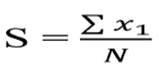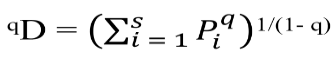INTRODUCTION
On the Pacific and Caribbean coasts of Colombia, there are rivers typical of large continental masses, as well as small coastal mountain streams. These latter systems are similar in geomorphology and hydrology to rivers in Mesoamerica and the Caribbean islands in terms of their small watersheds, steep slopes, and the fact that they flow directly into the sea without passing through large plains and estuaries (Blanco et al., 2013).
Species diversity in large rivers is high (Mojíca, 1999) in proportion to streams in Mesoamerica (Esselman et al., 2006) and the West Indies (Hein et al., 2013). In contrast, highland watersheds are generally less species diverse than their continental counterparts and have higher abundance of amphidromous than primary species (Arango-Sánchez et al., 2019; Bolaños-Domínguez, 2015; Castellanos-Galindo et al., 2013; Chinchilla et al., 2002; Guardiola and Torrealbo, 2013; and Sánchez-Garcés et al., 2013 in Lasso et al., 2011).
Colombia's megadiversity can be seen in the SNSM, the highest mountain in the country and the highest at the seashore in the world, which is an extension of 42 km and ascends to 5775 m.a.s.l. causing its rivers to descend rapidly. There are three hydrographic slopes or macro basins in this mountain massif: the Caribbean macro basin, with 18 basins, the Western macro basin, with six basins and the Ciénaga Grande de Santa Marta (CGSM), and the Eastern macro basin, with 10 basins, including the Cesar River. These watersheds make up more than 30 rivers and countless streams and creeks, in which more than 95 fish species are found. Of these 95 species, more than 20 are exclusively freshwater, about ten are estuarine and the rest are marine, with the presence of fish with marine characteristics towards the North (Caribbean) watershed, estuarine characteristics towards the Northeastern (Western) and freshwater towards the Southeastern (Eastern) (Fundación Pro-Sierra Nevada de Santa Marta, Prosierra, 2018).
The ecological aspects of tropical dry forest fish populations have been poorly documented, so determining the spatial and temporal variations of fish assemblages in these ecosystems is a necessary aspect to manage their protection and conservation (López-Delgado, 2013). In this sense, López-Delgado (2013) argues that fish assemblages are largely influenced by the ecological variables of the habitat such as light in the water, temperature, current, or population density, so that disturbances such as a decrease in water quality can be a determinant in characterizing the structure of fish assemblages (in distribution, feeding, reproduction, and behavior) (Aguirre-León et al., 2014). Ecological variables also influence changes in environmental variables such as salinity, temperature, dissolved oxygen, conductivity and altitude that are strongly implicated in the regulation of size, survival, distribution and structure of tropical fish (Ortiz-Arroyave, 2010; Sandoval-Huertas et al., 2014). In addition to the above, the heterogeneity of the environment is one of the most important factors in adjusting tropical fish communities, influencing their distribution and spatial segregation (Espinoza and Salas, 2005). In lotic systems, physicochemical parameters are related to physical variables, such as the relationship between water flow and flow rate with temperature and pH, which affect the distribution and abundance of species and can fluctuate temporally and spatially. Considering the above, the objective of this work was to determine the composition of the fish fauna present in the lower section of the Gaira River (SNSM), and to establish possible correlations in the structure, and between fish and the substrate in the two climatic periods evaluated.
MATERIALS AND METHODS
Study area
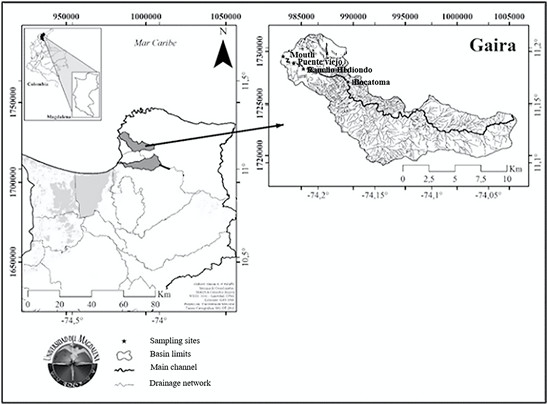
The present study was carried out in the Gaira River (Figure 1) (11°52'06" N-74°11'07" W) corresponding to the northwestern slope of the SNSM, San Lorenzo cut-off, Magdalena department, Colombia. The river has an area of 10,464.3 ha and the course runs east to west, with a length of approximately 32.53 km from its headwaters to its mouth at Salguero beach (urban area of the town of Gaira) in the Caribbean Sea (Frayter et al., 2000). The basin has a unimodal hydrological regime, which includes a dry season (low rainfall) period that begins in December and lasts until April, followed by a rainy season from May to November. Rainfall within the annual cycle generally begins in May, then there is a considerable continuous increase until June, where rainfall remains almost constant until July in the middle and upper part of the mountain massif, which can be considered as a "pluviometric stability". In August, the increase in rainfall continues, and in September the highest rainfall of the annual cycle is evident, and a maximum in October and November as occurs in much of the north of the country (Tamaris-Turizo, 2018).
