INTRODUCTION
Globalization, technology and human intervention in ecosystems have generated changes in the distribution of species, particularly invasive species that have environmental, social and economic impacts, and are perceived differently depending on the actors (Atlan and Darrot, 2012); heavily transformed sites, damaged, degraded and destroyed spaces are places highly sensitive to invasion (Ceccon, 2014).
According to Sher and Hyatt (1999), the three aspects that characterize an invasive plant species are: advantages in its reproductive attributes, environmental tolerance and competitive ability; U. europaeus fulfills each of these (León and Vargas, 2009a), taking into account the social and political recognition of its impacts, which is why it is considered one of the 100 most dangerous invasive species in the world by the International Union for Conservation of Nature (Lowe et al., 2000) and according to the I3N database it is in the potential impact category (Zalba and Ziller, 2007).
Ulex europaeus is native to Western Europe and the Atlantic coast of Europe (the British Isles, including Ireland) and northwestern Africa (Hill et al., 2008). This distribution is due to geographic isolation after the early Miocene when the Alpine mountain belt collapsed and the formation of the Mediterranean Sea separated these two places (Cubas et al., 2005).
The genus Ulex L., which belongs to the Fabaceae family, consists of 34 taxa, 30 species and four hybrids worldwide. In Latin America, only the species U. europaeus is reported (POWO, 2021). This species is hexaploid and originated from hybridization between an ancestor belonging to two different lineages (Muthulingam and Marambe, 2022).
The objective of this research is to identify the eco-physiological attributes, current geographic distribution and management actions that have been proposed for the control of U. europaeus globally.
MATERIALS AND METHODS
Literature review
A review of the literature on U. europaeus at the global level was conducted, taking into account studies of a biological nature, for which the Google Scholar, Web of Science and Scopus databases were consulted in the same proportion, using as search terms the scientific name (Ulex europaeus) and three common names that were selected from the 67 reported in different databases as the most used in America and Europe: prickly broom and gorse (Bernal et al., 2017). Given the objectives and the availability of data, we took into account those documents that evaluate the life history, geographic distribution and management actions of the species and its use, searching the title and keywords of the documents in Spanish and English.
Information analysis
Initially, the articles provided by the search were identified, then refined by eliminating irrelevant articles (those that were repeated or dealt with other topics) and subsequently, additional sources with the same search element were identified in blogs, technical reports, diagnostic reports, thesis reports and books. Finally, the information was consolidated by number of documents of each type of research, where each one contributed with information on ecology, life cycle, reproduction and seeds, geographic distribution, uses or management actions.
Distribution
Records of U. europaeus occurrence from 1990 to September 2022 were downloaded from the platform of the international organization Global Biodiversity Information Facility (GBIF, 2022) in complement with Natusfera and Species Explorer. Taking into account the records, a map of density of occurrences at a global level was elaborated with the Geographic Information System Quantum GIS (QGIS) version 3.16, a base map was added (QuickMapServices) and on it, the records of the species establishing ranges with a radius of 1100 km2. Finally, the points that presented errors in the precision of the coordinates were eliminated.
RESULTS AND DISCUSSION
Literature reviewed
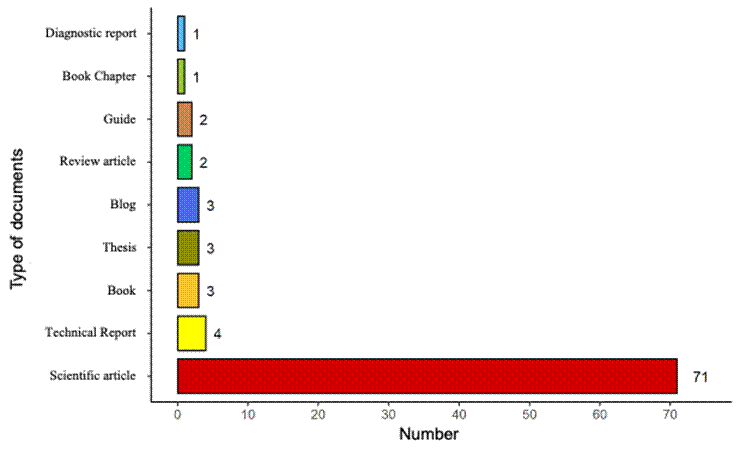
From the search in specialized engines, a total of 2648 results were obtained, more than 180 articles were identified in the first search, repeated and irrelevant articles were eliminated, leaving 70 and 20 additional documents obtained from other sources such as blogs, technical reports, diagnostic reports, thesis reports and books, for a total of 90 published documents, covering the period from 1976 to 2022. Of these documents, 77.7% were scientific articles, 4.44% were technical reports and 3.33% were graduate papers (Figure 1).
