INTRODUCTION
The destruction of mature forests often reduces the diversity of native species (Gibson et al., 2011), and negatively affects soil nutrient retention, biomass accumulation and climate regulation (Foley et al., 2007; Lima et al., 2014; Raiesi and Beheshti, 2022). Perhaps the biggest bet that has been made to prevent the loss of natural forests is the generation of protected areas. However, it is clear that even these measures are not entirely successful (Wade et al., 2020), such as what is happening in Colombia's National Parks (Clerici et al., 2020). It has also been suggested that in order to avoid serious environmental problems, it is necessary to recover destroyed forests through reforestation and ecological restoration processes (Chazdon, 2008). Within these processes, the most economical to implement is passive restoration, which involves the natural recovery of systems from ecological succession processes, without further human intervention (Bechara et al., 2016). This can occur in both government and private conservation areas, where these processes tend to be successful, especially when soil fertility has not been greatly altered, there are fragments of natural forest close to the deforested areas, and there are seed dispersers that collaborate in the colonization of these environments (Etter and Botero, 1990; Norden et al., 2009; Poorter et al., 2021; Rozendaal et al., 2019).
The Serranía de las Quinchas is located in the middle of the Magdalena River Valley, between the departments of Boyacá and Santander. The region has been subject to deforestation since the beginning of the 20th century (Díaz-Galindo, 1992) and suffered devastation of relatively flat areas and strong influence even in places with mountainous slopes (Burgos et al., 1980). In fact, the Magdalena basin is the most deforested basin in South America and the tenth most deforested basin in the world (García-Romero, 2013). In 2003, the ProAves Foundation established a private reserve in this region, the El Paujil Bird Reserve, which has been growing and currently includes 3419 hectares protected under the figure of Private Reserve of Civil Society (ProAves, 2010). This reserve has been consolidated and expanded with the purchase of land with forest remnants and forest crops. Some of the properties acquired in 2010 were farms on the Ermitaño River watershed, which had some elements of natural vegetation and some evidence of species planted near the banks of the river.
Although the study area does not include extensive flood plains, since it is located in the lower foothills of the Serranía de las Quinchas, high rainfall seasons generate floods that deposit sediments that can contribute nutrients to the riverbanks. For this reason, these ecosystems were preferred sites for the establishment of cultivation zones, since there are no flood plains that are not affected by human activities.
The objective of this study was to describe the population dynamics in three permanent vegetation plots that were established between 2012 and 2013, and that were remediated in 2021 after a natural regeneration process, to understand how much the forest structure (biomass) has been recovered, which species accumulate the most and what sizes accumulate the most. Finally, biomass accumulation rates were compared with some passive and active restoration studies.
MATERIALS AND METHODS
Area of Study
This study was conducted in the El Paujil Bird Reserve, in the departments of Santander and Boyacá (74° 11' W 5° 56' N, Figure 1) with a geographic altitude of 150-170 m and a mean annual temperature of 27.8 °C (Aldana et al., 2008). There are two precipitation peaks during the year, one between April and May and the other between September and November. The reserve was created in November 2003 to conserve bird species and their habitats (ProAves, 2010). Prior to the establishment of the reserve, the reforestation company Bosques del Futuro practiced selective logging for 5 years in part of the forest (Silva-Herrera, 1999). At this site, 4 plots of 1 ha (100 x 100 m, each with 25 subplots of 20 x 20 m) were established in terra firme forests in 2006 (Aldana et al., 2008), which have been evaluated in terms of population dynamics (Restrepo et al., 2016). This forest is located in the department of Boyacá, in the Puerto Pinzón village of the municipality of Puerto Boyacá. These plots serve as a frame of reference to compare the dynamics presented in this study, which correspond to three plots of 0.1 ha (20 x 50 m), in flood forests in the department of Santander, municipality of Bolivar on the Ermitaño River, a river that separates both departments. Two plots were established in 2012 and a third, in 2013, approximately 500 m upstream on the same bank (Santander department). The limited sampling was due to the difficulty of finding sites with native forest vegetation in the floodplains. The sampled forests can be considered as remnants of native forest as they included large trees up to 65 cm in diameter, but with the presence of human intervention (e.g., some banana plants) and present a flat topography including some small streams that generate slopes up to about 2 m high. The distance between these plots and the mainland plots varies between 1 and 7 km (Figure 1).