Sampling stations
Four sampling sectors were selected in the lower part of the river, the first sector corresponded to the mouth of the river (11°11'34.1" N- 74°13'51.5" W) where water is discharged into the Caribbean Sea, the little riparian vegetation is made up of Prosopis juliflora (trupillo) and Rizofora mangle (red mangrove) shrubs. The river's habitat is of the backwater type with a muddy substrate and aquatic vegetation consisting of Phragmites australis (carrillo), Eichhornia crassipes (tarulla) and Typha dominguensis (enea).
The second station corresponded to Puente Viejo (11°11'11.5" N- 74°13'19.3" W), a habitat mainly of backwaters, but with the presence of rapids on a substrate formed by gravel, decomposing organic matter and aquatic vegetation. It also has riparian vegetation consisting of Senegalia tamarindifolia (chicho), Guadua angustifolia (guadua), Guazuma ulmofolia (guazimo), Muellera sanctae-marthae (Macurutú), Albizia niopoides (guacamayo) and Enterolobium coclocarpum (orejero). There is anthropogenic intervention in this sector; sand is extracted from several places in the river, which destabilizes the substrate, and it also receives sewage and organic and inorganic waste from houses along the riverbank, keeping the area eutrophicated.
Station three, Rancho Hediondo (11°10'37.7" N-74°11'0.26" W), is a riffle habitat with a substrate of coarse sand, rocks, and organic matter. The area has two biotopes; the backwaters with medium current velocities, and the riffles, which have areas with current rapids and shallow depths characterized by rocky substrate. Anthropogenic intervention persists in this zone, receiving water discharges from nearby farms and homes.
Finally, at the Bocatoma, the fourth station (11°10'0.85" N- 74°10'24.0" W), as in station three, rapids and a substrate of coarse sand and rocks dominate. The area has backwater habitats with low current velocities, areas with rapids and shallow depths characterized by rocky substrate, and there are many tourist resorts in the sector.
Four samplings were conducted (twice per month) in the rainy season (June-November 2017) and in the dry season (December 2017-April 2018). These were conducted between 07:00 and 15:00 hours, taking place in habitats such as backwaters and rapids, and in different substrates such as silt, sand, gravel (<3 cm), boulders or pebbles (3-11 cm), stones (11-25 cm), rocks (>26 cm) debris, decomposing organic matter and aquatic vegetation. To collect fish, 30 sets per sampling station were made with two 1 and 2 cm diameter mesh-eyed trawls, respectively, and a four-meter trawl net with a one-centimeter diameter mesh eye, dragging it from riverbank to riverbank. Samplings with a trawl and a net were carried out over a length of approximately 200 meters (100 m upstream and downstream). After the captures, physicochemical water parameters such as pH, conductivity, dissolved oxygen and temperature were measured with multiparameter TW. Mean depth, flow and river width were measured with a tape measure, while current velocity was measured using the float method (Castro, 2009). The collected fish were deposited in jars with 10% formalin and transported to the Animal Biology and Physiology laboratory of the University of Magdalena for subsequent identification.
Identification and processing of individuals
Fish preserved in 10% formalin were transferred to a 70% alcohol solution and identified with keys from Dahl (1971), the update of Fricke et al., (2021), Maldonado-Ocampo et al., (2005), and Nelson et al., (2016). They were then transferred to the fishing pilot plant of the Universidad del Magdalena located in the corregimiento of Taganga (Santa Marta) for conservation.
Statistical analysis
The representativeness of the sampling was evaluated by estimating the number of expected species by means of the species accumulation curve, using the Chao 1, ACE and Cole estimators using the EstimateS 9.0 program (Colwell, 2013).
Relative abundance (RA%)
Relative abundance was determined from the number of individuals collected of each species with respect to the total number of individuals in the sample. This parameter was calculated in order to determine the importance and proportion in which each species is found with respect to the community in the different sampling stations. The analysis was carried out using the following formula:
RA = (ni / N) x 100
Where: RA = Relative abundance of the species 1.
ni = the number of captured individuals of the species.
N = the total number of individuals captured.
Abundance distribution
Based on the presence and abundance of each species in the sampling sites, the frequency of occurrence was estimated and its fit to the theoretical abundance models geometric series, log-normal series and MacArthur broken-rod (Moreno, 2001) was verified. To determine which of these models best explained the distribution of the data, the Chi goodness-of-fit test2 was used.