Ecology of Ulex europaeus
34.4% of the documents consulted (31) provided information on U. europaeus morphology, life cycle, reproduction and seeds.
Morphological description
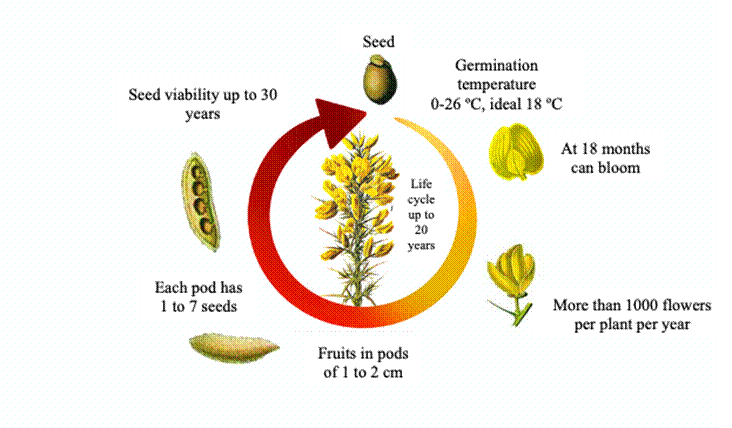
Ulex europaeus is a shrub that can reach up to four meters in height approximately, has a green, thick stem, which generally branches when there is no competition (Ríos, 2005; Salgado-Negret et al., 2017) and with roots that reach depths of up to 50 cm. The leaves are acicular phylloids and in mature plants form spines that are one to three centimeters long, which tend to branch into primaries, secondaries, and tertiaries (Lee et al., 2011). These spines play both defensive and photosynthetic roles. Medina-Villar et al. (2021) reported that spine length, spine width and spine biomass allocation are greater in the invaded than in the native range of the species.
The flowers have a coconut oil aroma, are observed in yellow clusters, the corolla measures between 15 and 18 mm, the calyx measures approximately 12 mm (Clements et al., 2001), the fruits are dehiscent, oblong brown pods measuring one to two cm, each pod produces one to seven smooth seeds, which are characterized by being brown, with six mg of weight and two mm long approximately. Seeds may remain dormant when environmental conditions are not suitable for germination (Sixtus et al., 2004).
Life cycle, reproduction and seeds
Ulex europaeus reproduces asexually by vegetative regeneration through root resprouting and sexually by seeds that spread by different dispersal mechanisms such as: entomocoria, ornithocoria, anemocoria and hydrocoria (Broadfield and McHenry, 2019) thus facilitating the species to colonize over long distances (Portilla et al., 2019). Ulex europaeus reaches first flowering at approximately 18 months, and each plant is estimated to produce 500 to 1000 flowers per season (Figure 2). Natural U. europaeus populations in Britain and France are observed to flower twice a year, a long flowering producing few flowers at a time and a short flowering with masses of flowers. Rathcke and Lacey (1985) named these processes as steady-state flowering and mass flowering, respectively.
Source: own elaboration based on Broadfield and McHenry, 2019
However, for both invasive and natural populations, it is difficult to establish with certainty flowering onset and duration because of variation among individuals within a population and adaptations to environmental conditions in different regions.
In New Zealand, flowering occurs twice a year in the summer and winter (Hill, 2014). On the contrary, in the tropics U. europaeus presents flowering and seed production throughout the year, with alternation of production among individuals of the same population. It is estimated that each plant can produce up to 40,000 seeds per year, but this amount can also change depending on the altitudinal gradient (Portilla Yela, 2019).
León et al. (2016) mentions that the soil seed bank of U. europaeus can exceed 10,000 seeds per square meter, and can remain viable for more than 30 years, making this species more competitive and easily dispersed. Soto and Diaz-Fierros (1997) and Beltrán (2012) determined that 90% of U. europaeus seeds are located in the first six cm of soil depth, Rees and Hill (2001) found 75% of seeds in the first five cm, and Castillo-Díaz et al. (2016) 54% with the same depth in the Embalse del Neusa Forest Park in Colombia. Local studies are necessary to generate inputs for the management of the species taking into account the particular conditions of each location (Pinzón-García et al., 2018).