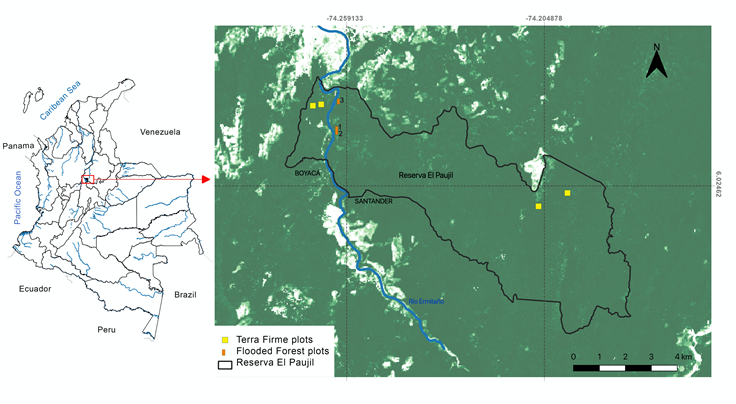
Figure 1 Location of the study area within Colombia and the 20 x 50 m plots in flooded forest (reddish rectangles, within the Paujil Reserve (Colombia). The map also indicates the perimeter of the reserve at the time the plots were established and the location of the terra firme plots with which they were compared (yellow squares). The dark green color refers to forests, the light green to intervened forests and the white color to open areas.
Data collection
At the time of establishment, diameter at breast height (DBH) measurements were taken in each plot for all individuals with DBH greater than 10 cm, which were identified to species (or morphospecies if not possible) and marked with metal tags. Although smaller plants may be important elements of diversity that contribute to the regeneration process, they were not considered given that large trees have the greatest effects on biomass accumulation (Lutz et al., 2018) and it is quite frequent to use this limit in monitoring plots (Nascimento and Laurance, 2004). In 2021, they were censused again, measuring DBH for individuals sampled in the first census, reporting dead or missing individuals and noting causes of mortality where possible, following the protocol of Phillips et al. (2009). All individuals that entered the size category equal to or greater than 10 cm DBH were included as new recruits. The cumulative biomass of each tree was calculated using the equation of Zianis (2005): Biomass = 0.1424 x DBH2.3679, which only takes into account diameter; since only in the re-survey were heights estimated with a laser meter. Additionally, this equation was used because in the information compiled for the country (Cárdenas-Camacho, 2014), not all the species that were present in the plots are found, and the Zianis model is the one that shows on average the lowest biases for the estimation of aerial biomass for Colombian ecosystems (-9.5% according to Alvarez et al., 2012).
Data analysis
Calculations of annual rates of tree community growth, mortality, and recruitment were made for each plot using the formulas presented by Sherman et al. (2012). Then, to statistically compare these calculations and make them comparable to the information obtained in the dryland plots, comparisons were made at a 20 x 20 m scale. To describe soil fertility in the re-sampling, about 1 kg of sample was taken from the center of each subplot (or 20 x 20 quadrat) at a depth of 5 - 15 cm (or on one side in 20 x 10 plots; N = 9). The samples were dried at room temperature and taken to the Water and Soil Laboratory, Faculty of Agricultural Sciences, National University of Colombia (Bogotá), where the effective cation exchange capacity (ECEC) was quantified, estimated as the sum of bases and exchangeable acidity. The data were analyzed using the statistical programs JMP and R (R Core Team, 2013). Using Kruskal-Wallis tests, demographic rates were compared between floodplain and upland forests, and between mortality and recruitment rates of floodplain plots. Linear regressions were used to compare the effect of CICE on biomass change.
Finally, the most important species sampled were identified with the importance value index determined by the density, biomass and relative frequency of each species (using ten 10 x 10 m subplots for each of the three 20 x 50 m plots). Since pioneer species play an important role as indicators of disturbance (e.g., logging), sampled individuals were categorized into two groups depending on seed size (< 4 mm long) and dependence on light for germination and establishment.
RESULTS
General information
Thirty-five species were recorded in the vegetation surveys, where the dominance of Anacardium excelsum (Anacardiaceae) stands out according to the importance indexes of the first census (Table 1). Also noteworthy is the density of Guadua angustifolia (Poaceae) stems. Although Guadua angustifolia was not present in several subplots of the sampling, it had a high density where present. Additionally, some specialist species of floodplains were found, such as Cecropia membranacea (Urticaceae) and Inga cecropietorum (Fabaceae). Typical pioneer species were also found, including two Cecropia species, Vismia baccifera (Hippericaceae), Apeiba tibourbou (Malvaceae), Luehea seemannii (Malvaceae) and Ficus insipida (Moraceae). About half of the species have seeds that are dispersed by animals (52%), followed by abiotic dispersal (Anemochory = 31%), and explosive dehiscence (11%) (Table 1). The floristic and dominance patterns were maintained in the second census.