Fish community and habitat structure
In order to determine the relationship between the presence of fish and the type of substrate, a principal component analysis (PCA) was performed. For this purpose, a matrix was constructed taking into account the presence (one) and absence (zero) of some components such as: rocks, stones, pebbles, gravel, coarse sand, fine sand, silt and decomposing organic matter (leaf litter) in each sampling station, and then a canonical correspondence analysis (CCA) was performed. All multivariate analyses were performed with the statistical program Past-Program® 3.0 (Hammer et al., 2001). Subsequently, substrate composition (S) and habitat structural complexity indices were found, according to Winemiller et al., (2008) which standardized the (S) index by means of the following formula:
Where S is the index of substrate composition and habitat structural complexity, x1 represents each of the substrate components and habitat structure for each sampling station, and N represents the number of combined components or habitat structure observed at the sampling stations. Values close to 0 indicate minimum complexity and close to 1 indicate maximum complexity.
To measure diversity at the spatial level, the effective numbers of species or Hills numbers were found through the equation called qD (Jost, 2006):
Where qD is the diversity. The exponent q determines the sensitivity of the index to the relative abundances of the species and has three components, the diversity of order zero (q = 0), which is insensitive to the abundances of the species, whose value is equivalent to the species richness; the diversity of order 1 (q = 1) that includes all the species with a weight exactly proportional to their abundance in the community and takes into account the common species, and the values of q greater than 1 (q = 2), takes more into account the dominant species (Hill, 1973). The above was found with the statistical program PRIMER + 6 version 1.0.1 (Clarke and Warwick, 2006).
To determine if there was a significant statistical difference at the spatial and temporal level in diversity, both in the sampling sectors and in the periods evaluated, and between physicochemical parameters, a Friedman ANOVA was applied after a Tukey test. This test was performed because the data did not meet the assumptions of normality and homogeneity of variances and were evaluated with the Shapiro-Wilk test using the Past-Program® 3.0 statistical package (Hammer et al., 2001).
RESULTS
Physicochemical variables
The physical characteristics of the stations during the rainy season showed that the riverbed was wider (30.6 m), deeper (105 cm) and more flowing (4.25 cm3 /s) at the mouth of the river (Table 1), less wide (16 m) and less flowing (1.3 cm3 /s) at the Bocatoma and shallower (45 m) at Rancho Hediondo. Current velocity was higher at the Bocatoma (0.45 m/s) and lower at the mouth of the river (0.55 m/s). During the low rainfall season, the basin continued to be wider (27 m), deeper (100 cm) and more abundant (4.65 cm3 /s) at the mouth. The smallest width was recorded at Puente Viejo (9.6 m), the shallowest depth at Rancho Hediondo (45 cm), and the lowest flow at the Bocatoma (3.29 cm3 /s). Friedman's ANOVA and Tukey's test showed no significant differences between any of the physical parameters measured (p>0.05).
Table 1 Average and standard deviation of the physicochemical parameters and variables measured in the Gaira River
| Variables | Mouth | Puente Viejo | Rancho Hediondo | Bocatoma | ||||
| Rain | Drought | Rain | Drought | Rain | Drought | Rain | Drought | |
| Temperature (°C) | 27.1±0.1 | 29.6±0.3 | 25.9±0.07 | 25.4±0.08 | 25±0.20 | 24.6±1.12 | 25.1±0.15 | 24.2±0.12 |
| pH | 6.67±1.15 | 6.85±0.057 | 6.6±0.04 | 6.92±0.05 | 7.07±0.103 | 7.2±0.05 | 7.1±0.081 | 7.3±0.050 |
| Conductivity (µS/cm) | 127±14.25 | 135.3±1.48 | 77.2±10.13 | 110.2±9.80 | 109±0.31 | 80.2±10.83 | 102.±0.09 | 85.3±0.129 |
| Oxygen (mg/l) | 6.52±0.05 | 6.4±0.08 | 6.1±0.238 | 6.45±0.03 | 6.38±0.15 | 6.82±0.08 | 6.8±0.07 | 7.01±0.47 |
| Speed (m/s) | 0.55±0.009 | 0.75±0.004 | 0.51±0.05 | 0.7±0.05 | 0.49±0.005 | 0.71±0.008 | 0.45±0.008 | 0.69±0.009 |
| Flow rate (cm3 /s) | 4.25±0.04 | 4.65±0.009 | 3.42±0.12 | 4.46±0.005 | 3.25±0.005 | 3.82±0.01 | 1.3±0.02 | 3.29±0.008 |
| Width (m) | 30.6±0.12 | 27±0.21 | 10.40±0.009 | 9.6±0.05 | 17.8±0.17 | 15.1±0.12 | 15.8±0.17 | 14±0.17 |
| Depth (cm) | 105.00±0.19 | 100±0.15 | 90±0.19 | 70±0.096 | 60±0.57 | 45±0.22 | 81±0.54 | 60±1.057 |
With respect to the physicochemical variables, during the rainy season the mouth of the river showed high temperature values (27.1 °C), conductivity (127 µS/cm), but lower dissolved oxygen (6.5 mg/l). The Bocatoma had low temperature values (25 °C); conductivity (102.7 µS/cm), high dissolved oxygen values (6.8 mg/l), and a basic pH. In the dry season, temperature (29.6 °C) and conductivity (135.4 µS/cm) values were also higher at the mouth; dissolved oxygen was higher at the Bocatoma (7.0 mg/l), and pH was basic at all stations. Friedman's ANOVA and Tukey's ANOVA showed significant statistical differences in pH (p < 0.05) during the rainy and dry seasons in the lower reaches of the river.
