aral et al., 2011). Embryonic development of B. cephalus, from fertilization to larval hatching, lasted 11 h at 26 °C (Alexandre et al., 2009) and 13 h at 26 °C for B. moorei according to David-Ruales et al. (2020). In the current study it lasted 14 h at 26.5-27.5 °C. The difference with B. cephalus is probably due to the species factor and the optimal temperatures for its development; with the work of David-Ruales et al. (2020) on B. moorei, the one-hour difference is possibly due to the controlled conditions they worked with. We could say that the optimum temperature for embryonic development of B. moorei would be 26 ºC, since not controlling this variable could delay its development.
The identification of blastomere closure is important in aquaculture, as it indicates that fertilization was successful (Gomes et al., 2011). For B. moorei, it was possible to identify this event at 6.4 HPF, a time similar to what occurs in other Brycon species. For example, 7.5 HPF for B. orthotaenia at 24 ºC (Gomes et al., 2011), but very different from B. nattereri (25 HPF at 19ºC; Maria et al., 2017); the latter species inhabits colder waters and these temperatures slow development. Some authors consider the epiboly stage at 100% to be equivalent to blastopore closure and attribute this moment to the opening of the primitive gut in larvae (Sampaio-Nakauth et al., 2016).
In some species the organs differentiate after hatching, during or after metamorphosis. Larvae of such species are called altricial larvae (Çelik et al., 2014; Falk-Petersen and Hansen, 2001) and this is what occurs with B. moorei.
The embryogenesis of B. moorei, as mentioned by Gomes et al. (2011) for B. orthotaenia is considered rapid, similar to other migratory Characiformes that have spawning reproductive strategies without parental care, such as Prochilodus lineatus (Ninhaus-Silveira et al., 2006 cited in Gomes, 2011) and Leporinus piau (Borçato et al., 2004 cited in Gomes, 2011).
At hatching, the embryo always emerges with the head and the front part of the body (Aral et al., 2011). With the help of the tail detachment the embryo can break the chorion thanks to the movement it generates. The detachment occurred at 11 HPF and is maintained for the rest of its growth (Figure 6). Relatively rapid embryonic development is a common feature among species with reproductive strategies involving migration and spawning in lotic environments (Sampaio-Nakauth, 2016; Sanches et al., 2001), and for the genus Brycon it can be considered an advantage in the reproductive process (Andrade-Talmelli et al., 2001; Romagosa et al., 2001; Sampaio-Nakauth, 2016).
As for the digestive tract, the formation of the intestine began at 13 HPF (Figure 6) and at 43 HPF stomach contents were observed, which could indicate normal functioning. Rapid formation is to be expected since it is an organ related to food processing and is one of the main activities for survival; larvae face the need to capture their food quickly (Rivera and Botero, 2009), providing energy necessary to maintain their metabolism, growth and to ensure their survival (Civera et al., 2004; Rivera and Botero, 2009). Mouth opening, also related to feeding, began at 23 HPF. At 43 HPF, the larva is able to chase, capture, swallow and digest prey (live food) (Rivera and Botero, 2009; Verreth, 1999). The open anus was evident at 24 HPF and consolidated at 43 HPF when feces were observed exiting the digestive tract. Teeth were evident at 30 HPF and exogenous feeding occurred at 43 HPF. Yolk absorption occurred between 30 HPF and 50 HPF (Figure 6); the larva still uses yolk reserves (Rivera and Botero, 2009) in the preflexion phase, but initiates the feeding process exogenously (Rivera and Botero, 2009). Eye pigmentation occurred from 29 HPF (Figure 6), a necessary condition for visual acuity, essential for food capture and to escape predators.
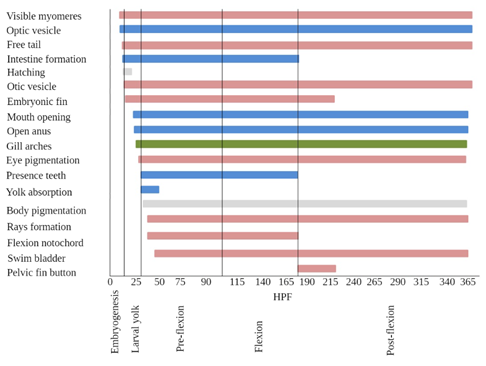
Figure 6 Phases in which structures are observed in relation to hours-post-fertilization (HPF). Blue: structures associated with feeding. Pink: structures associated with swimming and movement. Green: structures associated with respiration.
The hatching time for B. moorei, according to Baras et al. (2000), was 17-19 HPF at 27 ºC, which differs from our result of 13-14 HPF at 26.3 ºC. This difference may be due to temperature since in this study this variable could not be controlled (incubation at 27.4-26.3 ºC). These results could suggest that by gradually decreasing the incubation temperature the development process is accelerated and, therefore, hatching occurs faster. As mentioned above, 26 ºC is considered the optimum temperature for embryonic development.