Table 1 Most important species in the three 0.1 hectare plots established in the El Paujil reserve with the values of biomass (kg), density, and relative frequency, ordered according to the importance index
| Species | Biomass | Dom. Rel. | Stems | Den. Rel. | Plots 20 x 20 | Freq. Rel | IVI | Main dispersers |
| Anacardium excelsum | 43205 | 65.7 | 43 | 26.9 | 6 | 66.7 | 159.3 | Bats |
| Guadua angustifolia | 2530 | 3.9 | 39 | 24.4 | 3 | 33.3 | 61.6 | Unassisted |
| Cecropia membranacea | 6150 | 9.4 | 8 | 5 | 4 | 44.4 | 58.8 | Bats, birds and primates |
| Schizolobium parahyba | 4892 | 7.4 | 11 | 6.9 | 4 | 44.4 | 58.8 | Wind |
| Luehea seemannii | 2015 | 3.1 | 7 | 4.4 | 4 | 44.4 | 51.9 | Wind |
| Bauhinia picta | 433 | 0.7 | 8 | 5 | 4 | 44.4 | 50.1 | Explosive dehiscence |
| Cecropia peltata | 607 | 0.9 | 5 | 3.1 | 4 | 44.4 | 48.5 | Bats, birds and primates |
| Pterocarpus rohrii | 415 | 0.6 | 4 | 2.5 | 4 | 44.4 | 47.6 | Wind |
| Terminalia oblonga | 1304 | 2 | 3 | 1.9 | 3 | 33.3 | 37.2 | Wind |
| Vismia baccifera | 419 | 0.6 | 5 | 3.1 | 3 | 33.3 | 37.1 | Bats and birds |
| Zanthoxylum rohifolium | 434 | 0.7 | 3 | 1.9 | 2 | 22.2 | 24.8 | Birds |
| Hura crepitans | 788 | 1.2 | 2 | 1.3 | 2 | 22.2 | 24.7 | Explosive dehiscence |
Demographics
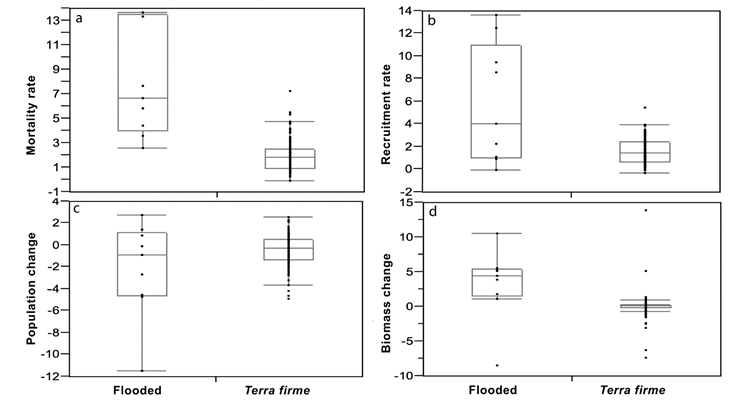
In the first census, 160 individuals were found, with an average of 53 individuals per plot (Table 2). In the second census this value decreased to 144 individuals ( X = 48) and 78 deaths were recorded ( X = 26). Given the times between censuses, we estimated high annual mortality rates (7.98( 4.50 SD), which were significantly higher than those of non-flooded forests (KW Chi2 = 20.1, df = 1, p <0.001; Figure 2a). In floodable areas average mortality rates were higher than recruitment rates (5.89( 5.29 SD). However, the variation was high, and this difference did not yield significant values (Chi2 = 1.32, df = 1, p = 0.25). Thus, a negative change in the mean number of individuals over time was found, but the range of variation included zero (-2.09( 4.36 SD) (Table 2). On the other hand, annual recruitment rates were higher in floodplains than in upland forests (KW Chi2 = 4.2, df = 1, p <0.04; Figure 1b). However, in terms of overall population change, no differences were found between forests (KW Chi2 = 0.6, df = 1, p = 0.42; Figure 2c).