Sampling representativeness
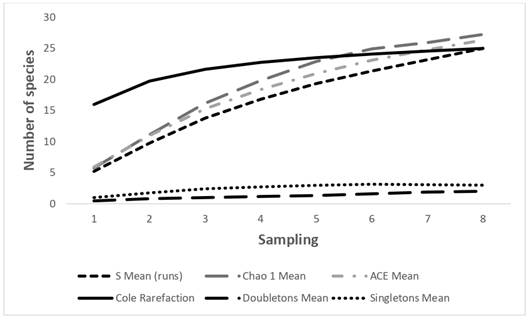
The number of species observed in the Gaira River for the climatic periods was 23 (Figure 2), which corresponded to 95% of the expected value according to the Chao 1, ACE and Cole richness estimator; the singletons curve showed a tendency to decrease, and the doubletons curve showed an asymptotic behavior, indicating a good representativeness of the sampling.
Fish community composition
A total of 967 individuals were captured, 699 in the rainy season and 268 in the dry season, distributed in 11 orders, 13 families and 23 species (Table 2), of which six species (26.08%) are considered purely freshwater, 15 (65.21%) are estuarine species and two (8.69%) are marine species that enter coastal lotic systems. During the rainy season, eight orders, 11 families and 20 species were captured, while during the dry season, seven orders, 12 families and 16 species were captured. Of the species captured, one exotic species (Orechromis niloticus) and Joturus pichradi were reported in the Bocatoma, which is below 200 m above sea level. Friedman's ANOVA and Tukey's test showed no significant differences for the number of species (p > 0.05), but significant differences for the number of individuals (p < 0.05) in the two seasons studied. On the other hand, more estuarine organisms were captured in the rainy season (15 species, 72%) than in the dry season (six species, 12%).
Table 2 Composition and abundance of the fish fauna of the lower sector of the Gaira River.
| Order | Family | Species | Habitat | Abundance | |
|---|---|---|---|---|---|
| Rain | Drought | ||||
| Characiformes | Characidae | Psalidodon sp. (Cuvier, 1819) | Fresh water | 146 | 170 |
| Hemibrycon jabonero (Schultz, 1944) | Fresh water | 57 | 1 | ||
| Cichliformes | Cichlidae | Andinoacara latifrons (Steindachner,1878) | Fresh water | 18 | 10 |
| Oreochromis niloticus (Linnaeus, 1758) | Fresh water | 8 | 0 | ||
| Caquetaiia kraussii (Steindachner, 1878) | Fresh water | 2 | 0 | ||
| Gobiiformes | Gobiidae | Sicydium antillarum (Ogilvie-Grant, 1884) | Estuarine | 17 | 0 |
| Awaous banana (Valenciennes, 1837) | Estuarine | 27 | 5 | ||
| Eleotridae | Eleotris amblyopsis (Cope, 1871) | Estuarine | 4 | 2 | |
| Eleotris pisonis (Gmelin, 1789) | Estuarine | 16 | 4 | ||
| Gobiomorus dormitor (Günther, 1859) | Estuarine | 14 | 0 | ||
| Carangiformes | Carangidae | Caranx sp. | Marine | 3 | 2 |
| Perciformes | Centropomidae | Centropomus undecimalis (Bloch, 1792) | Marine | 0 | 4 |
| Heamulidae | Pomadasys croco (Cuvier, 1830) | Estuarine | 5 | 2 | |
| Mugiliformes | Mugilidae | Mugil curema (Valenciennes, 1836) | Estuarine | 32 | 8 |
| Mugil incilis (Hancock, 1830) | Estuarine | 25 | 28 | ||
| Dajaus monticola (Bancroft, 1834) | Estuarine | 254 | 29 | ||
| Joturus pichardi (Poey, 1860) | Estuarine | 1 | 0 | ||
| Cyprodontiformes | Poecilidae | Poecilia caucana (Steindachner, 1882) | Fresh water | 19 | 0 |
| Clupeiformes | Engraulidae | Anchova sp. | Estuarine | 4 | 1 |
| Elopiformes | Megalopidae | Megalops atlanticus (Valenciennes, 1847) | Estuarine | 12 | 1 |
| Plauronectiformes | Achiridae | Achirys lineatus (Linnaeus, 1758) | Estuarine | 0 | 1 |
| Syngnathiformes | Syngnathidae | Pseudophallus mindii (Mee and Hildebrad, 1923) | Estuarine | 2 | 0 |
| Microphis Brachiurus (Bleeker, 1854) | Estuarine | 0 | 1 |
The relative abundance of species varied between seasons. The highest relative abundance of species in the rainy season (Table 3) was presented by Dajaous monticola (0.42), Psalidodon fasciatus (0.27), Hemibrycon jabonero (0.09) and Andinoacara latifrons (0.08). During the dry season (Table 4), P. fasciatus (0.64), D. monticola (0.11), Mugil incilis (0.10) and A. latifrons (0.03). No significant differences were found between sampling sectors in the river in the rainy season (p > 0.05) or in the dry season (p > 0.05). The species with the highest abundance in the two climatic seasons was P. fasciatus with 332 individuals (37%), with Rancho Hediondo, with 172 individuals, contributing the highest abundance (51.80%).