Despite this, optimal developmental temperatures are different for each species. The hatching time and temperature of B. moorei in this study are similar to other studies with B. moorei (12 HPF at 26 ºC; David-Ruales et al., 2020), and to B. insignis (14 HPF at 26 °C; Andrade-Talmelli et al., 2001), B. gouldingi (14 HPF at 26 °C; Faustino et al., 2015); and differs from B. amazonicus (13 HPF at 28 °C; Mira-López et al., 2007; Nakaghi et al., 2014).
Optic and otic vesicles emerged at 9 and 14 HPF, respectively (Figure 6). As observed by Sampaio-Nakauth et al. (2016) and Falk-Petersen (2005), at the stage when these capsules emerge, larvae do not have stability in water, coordination of movement or visual acuity. In B. amazonicus, Sampaio-Nakauth et al. (2016) observed optic capsules between 6 and 8 HPF, coinciding with the results for B. cephalus, as observed by Alexandre et al. (2010) (26 °C), Romagosa et al. (2001) (26 °C) and Lopes et al. (1995) (30 °C). The formation of these structures takes a little longer for B. moorei; observation is difficult from the stereoscope and it is recommended to use diaphanization methods to improve the analysis.
Myomeres were observed at 9 HPF before hatching, these were not counted until hatching occurred. The larva yolk of B. moorei presented 23.17 ± 1 preanal myomers. Bettinelli-Nogueira et al. (2012) reported 26.5 ± 1.2 preanal myomers for B. orbignyanus, Dorada presents fewer myomers than B. orbignyanus. Again, to improve the observation of this feature, it is advised to use staining methods due to the inconsistency between this study and that of David-Ruales et al. (2020) regarding the difference in the number of myomeres (pre-anal 23.17 ± 1 and 28.65 ± 4.7, respectively) for the same species. Since the number of myomeres represents a character commonly employed in the identification and taxonomic differentiation of fish larvae (Sousa and Severi, 2002), this aspect needs to be clarified. The larval yolk does not present melanophores which is a common feature in freshwater Neotropical teleosts (Bettinelli et al., 2012; Godinho et al., 2003; Nakatani et al., 2001). Total length was 3.759mm ± S.D 0.095, slightly smaller than B. nattareri (6.32 ± 0.69 mm; Maria et al., 2017), but similar to other Brycon species, such as B. orbgnyanus (4.46 mm; Reynalte-Tataje et al., 2004). According to David-Ruales et al. (2020) total length is 4.74 ± 0.05 mm for B. moorei. This may be due to the methodologies used, as individuals were measured at different times within the larval yolk stage.
The dendritic melanophores of the larvae in preflexion phase range from a few pigments in the anterior-superior part of the vitellum following a line above the vitellum, and increasing in the gut area, which diffuse in the flexion phase to concentrate above the swim bladder. In postflexion, the pigments become more distant and are not so clustered. New pigments form in the dorsal and anal fin and those of the head intensify. These observations coincide with those made by David-Ruales et al. (2020) in the same species. Detailed descriptions of the distribution of chromatophores on the body can be used to identify species at an early stage of development (Bettinelli et al., 2012; Meijide and Guerrero, 2000; Nakatani et al., 1997; Santos and Godinho, 2002). Pigments above the gut were also observed by Bettinelli et al. (2012) in larvae of B. orbignyanus. In Piracanjuba (B. orbignyanus). Dendritic chromatophores were observed in larvae from 12 h post-hatching (HPE) and by 24 HPE had spread throughout the skin of the embryo as previously recorded by Bettinelli et al. (2012) and Maciel et al. (2010).
The primordial fin is possibly an adaptation for locomotion in smooth, less turbulent water and may also function in respiration by increasing the larval surface area ratio (Bettinelli et al., 2012; Geerinckx et al., 2008; Snik et al., 1997). This fin begins to develop from the larval yolk stage at 14 HPF (initiates vertical swimming) and is reabsorbed over time until 228 HPF.
During the larval stage, weight and length increase due to the progressive differentiation of body systems and the characteristic development from larva to adult (Gomes et al., 2011; Kendall et al., 1984). Figure 4 shows a positive and significant linear fit of the total length of individuals at each larval stage with respect to post-fertilization hours. The same relationship is also observed with other body variables. According to Baras et al. (2010), growth in body length is almost linear in young fish, so simple comparisons can be made between fish of different sizes.
Flexion of the notochord gives way to the complete formation of the caudal skeleton (Hernández et al., 2012; Kendall et al., 1984). The fin supports begin to form at 43.4 HPF with the hypural in the caudal fin, this formation extends to 348.7 HPF (Figure 6) where the caudal, dorsal and anal fin rays are already formed, but not yet those of the pelvic and pectoral fins. These structures are essential as they are the propulsive structures that give direction to swimming.