Hornoy et al. (2011) state that U. europaeus has modified some morpho-physiological characteristics due to different factors of ecological pressure. For example, it has been found that in sites with longer invasion time with elevations below 2700 m.a.s.l. the plants produce larger seeds in order to accumulate resources that guarantee successful germination. In places with less time of permanence and altitudes above 2700 m.a.s.l., the species produces more seeds of small size and with limited germination speed (Atlan et al., 2010).
The behavior of seeds in low areas can be attributed to the fact that the nutrient cycle is faster and the plant produces large seeds where resources are accumulated to guarantee successful germination, while in higher altitude areas the nutrient cycle is slower and the plant produces abundant seeds, but of smaller size (Imbert et al., 2004).
Similarly, Hornoy et al. (2011) demonstrated on a laboratory scale that aspects such as growth, reproduction and defense against predators in invaded sites versus native populations do not present statistically significant differences. However, some particular characteristics were observed that at an ecological level may have relevance according to the evolutionary potential of the species or to changes in the environmental conditions of each territory.
In the initial stage of fruiting, green soft pods are formed that mature eight weeks later and turn brown, producing up to five seeds each (Richardson and Hill, 1998). Seeds that are first detached from the plant have a high degree of hardness due to their tegument (which decreases as they are incorporated into the soil), are ovoid and laterally compressed (Udo et al., 2017), have a fleshy aril and present a smooth and shiny testa with two layers that protect the seed and increase its resistance to decomposing organisms such as bacteria and fungi (Cubas and Pardo, 1988).
Geographical distribution
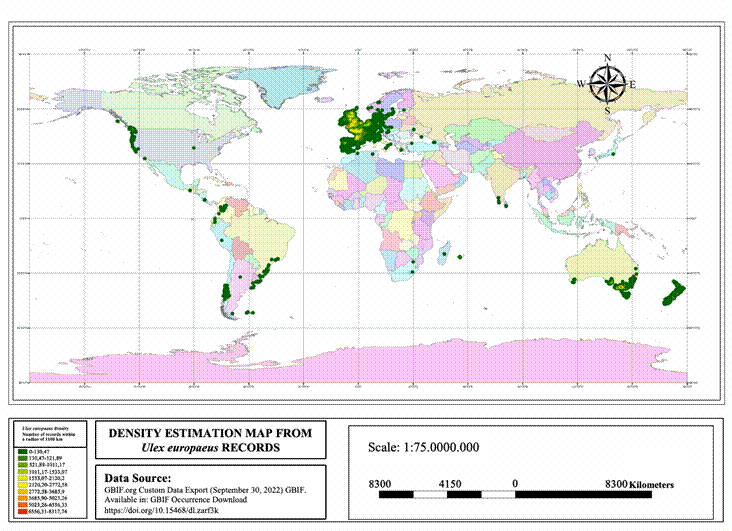
Data on the distribution of U. europaeus were found in 6.6% of the documents consulted (6), showing that this species is naturally distributed in heathlands (Richardson and Hill, 1998) where environmental and edaphic conditions are extreme, with constant winds, limestone substrates, acid soils and have evolved from the management of human communities. Ulex europaeus is one of the most naturally and exotically distributed species of the genus, naturally distributed in Portugal, Sweden, Austria, Belgium, Denmark, Norway and Great Britain, but it also occurs in other regions of the world, which is why it was initially established in southwestern Australia, central California, the coast of Chile and South Africa. Later it was reported that the species also invades mountainous regions such as Hawaii, Sri Lanka, Peru, Costa Rica, Ecuador, Mexico, Panama, Brazil and Colombia (León and Vargas, 2009b; Ramírez-Rodríguez et al., 2022). Currently, U. europaeus has been observed at approximately 3500 m.a.s.l., demonstrating its ability to invade páramo areas (Udo et al., 2016).
The distribution of the species up to September 2022 (Figure 3) has a total of 2,349,736 records. Within a radius of 1100 km2 the native regions have between 6556.33 and 8317.74 records. With respect to invaded areas, the most affected region is Australia where between 6556.33 and 8317.74 records were found in 1100 km2, followed by New Zealand, Washington, Hawaii and British Columbia and in South America, Colombia, Brazil, Chile, Peru and Argentina; immediate attention is required to control the spread of the species.