Table 3 Total abundance, relative abundance and category of species captured in the lower sector of the Gaira River during the rainy season.
| Species | Total Abundance | Relative Abundance | Category |
|---|---|---|---|
| P. fasciatus | 110 | 16.59 | Uncommon |
| H. soap maker | 57 | 8.5 | Scarce |
| A. latifrons | 53 | 7.9 | Scarce |
| O. niloticus | 3 | 0.4 | Scarce |
| C. kraussi | 1 | 0.1 | Scarce |
| S. antillarum | 28 | 4.2 | Scarce |
| A. banana | 27 | 4 | Scarce |
| E. pisonis | 16 | 2.4 | Scarce |
| G. dormitor | 4 | 0.6 | Scarce |
| Caranx sp | 3 | 0.4 | Scarce |
| P. croco | 5 | 0.75 | Scarce |
| M. curema | 32 | 4.8 | Scarce |
| M. incilis | 25 | 3.7 | Scarce |
| D. monticola | 254 | 38.3 | Abundant |
| P. caucana | 19 | 2.8 | Scarce |
| Anchova sp | 4 | 0.6 | Scarce |
| A. lineatus | 2 | 0.3 | Scarce |
| M. atlanticus | 12 | 1.8 | Scarce |
| M. brachyurus | 1 | 0.1 | Scarce |
| J. pichrdi | 1 | 0.1 | Scarce |
| E. amblyopsis | 4 | 0.6 | Scarce |
| P. mindii | 2 | 0.3 | Scarce |
Table 4 Total abundance, relative abundance and category of species captured in the lower sector of the Gaira River during the dry season.
| Species | Total Abundance | Relative Abundance | Category |
|---|---|---|---|
| P. fasciatus | 170 | 63.9 | Very abundant |
| H. soap maker | 1 | 0.37 | Scarce |
| A. latifrons | 10 | 3.75 | Scarce |
| A. banana | 5 | 1.87 | Scarce |
| E. pisonis | 4 | 1.5 | Scarce |
| Caranx sp. | 5 | 1.87 | Scarce |
| M. curema | 8 | 3 | Scarce |
| M. incilis | 28 | 10.5 | Uncommon |
| D. monticola | 29 | 10.9 | Uncommon |
| A. lineatus | 1 | 0.37 | Scarce |
| M. atlanticus | 1 | 0.3 | Scarce |
| M. brachyurus | 2 | 0.7 | Scarce |
| E. amblyopsis | 2 | 0.7 | Scarce |
Abundance distribution
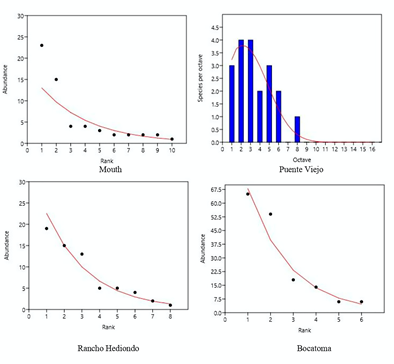
The distribution of abundances of fish communities (Figure 3) were adjusted to the geometric series models at the mouth of the river (Chi2 = 8.18, p = 0.06), Rancho Hediondo (Chi2 = 0.55, p = 0.75) and Bocatoma (Chi2 = 8.75, p = 0.11) stations. Puente Viejo to the log-normal model (Chi2 = 1.3, p = 0.51).
River structure
According to the PCA between fish and substrate type in the rainy season (Figure 4A), 72.36% of the variance was explained by two components. Component 1 (X-axis) contributed 47.87% of the variability, with silt, organic matter, aquatic vegetation, and fine sand as the most important. Species such as Mugil curema, M. incilis, O. niloticus, A. latifrons, Eliotris amblyopsis, Eliotris pisonis, Caranx sp., P. fasciatus, H. jabonero, Pseudophallus mindii, Poecilia caucana, Megalops atlanticus and Anchova sp. were associated with these microhabitats. Component two (Y-axis) accounted for 24.9%, with coarse sand, stones and rocks having the highest incidence. Sicidyum antillarum, Awaous banana, D. monticola and J. pichardi were associated with these microhabitats.