In B. moorei, cannibalistic behavior occurs at a very early age, 20-24 HPE at 27 °C (Baras et al., 2010, 2000; Baras and Jobling, 2002; Vandewalle et al., 2005), agreeing with David-Ruales et al. (2020) for the same species 22 HPE at 26 °C. In the current study, cannibalism was witnessed at 29 HPE at 25.6 °C. Although temperature is an important variable to consider, the values obtained are within the normal range despite the differences observed as exogenous feeding starts before 40 HPF (Baras et al., 2010, 2000; Vandewalle et al., 2005). Eye diameter and standard length for B. moorei in preflexion was 0.43 mm ± S.D 0.017 and 6.75 mm ± S.D 0.24, and 0.30 ± 0.13 mm and 6.17 ± 0.38 mm for B. amazonicus (Sampaio-Nakauth et al., 2016). These measurements by themselves do not work to differentiate species in early periods due to their great similarity; it is necessary to consider other characteristics. For example, in B. amazonicus, pigments are evidenced at the same time in the head and trunk at 36 HPF. However, in B. moorei, trunk pigments appear first at 34.4 HPF and then those of the head at 78.4 HPF, important aspects when identifying species as they represent specific characteristics used in taxonomy (Sampaio-Nakauth et al., 2016).
We found individuals at different stages of development at the same time, probably due to the different spawning times of each female. The difference in sizes is more noticeable in the larval period in the preflexion phase, a product of predation or audacity of the larvae in search of resources, since exogenous feeding begins.
At the onset of preflexion, Maria et al. (2017) reported a total absorption of the yolk sac for B. nattereri. In this study, larvae at this stage still have yolk, thus presenting a mixed feeding (i.e., exogenous feeding started before the total absorption of the yolk sac). For B. moorei this could be a disadvantage, because the presence of the yolk sac could hinder swimming and thus the capture of prey (Atencio, 2000; Mira-López, 2007). On the other hand, this would probably allow it to survive for more hours in the face of difficulties in finding food (Atencio, 2000; Mira-López, 2007). Vandewall (2005) mentions that at 25 HPE, B. moorei initiate piscivory and cannibalism (the yolk sac is only completely reabsorbed after 36 h). In our observations cannibalism began at 29 HPE and yolk absorption at 30 HPE. At this age the teeth are fully developed.
The filling of the swim bladder is considered one of the most critical stages of larval development (Çelik et al., 2014; Blaxter, 1992; Pelberg et al., 2008). For B. moorei it was present from 48 HPF (Figure 6). The swim bladder of B. orbignyanus was insufflated before yolk sac depletion (Bettinelli et al., 2012) as in this study for B. moorei, where it was able to achieve neutral buoyancy that is necessary for larvae to develop less costly energetic swimming activities (Bettinelli et al., 2012). Erratic vertical swimming is associated with the process of bladder filling (Maciel et al., 2010; Sampaio-Nakauth, 2016) and the lack of pectoral fins. This behavior, associated with consecutive jaw movements followed by frequent attacks, was witnessed at 34 HPF, and could be associated with the mechanism to develop muscular and skeletal structures involved in food capture (Herbing, 2001; Sampaio-Nakauth, 2016).
Most larvae inflate their swim bladders at the developmental stage corresponding to the time of yolk sac absorption and the onset of exogenous feeding (Battaglene and Talbot, 1990; Çelik et al., 2014; Doroshev and Cornacchia, 1979). Here, the formation of this organ started before exogenous feeding and yolk absorption. It would be interesting to perform studies from histochemical analysis to determine with certainty the total yolk sac absorption and the formation of the swim bladder.
Teeth were evident at 30 HPF (16 HPE); according to Vandewall (2005) for B. moorei the first oral teeth appear no later than 3 h after hatching, but they remain covered with epithelium until 45 h. With the stereoscope, it was not possible to observe the teeth covered with epithelium and the process of tooth formation was not followed, only observed when they were exposed.
Temperature is an important variable, especially during incubation; the difference in hours is evident when the blastopore closes at 6.4 HPF in B. moorei and at 25 HPF in B. nattareri, a species that inhabits cold waters. For other Brycon species, it is similar since they inhabit warmer waters. We speculate that each developmental stage will present an optimum temperature.
Another important feature is the formation of the organs that are part of the digestive system. The larval yolk stage is where the establishment of organs such as the opening of the mouth and anus, and the formation of teeth and intestine, important for the passage and permanence of the preflexion, where exogenous feeding begins.
Brycon moorei is a species that maintains body proportionality during the early stages as indicated by the linear relationships. The Dorada presented fewer preanal myomeres than B. orbignyanus. Pigments arise at the same time in the trunk and head of B. amazonicus while for B. moorei they occur at different times. These characteristics should be considered at the time of identification and should not be individualized. Great care should be taken when observing the number of myomeres and rays, especially when the larvae are in flexion, as these are characteristics used for identification, as well as the distribution and type of pigmentation present in the stages.
The results of this study can be used to explain the overall picture of early larval stages under culture conditions and may help in the development of better methods for rearing larvae in production (Çelik et al., 2014), finding diagnostic characters for taxonomy and breeding sites important for the conservation of the species.