Table 2 Annual mortality rate (m), recruitment (r) and population change (delta) in 3 plots of 0.1 hectares established in flooded forests in the Paujil Reserve (Magdalena Medio, Colombia)
| Plot 0.1 ha | 1st Census | 2nd Census | Dead | Mortality rate (m) | Rate (r) Recruitment | Population (delta) change |
|---|---|---|---|---|---|---|
| M | 53 | 51 | 26 | 8.43 | 7.95 | -0.48 |
| Q | 32 | 29 | 12 | 5.88 | 4.65 | -1.23 |
| R | 75 | 64 | 40 | 9.53 | 7.54 | -1.98 |
| Average | 7.94 | 6.71 | -1.23 |
The species showing the highest mortality rates were Guadua angustifolia, Bauhinia picta (Fabaceae), Cecropia peltata, C. membranacea and Schizolobium parahyba (Fabaceae). Interestingly, the species with the highest recruitment was also Guadua angustifolia, suggesting short-lived stems, which are rapidly replaced by asexual growth. Hasseltia floribunda (Salicaceae) and Bauhinia picta were the next species with the highest recruitment rate, corresponding to species of not very large trees (low canopy).
Biomass change
Despite the high mortality observed in flood plains, the relative growth of surviving individuals was high (0.028( 0.019 SD), which generated a positive rate of biomass accumulation per year (3.33 ton/year( 5.15 SD). This implies a biomass gain to accumulate 219.8 and 295.4 ton.ha-1. On the other hand, biomass accumulation in flooded areas resulted much higher than in dryland forests in the same area (KW Chi2 = 3.7, df = 1, p <0.002), with positive, but much lower accumulation values (0.10( 1.90 SD; Figure 2d). Similarly, the average effective cation exchange capacity (ECEC) was 12.6 (5.5 SD) in floodplains, about twice that estimated for plots in upland forests (6.0( 5.4). A positive correlation was found between cation exchange rate and biomass change (r = 0.46). By omitting the only quadrat with negative accumulation result, in order to work with the natural logarithm of biomass accumulation rate, a regression analysis indicated a high predictive power of CICE on biomass accumulation (R2 = 0.67, n = 8, p < 0.05; Figure 3).
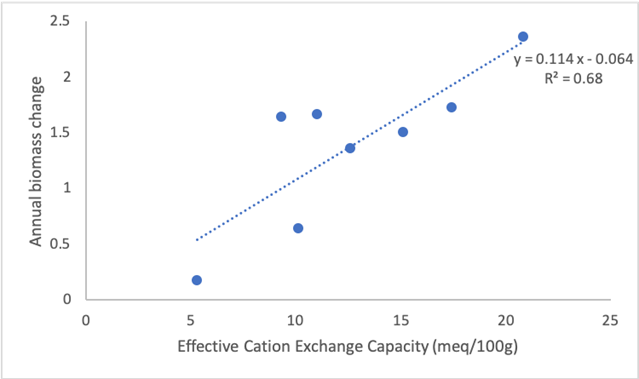
Figure 3 Relationship between the Effective Cation Exchange Rate (ECI) and aboveground biomass accumulation (with natural logarithm transformation) in 3 permanent plots of 0.1 ha in the El Paujil Reserve, Santander, Colombia.
The species that showed the highest relative growth rates in diameter were Apeiba tibourbou, Inga cecropietorum (Fabaceae) and Ficus insipida (Moraceae) (Table 3). Within the list of species with high individual growth, pioneer species were found [ex. Apeiba tibourbou, Ficus insipida, Rollinia dansforthii (Annonaceae), Jacaranda hesperia (Bignoniaceae), Zanthoxylum rohifolium (Rutaceae), Hasseltia floribunda and Cecropia membranacea]; but also species that require good lighting for their establishment, but that can last for a long time [Anacardium excelsum, Schizolobium parahyba, Inga spp. (Fabaceae), Hura crepitans (Euphorbiaceae) and Terminalia oblonga (Combretaceae)]. The species that showed the least growth in diameter was Guadua angustifolia.