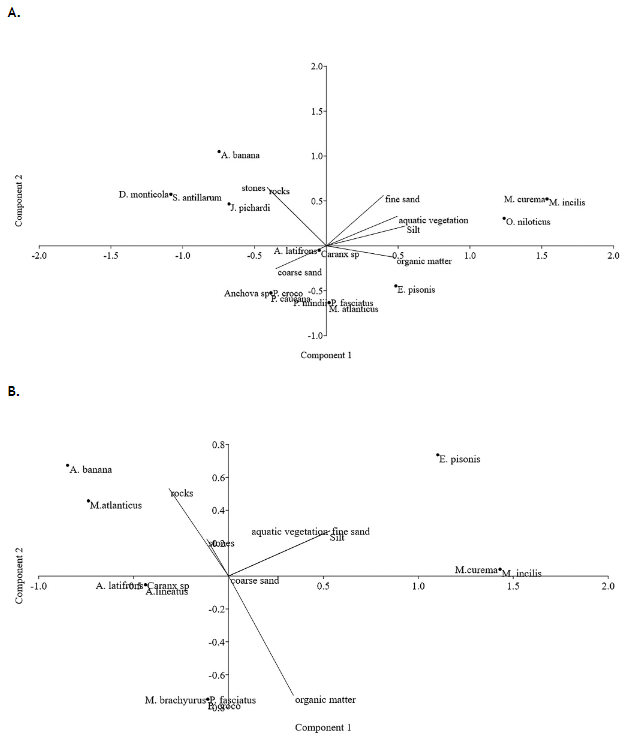
Figure 4 Principal Component Analysis between fish presence and substrate type in rainy (A) and dry season (B) in the lower sector of the Gaira River.
In turn, in dry season (Figure 4B), 77.13% of the variance is also explained by two components, component 1 (X axis) explains 49.43% of the variability, fine sand, silt, aquatic vegetation and organic matter being the most important, species such as E. pisonis, M. curema, M. incilis, Microphis brachyurus, and A. latifronns were associated with this component. Component 2 (Y-axis) explains 27.7% of the variability, with stones and rocks appearing as the most important, D. monticola, A. banana, and M. atlanticus were associated with these microhabitats.
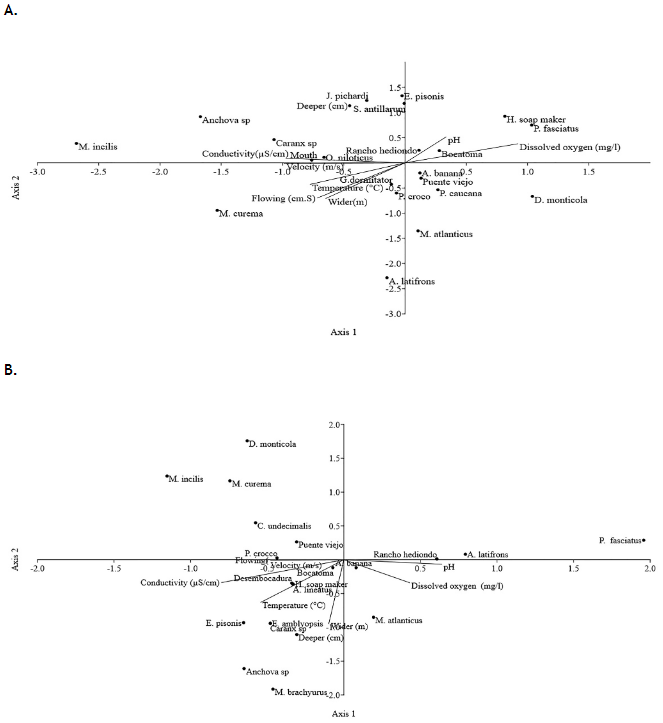
According to the ACC in rainy season (Figure 5A) 92.22% of the accumulated variance in the triplot of species and physicochemical parameters is explained by the first components. Component 1 (X-axis) contributes 63.19% of the variability, while component 2 (Y-axis) contributes 29.03%. The correlation values indicate that dissolved oxygen (X axis, 0.92%) and pH (0.34%) are the environmental variables of greatest relevance and incidence in the distribution of species. In turn, depth (0.97%), pH (0.5%) and dissolved oxygen (0.3%) registered the highest values in component 2 (Y-axis). Ichthyofauna composition was inversely related to dissolved oxygen and pH, with only P. fasciatus, H. jabonero, D. monticola, A. banana, M. atlanticus and P. caucana showing preference for these variables. P. fasciatus, H. jabonero, S. antillarum, J. pichardi and to a lesser extent O. niloticus, M. incilis, Caranx sp., and Anchova sp. showed preference for depth and conductivity. The direction of the vectors (dissolved oxygen, pH, and depth) was almost perpendicular, indicating that the effects of these factors on fish assemblages are independent.
In drought season (Figure 5B), 96.99% of the accumulated variance in the triplot of species and physicochemical parameters is explained by two components. Component 1 (X-axis) contributes 75.56% of the variability, and component two (Y-axis) 21.43%. Correlation values showed that pH is the environmental variable with the greatest impact on species distribution (X-axis, 0.64%). The composition of the ichthyofauna was negatively related to pH, only species such as P. fasciatus, A. latifrons, M. atlanticus and to a lesser extent A. banana showed a preference for this variable.