In Mexico, Ramírez-Rodríguez et al. (2022) reported for the first time the presence of U. europaeus through botanical collections. Although the species does not report occurrence in the mountains of New Guinea, Kenya or Ethiopia, Christina et al. (2020) show that these regions are susceptible to invasion given the environmental conditions there. Similarly, predictive models have been developed for mapping the distribution of U. europaeus in various geographic areas (Gränzig et al., 2021; Thapa et al., 2018).
Uses
In natural conditions, U. europaeus is part of the Mediterranean shrub communities known as heathlands (Beltrán and Barrera-Cataño, 2014). These plant formations emerged before the development of agriculture during the Mesolithic when human communities began to generate settlements and grazing. In the Neolithic, due to the improvement of agricultural systems, the species was used as construction material, forage and fuel, which favored dispersal to new geographical areas (Richardson and Hill, 1998). In the selected research, it was found that 3.45% (3) mention that U. europaeus affects natural forest components and decreases pasture cover (León et al., 2016). Additionally, this species was introduced as a living fence in many areas globally, but its vital traits have allowed it to spread rapidly due to the intense human introduction worldwide and the vulnerability of the degraded ecosystems it has colonized.
In Spain, efforts continue to implement U. europaeus as an alternative forage for cattle, horses and sheep at different stages of growth and under crushing processes that provide animals with the proteins contained in the plant, without causing adverse effects. Similarly, publications of medicinal applications were found that are not limited to the use of the seed of the plant, but also the leaves, stem and flowers to extract terpenoids and glycosides (Carvajal et al., 2021; López-Hortas et al., 2016) and exploration of their bioherbicidal potential (Pardo-Muras et al., 2018).
On the other hand, in Colombia, Bonilla and Bonilla (2021) and Salgado-Negret et al. (2017) have developed alternatives as raw material for agromanto production and fuel to produce electricity by gasification due to its physicochemical characteristics, its low percentage of moisture and ash and high calorific value (Niño et al., 2018).
Table 1 Biological control agents of Ulex europaeus
| Biological controller | Comments | Native distribution of the species | Impact | Country of study | Reference |
|---|---|---|---|---|---|
| Bandicota spp. (Gray, 1873), Rattus rattus (Linnaeus, 1758), Mastomys natalensis (A. Smith, 1834), Xenus erythropus (É. Geoffroy, 1803), R. sordidus (Gould, 1858), R. tiomanicus (Miller, 1900), R. argentivemter (Robinson and Kloss, 1916). | Traces of Ulex europaeus were recorded on the trunks of Ulex europaeus | Tropical Asia | The study was temporary and no follow-up is reported | Colombia | (Vargas, 2018) |
| Cavia aperea (Erxleben, 1777) | In the municipalities of Cogua and Tausa in the northwest of Bogotá, it was observed that this species makes use of thorny broom | Argentina, Brazil, Colombia, Ecuador, Peru, Bolivia, Paraguay, Uruguay, and Venezuela | The study was temporary and no follow-up is reported | Colombia | (Vargas, 2018) |
| Agonopterix ulicetella (Fabricius, 1794) | Larvae feed on thorny broom shoots | Western Europe | Attack in spring, but the plant recovers in the medium term | Chile, New Zealand, Hawaii, Australia, USA, Sri Lanka, Hawaii, Sri Lanka | (Markin and Yoshioka, 1998; Muthulingam and Marambe, 2022; Norambuena et al., 2001). |
| Pempelia genistella (Duponchel, 1836) | Larvae feed on the thorns of prickly broom | Southwest Europe and northwest Africa | In Hawaii only a small population has been recorded since its release, in New Zealand it was released throughout the country, but has only become established in a few places; there are doubts about its persistence and its future role in the control of the thorny broom | New Zealand, USA, Hawaii | (Culliney et al., 2003; Markin et al., 2002). |
| Sericothrips staphylinus (Haliday, 1836) | Insect described in 1836 | Sweden | Laboratory studies have shown 93% mortality of thorny broom seedlings, only tested in the field in Australia | New Zealand, Australia, Hawaii and Canada. | (Hill et al., 2000; Ireson et al., 2018; Markin et al., 2002; Memmott et al., 1998). |
| Tetranychus lintearius (Dufour, 1832) | Known as the red spider mite, its populations are susceptible to other natural predators | Originating in Europe | Acts on foliage | Northeastern USA, Australia, Tasmania, Hawaii, Chile, Oregon, USA, New Zealand, St. Helena | (Broadfield and McHenry, 2019; Ireson et al., 2008; Martinez, 1998; Rice, 2004). |