Species diversity
Species diversity 0D at the spatial level determined that in the rainy season, Puente Viejo (20 ssp.) and the mouth of the river (9 ssp.) showed the highest richness. From the number of typical or common species (1D), the mouth of the river (6 ssp.) and Puente Viejo (8 ssp.) showed high values, while Rancho Hediondo (5 ssp.) and Bocatoma (3 ssp.), the lowest; with respect to the number of dominant species (2D), Puente Viejo (2 ssp.) presented the highest number.
During the dry season, it was determined that the mouth of the river (7 ssp.) and Puente Viejo (12 ssp.) had the highest species richness. The highest number of typical or common species was found in the mouth of the river (6 ssp.) and Puente Viejo (5 ssp.) and in terms of the number of dominant species Puente Viejo had the highest number (2 ssp.). Friedman's ANOVA and Tukey's ANOVA showed no statistical differences in the effective number of species in orders 0 (q = 0), 1 (q = 1), and 2 (q = 2) between sampling stations (p > 0.05), nor between the two climatic epochs evaluated.
DISCUSSION
The number of species identified in this research represents 26.6% of the species described for rivers in the SNSM. However, in this study more species are reported than in the works carried out in rivers of the Tayrona National Natural Park by Bolaños-Domínguez (2015), Galvis (1996) in the SNSM park, to those obtained by Guardiola and Torrealbo, 2013; Lasso et al., 2015, and López and Pulido (2002). However, they differ from those reported by Blanco-Cervantes and Blanco-Cervantes (2022) in the Córdoba River, who report 27 species, with a greater abundance of freshwater species than estuarine and marine species.
In this research, the Characidae family presented the highest abundance with 76.48% of the captures. The results are congruent with those obtained by Espinoza (2007), Espinoza (2008), Esselman et al., (2006), and Trujillo et al., (2010), who report individuals of this family as the most dominant and widely distributed in rivers and streams of Central America and Mexico, which would indicate a greater diversity of this family within the Characidae. Maldonado-Ocampo et al. (2008) found that this family has a better adaptability and specialization to the habitats offered by rivers. With respect to the total number of individuals, P. fasciatus had the highest abundance, a fact that could be attributed to its continuous reproduction during the year, keeping the number of individuals in the population high (De Carvallo et al., 2009).
According to the species distribution models by sampling stations, the mouth of the river, Rancho Hediondo and the Bocatoma presented a better fit to the geometric series model, characteristic of communities with few species (low specific richness) and with a certain level of disturbance (Hill and Hamer, 1998; McGill et al., 2007). However, the pattern may be a product of sampling effects. Water is collected at the intake for the aqueduct of the town of Gaira, which would modify the hydrobiological patterns of the channel, generating changes in the fish community (He and Tang, 2008). The Puente Viejo station was adjusted to the log-normal model, which indicates a minority of dominant species, a greater proportion with average abundances and a small group of rare species, which have different habitat requirements (Uribe and Orrego, 2001). In addition, sand is extracted at this station to be sold in the town of Gaira and in the city of Santa Marta, which destabilizes the ecosystem and modifies the habitat of the fish community (He and Tang, 2008).
Influence of habitat on the river's fish community
The PCA allowed for the identification of two biotopes and some associations of species particular to the backwaters such as P. caucana, A. latifrons, M. curema, M. incilis, G. dormitator, P. fasciatus, H. jabonero, C. craussi, and O. niloticus. The association of these species by these habitats is possibly due to their specific adaptations, highlighting the species' swimming and feeding (Casatti and Castro, 2006; Douglas and Matthews, 2012; López-Delgado, 2013; Oliveira et al., 2010). The main characteristic of the species that inhabit these ecosystems is that they have laterally compressed bodies, which, together with a well-developed anal fin, gives them greater maneuverability moving up and down in the water body, in places with macrophytes, leaf litter and roots. In addition to the above morphological characteristics, the trophic level of the species must be specified, since most of the individuals recorded in these areas have as their main food detritus, algae, litter (decomposing organic matter) and insects of allochthonous origin, from riparian vegetation and only found in shallow areas, since they can settle and are not carried by the current downstream (López-Delgado, 2013).
The species collected at the mouth of the river and Puente Viejo (Tables 5 and 6) are faithful representatives of the ichthyofaunal composition of tropical estuarine habitats (Aguirre-León, 2014; Vieira and Musick, 1994). In estuarine environments, conductivity is one of the variables that most affects both the spatial and temporal distribution of ichthyofauna, due to the physiological conditions required to be tolerated (Amadi, 1990). According to Benavidez (2008), conductivity is a determining parameter for the distribution of all estuarine species because they are euryhaline. The aforementioned could explain the decrease in catches and affect the distribution of these species during the dry season, mainly representatives of the Gobiidae and Eleotridae families. The gobies of the genus Sicydium are typical secondary freshwater fishes, with distribution patterns that indicate saltwater dispersal, and their development involves euryhaline larval stages (Silva-Melo and Acero, 1990) and the typical amphidromous migratory behavior of the Sicydiinea (Berra, 2001).