| Exapion ulicis (Forster, 1771) | The adult feeds on the stem and flowers of the broom, reducing the seeds from 3 to 1 in 95% of the plant | Western Europe | Reduces the reproductive performance of the plant, it is the main predator | New Zealand, California, Western USA, California, Chile, Hawaii, Australia | (Barat et al., 2007; Cowley, 1983; Davies et al., 2008; Markin and Yoshioka, 1998; Norambuena, 2007). |
| Cydia Succedana (Denis and Schiffermüller, 1775) | They feed on the seeds of prickly broom inside the pods. Each larva can destroy up to three pods (90% of the plant) | Europa | Reduces the reproductive performance of the plant | New Zealand, Sri Lanka | (Hill and Gourlay, 2002; Ireson et al., 2008; Muthulingam and Marambe, 2022). |
| Condrostereum purpureum (Pouzar, 1959), Fusariumtumidum tumidum (Link ex Grey, 1821) | They were applied as micellar agar cultures to decapitated stem wounds | N/A | Reduces by half the survival of stem stump. Act independently | New Zealand, Chile | (Herrera-Murillo and Picado-Arroyo, 2022; López-Rodríguez et al., 2022; Muthulingam and Marambe, 2022; Shamoun and Elliott, 2022; Yamoah et al., 2008). |
| Uromyces pisi (Liro, 1908) | Introduced for the control of Ulex europaeus | USA | There is only a record of two years after release, so the establishment of the species is in doubt | Hawaii | (Culliney, et al., 2003). |
Management actions
For the control and elimination of U. europaeus, it is necessary to characterize the invasion and establish its age in order to make management decisions (Beltrán and Barrera-Cataño, 2014). The literature consulted shows physical, chemical and biological control strategies and 55.55% of the documents (50) mention that these are generally costly and ineffective strategies when the conditions of each territory are not evaluated (Hill et al., 2008; Beltrán, 2012). In addition, the analysis of social and economic factors suggests addressing the problem from an integrated approach (Atlan and Darrot, 2012).
The most commonly used methods for the control and eradication of the species are described below.
Manual eradication
Manual eradication is mentioned by 11.11% of the research reviewed (10) as one of the most effective mechanisms for the control of U. europaeus populations. In this regard, Jayasekara et al. (2021) conducted a study in Sri Lanka and confirmed that this process decreases the invasion density and does not negatively affect the surrounding flora and fauna. However, it requires considerable manpower, high costs to achieve total eradication, and continuous monitoring due to the high propagation and germination capacity of the species (Barrera-Cataño et al., 2019).
In Colombia, successful results have been achieved such as those obtained by Rivera-Díaz (2015) by complementing manual eradication with shading on seedling development; changing light, humidity and temperature conditions (León and Vargas, 2009a) and with ecological restoration processes (Aguilar-Garavito, 2010) that decrease soil erosion and land degradation.
Biological control
Successful biological control of the species requires previous studies on the behavior of U. europaeus in the locality where eradication is sought (Table 1). Successful mechanisms in one country do not guarantee the same percentage of efficiency in other territories. However, it is the management action with the highest number of investigations, present in 29% (26) of the documents reviewed.
Studies with the weevil Exapion ulicis controlled U. europaeus in its native range and was the first phytophagous introduced to other localities. This insect lays its eggs in spring and acts as an impact controller limited by the phenology of the plant. When it coincides with flowering, there is a peak infestation that reduces U. europaeus populations by approximately 65% (Richardson and Hill, 1998). When flowering is long it tends to recover with time and if flowering is short the plant plays with the satiation of this insect. Laboratory experiments and introduction of field populations have been conducted and it is widely established in New Zealand, Australia, USA (West Coast and Hawaii) and Chile (Hill et al., 2000). Subsequently, the moth Cydia succedana was introduced in New Zealand, seeking to strengthen control, taking into account that this species reproduces twice a year; in this case the moth affected 80% of the seeds of U. europaeus (Atlan et al., 2010).
Introductions of the phytophagous red spider mite, Tetranychus lintearius, have been successful in New Zealand, Australia and Hawaii (Davies et al., 2004), because it wears away the foliage of U. europaeus and reduces the dry weight of the plant. However, it has been affected by predators such as Stethorus bifidus, Kapur and Phytoseiulus persi milis (Hill et al., 2008). On the contrary, in Chile the spider populations are maintained by the absence of predators, fulfilling the objective of its introduction (Norambuena et al., 2007).
The insects Sericothrips staphylinus and Agonopterix ulicetella as controllers of U. europaeus have been studied in laboratory conditions in Australia and Chile, respectively, where they have shown an efficiency of about 80%. In the field they are distributed in New Zealand and Hawaii, but research does not report constant monitoring of populations (Norambuena et al., 2001). Likewise, Pempelia genistella, a moth introduced for biological control in New Zealand and Hawaii approximately 15 years ago, has decreased its effectiveness in controlling U. europaeus (Markin and Yoshioka, 1998).
Other research finds that fungi have mechanisms of action that allow them to control U. europaeus such as Condrostereum purpureum and Fusarium tumidum studied in New Zealand under laboratory conditions and Uromyces pisi in Hawaii. Since then, they have not been recorded again, so it is inferred that their strains were not adequately established (Culliney et al., 2003).
In Colombia, the rodent species Cavia aperea is postulated as a potential biological control agent, which has demonstrated a relationship of use with U. europaeus (Vargas, 2018), so far its efficiency has not been quantified with case studies.
Herbicides
Its use is regulated in each country, this mechanism allows the removal of leaf tissue directly, however, large-scale application requires more labor and may indirectly affect water sources, native plant species and animals (McAlpine et al., 2018) that fulfill specific ecological functions (Castro, 2011).
For the control of U. europaeus, 12.2% of studies (11) were found using herbicides that target seeds, seedlings, juveniles, leaf areas and stump cuttings. These studies were conducted in Australia, New Zealand, Uruguay, South Africa and the United States (Table 2).
Table 2 Herbicides used for the control of Ulex europaeus
| Commercial Name | Active ingredient | % of Efficiency | Affected plant organ | Country where the research | Reference |
|---|---|---|---|---|---|
| Reglone® 200 g/l | Diquat | 100% | Seeds | Australia | (Moore and Kennewell, 2010) |
| Spray.Seed 250 | 135g/L Paraquat 115 g/L Diquat | 100% | Seeds | Australia, United States | (Moore and Kennewell, 2010) |
| Grazon™ Extra | Triclopyr + picloram | 68% | Juvenile leaves and roots | Australia | (Moore and Kennewell, 2010; Rolando et al., 2011). |
| Buctril®. | Bromoxynil | 54% | Sheets | Australia | (Moore and Kennewell, 2010) |
| MCPA | Phenotil | 45% | Seeds | Australia | (Moore and Kennewell, 2010) |
| Tordon | 2,4-D + picloram | 54% | Seeds | Australia, United States, Canada | (Moore and Kennewell, 2010) |
| Picloram | Picloram 240 g/L | 100% | Mature leaves and stems | South Africa, Uruguay | (Castro, 2011; Viljoen and Stoltsz, 2013). |
| Clopyralid | Picolinic acid | 70% | Sheets | California | (Viljoen and Stoltsz, 2013) |
| Triclopyr | Butoxyethyl ester | 100% | Mature leaves and stems | South Africa, Uruguay, New Zealand | (Balneaves and Davenhill, 1990; Castro, 2011; Viljoen and Stoltsz, 2013). |
| Imazapyr | Imidazolinone | 100% | Mature leaves and stems | South Africa | (Viljoen and Stoltsz, 2013) |
| Glyphosate | Glyphosate | 65% (1 application) 100% (2 o more applications) | Mature leaves and stems | Uruguay, New Zealand | (Castro, 2011; Preest, 1980). |
| 2, 4, 5- trichlorophenoxyacetic acid | 99.4% | Juvenile leaves and stems | New Zealand | ( Preest, 1980; Rolstony Devantier, 2012). | |
| Hexazinone | 100% | Juvenile leaves and stems | New Zealand | (Preest, 1980) | |
| TTA Terbuthylazine+ terbumeton+amitrol | 100% | Juvenile leaves and stems | New Zealand | (Preest, 1980) |
The efficiency percentages of herbicides are related to the application, environmental conditions, soil characteristics, and the dilution ratio of the herbicide, for which it is suggested to follow the technical data sheet of each product in order to obtain favorable results; some products are not specific and have been applied by trial and error. Of the herbicides mentioned, Reglone® is not affected by rain and acts by breaking cell membranes. Just like Grazon™, the ingredients remain active for a long time and for several months inhibit growth of U. europaeus. On the other hand, Viljoen and Stoltsz (2007) point out that Picloram is efficient in foliar treatments and cut stumps in mature plants. Other studies affirm that it is more efficient in the first stages of development (Rolston and Devantier, 2012). Similarly, other products, such as spray seed 250 on seeds and Clopyralid in foliar treatments cause metabolic alterations and increase oxidative stress.