Table 5 Spatial distribution and abundance of species captured in the sampling sectors during the rainy season in the lower sector of the Gaira River.
| Species | Mouth | Puente Viejo | Rancho Hediondo | Bocatoma |
|---|---|---|---|---|
| P. fasciatus | 0 | 41 | 16 | 54 |
| H. soap maker | 0 | 20 | 19 | 18 |
| A. latifrons | 3 | 50 | 0 | 0 |
| O. niloticus | 1 | 2 | 0 | 0 |
| C. kraussi | 0 | 2 | 0 | 0 |
| S. antillarum | 4 | 6 | 4 | 14 |
| A. banana | 2 | 17 | 2 | 6 |
| E. pisonis | 2 | 3 | 5 | 6 |
| G. dormitor | 0 | 4 | 0 | 0 |
| Caranx sp. | 2 | 1 | 0 | 0 |
| P. croco | 0 | 5 | 0 | 0 |
| M. curema | 15 | 17 | 0 | 0 |
| M. incilis | 23 | 2 | 0 | 0 |
| D. monticola | 0 | 176 | 13 | 65 |
| P. caucana | 0 | 14 | 5 | 0 |
| Anchova sp. | 4 | 0 | 0 | 0 |
| M. atlanticus | 0 | 12 | 0 | 0 |
| J. pichardi | 0 | 0 | 0 | 1 |
| P. mindii | 0 | 2 | 0 | 0 |
In addition to estuarine species, species representing the Cichlidae family were captured at the mouth of the river and Puente Viejo stations. This family is classified as secondary freshwater species due to the tolerance of many species to brackish water, even totally marine. Other widely distributed species were the mugilids, species that inhabit shallow lakes and estuarine zones of temperate and tropical regions, moving to sites farther from the coast during the reproductive period. They are usually the most dominant species in these environments, both in number and biomass (Cardona, 2006), constituting a primordial factor in the functioning of these ecosystems, as well as a fundamental fishing resource.
Table 6 Spatial distribution and abundance of species capture by sampling sectors during the dry season in the lower sector of the Gaira River.
| Species | Mouth | Puente Viejo | Rancho Hediondo | Bocatoma |
|---|---|---|---|---|
| P. fasciatus | 0 | 8 | 156 | 6 |
| H. soap maker | 0 | 1 | 0 | 0 |
| A. latifrons | 0 | 2 | 8 | 0 |
| A. banana | 0 | 2 | 8 | 0 |
| E. pisonis | 0 | 2 | 2 | 1 |
| M. curema | 2 | 2 | 0 | 0 |
| M. incilis | 3 | 25 | 0 | 0 |
| D. monticola | 0 | 25 | 2 | 2 |
| A. lineatus | 0 | 1 | 0 | 0 |
| M. atlanticus | 0 | 0 | 1 | 0 |
| M.brachyurus | 2 | 0 | 0 | 0 |
| E. amblyopsis | 1 | 1 | 0 | 0 |
| C.undecimalis | 0 | 4 | 0 | 0 |
| Caranx sp. | 1 | 1 | 0 | 0 |
| Anchova sp. | 3 | 1 | 0 | 0 |
| P. croco | 0 | 2 | 0 | 0 |
According to the effective number of species, most taxa were recorded at Puente Viejo and at the mouth of the river, both in the rainy and dry seasons, and the lowest taxa were recorded at the Bocatoma, despite the fact that the station was below 100 m above sea level. This could be related to the continuous river concept of Vannote et al. (1980) and the flood pulse of Junk et al. (1989), which foresees the existence of a biotic and abiotic gradient from the source to the mouth. The increase in species with decreasing altitude is due to a greater availability of space, complexity of the environments in the lower sectors, and available nutrients, conditions that favor the growth of periphyton and aquatic macro-invertebrates in the food web. Similarly, factors such as flow, temperature, and geographic barriers influence the distribution of species, with ichthyofauna showing changes in their distribution patterns and habitat use (Oliveira et al., 2010), which is why species are structured with respect to the way they exploit a resource and its diversity is generally determined by the different ways of using the environment; therefore, as supply is reduced, so is diversity and competition (Herder and Freyhof, 2006).
CONCLUSION
The change in seasonality caused variations in the flow of water that affected abundance and affected the structure of the habitat in the study reaches. This alteration generated hydrological variations that were reflected in the fish communities during the dry season due to the interruption of water flow between the upper, middle and lower reaches of the river. The values of width, depth and current velocity decreased drastically in the dry season. These variations in environmental components influenced fish composition in the samples and are consistent with studies showing a directly proportional relationship between river width and depth with species composition and abundance. The new report on the distribution of Joturus pichardi and, above all, the greater number of species in the same community of Gaira, where contamination and sand extraction may be affecting the ichthyofauna, demonstrates the importance of carrying out this type of work in order to take conservation measures and management plans for the river basins.