Fire
The use of fire as a control mechanism is quick to apply, economical and paves the way for other techniques. However, it has had limited success: 2.7% of the studies (3) reviewed classify fire as a control mechanism that can fail, be marginal or successful. Failure occurs when ignition does not spread and thorns remain intact, marginal when only soil seeds and plant edges are consumed and successful when flames develop and spread to the top of the plant (Anderson, 2010). Ulex europaeus was found to fix atmospheric nitrogen and is susceptible to numerous fires from necromass and fine suspended fuels, which accumulate in particles of less than five mm, on branches, within the canopy and in ground litter. Published articles evidence that it is not feasible to perform this technique in protected areas, because it modifies ecosystem behavior and generates collective panic (Aguilar-Garavito et al., 2018). In laboratory tests it has been found that U. europaeus has a high rate of combustion and propagation (Anderson, 2010), which favors its regeneration and threatens communities and populations of native species (De Luis et al., 2005).
Ulex europaeus, being a heliophilous plant, requires constant sunlight, which is why it adapts easily in open, disturbed, deforested and intensively grazed areas (Poveda, 2020), where the low availability of organic matter allows the plant to accelerate its growth (Clements et al., 2001). It should be noted that scarification of U. europaeus seed by fire or water action increases the percentage and speed of germination regardless of the presence or absence of sunlight (Ocampo-Zuleta and Solorza- Bejarano, 2017).
On the other hand, fire can stimulate the germination of seeds stored in the seed bank if it is not complemented with other control techniques. In this sense, Ocampo-Zuleta and Beltrán-Vargas (2018) mention that fire can decrease 62% the seeds of U. europaeus available above ground, but buried seeds do not die and germinate in a short time.
CONCLUSIONS
Most research focuses on studies of the ecology and life history of U. europaeus, the species becomes an invasive species in ecosystems transformed by humans for agricultural uses that have characteristics such as a predominance of grasslands, degraded soils, with little or no native species cover, open and deforested areas. It has numerous vital attributes that guarantee its survival in adverse environmental conditions, among them are: being a heliophilous plant, having low soil moisture requirements, the ability to form seed banks with high viability for long periods of time, and the absence of competitors and predators in the ecosystems it invades.
In terms of management actions, biological control is leading research in Europe and North America. In Central and South America, it is recent and most of it is still under development. Studies show that it is possible to eradicate the species with the use of herbicides, some of which are 100% effective, but with the possibility that it may become established again and cause harmful effects on native species and soil fauna.
Despite the high costs, manual removal is still the main alternative at a global level for the control of U. europaeus, in complement with ecological restoration processes in places where the density of invasion is 130.47 to 521.89 records per 1100 km2 , the plant material obtained from manual removal can be used for the extraction of essential oils, extraction of antioxidants or raw material for agromanto production, in order to provide sustainable management.
In the documents analyzed, only 6.6% work on the distribution of U. europaeus, a range that is constantly expanding at latitudinal and altitudinal levels. The map generated in the research shows that in South America the country with the highest density of records of presence is Colombia, given the environmental and territorial conditions, this country is prone to increased invasion. Some information gaps are: the scarcity of protocols for the introduction of control species according to the experiments carried out, the responsibility in the use of herbicides, the use of fire as a management action taking into account the seed bank, the area to be intervened and studies of geographic information systems that allow predicting and modeling the potential distribution of the invasive species before its colonization.
To fill these gaps and obtain favorable results in the control of U. europaeus from the analysis of the information, it is suggested to combine the different techniques, taking into account the environmental conditions of each place and the characteristics of each territory.