Table 3 Annual relative diameter growth rates for the fastest growing species found in 3 plots of 0.1 hectares established in flooded forests in the Paujil Reserve (Magdalena Medio, Colombia)
| Species | Relative growth rate | No. Individuals |
|---|---|---|
| Apeiba tibourbou | 0.076 | 3 |
| Inga cecropietorum | 0.065 | 1 |
| Ficus insipida | 0.057 | 1 |
| Schizolobium parahyba | 0.049 | 8 |
| Rollinia danforthii | 0.044 | 3 |
| Pterocarpus rohrii | 0.036 | 6 |
| Hura crepitans | 0.03 | 2 |
| Xylopia poliantha | 0.026 | 1 |
| Jacaranda hesperia | 0.025 | 2 |
| Zanthoxylum rohifolium | 0.025 | 3 |
| Hasseltia floribunda | 0.023 | 2 |
| Anacardium excelsum | 0.022 | 39 |
| Cecropia membranacea | 0.022 | 4 |
| Casearia aculeata | 0.022 | 2 |
| Terminalia oblonga | 0.021 | 3 |
The only two species that showed negative growth in biomass accumulation were Cecropia peltata (-20.7) and Cecropia membranacea (-9.4), due to loss of individuals. Another pioneer species not recorded in this calculation was Vismia baccifera, because the individuals present did not survive the second census. On the other hand, the species with the greatest increase coincided with those that showed high growth in diameter.
DISCUSSION
The floristic composition of the floodplain plots includes species that have already been recorded for the area (Balcázar et al., 2000), but quite different from those of terra firme forests (Stevenson et al., 2018), with high dominance of generalist species (e.g., Anacardium excelsum, Guadua angustifolia, and Hura crepitans), floodplain specialists (e.g., Cecropia membranacea and Inga cecropietorum), and other pioneer species (Cecropia peltata, Ficus inspida, Luehea seemannii, and Vismia baccifera). The presence of these species and their turnover patterns corroborate the intervention history of these areas. This floristic composition is similar to that found for a series of plots totaling one hectare (Millán-Cáceres, 2021), which is not surprising because this study included these same plots (and an additional 0.7 ha) as they are all in the same reserve. That study also compares with other floodplains in the Magdalena Medio, which showed floristic differences. For example, the four species with the highest density in the floodplains in San Juan del Carare were Cordia collococca (Boraginaceae), Spondias mombin (Anacardiaceae), Genipa americana (Rubiaceae) and Pouteria procera (Sapotaceae), which were not recorded in this sampling (except S. mombin in the 1 ha compilation). Also, within the most important, other species such as Ficus insipida, Hura crepitans, and Luehea seemannii are shared (Stevenson et al., 2018). It is possible that some of these differences are due to the duration of flooding periods, which usually extend for periods of weeks, while in the El Paujil Reserve, flooding periods rarely exceed one or two days. Other differentiating aspects are the low anthropogenic intervention and high density of seed-dispersing organisms in San Juan del Carare, such as primates (Link et al., 2010), compared to the Serranía de las Quinchas area, where there are many secondary forest elements and although the same primate species are present, they are found in lower densities (Aldana et al., 2008). Although primate populations have recovered in the floodplain areas (e.g., spider monkeys that had not been reported before are already observed), sporadic observations made during re-sampling suggest that they are lower than those of San Juan.
Demographics
As predicted, demographic rates were generally high, which can be expected for sites with disturbances, which in this case were generated by anthropogenic effects and flooding (Marques et al., 2009). Comparison with demographic rates of plants in terra firme forests, showing higher annual mortality and recruitment rates, suggests that these factors may depend largely on soil fertility and by species type (since the climate is basically the same). For example, one of the common species in floodplains is Guadua angustifolia, which showed the highest mortality rates per stem, and the highest recruitment rates. This indicates that the life span of each guadua stem is relatively short (only two marked stems were found after 8 years of sampling). But the fact that the population change was similar indicates that new stems are generated by asexual growth, since the subplots with high density of guadua were maintained. It is also noteworthy the high mortality of pioneer plants such as C. peltata, C. membranaceae and Vismia baccifera, all with significant reductions in the number of individuals. These were among the few species that showed a reduction in biomass, which is expected to occur with short-lived pioneer plants (Norden et al., 2011).
Among the tree species that recruited new individuals in the plots, Rollinia danforthii, Hasseltia floribunda, Nectandra turbacensis, Piper laevigatum, Apeiba tiborbou, Ruizodendron ovale, Pterocarpus rohrii and Bauhinia picta were found; all are animal-dispersed except the last two. This suggests that as these secondary forests develop, they may be visited by animal species that may be dispersing species to these systems. Interestingly, some of the species found in floodplains are recognized by local farmers as having been part of reforestation projects (e.g., Schizolobium parahyba and Cordia gerascanthus). Future work should check if the succession process is different in places where these crops were cultivated, since it is expected that diversity will be higher in places where seed dispersers visit frequently (Stevenson, 2011), which is not expected for places with wind-dispersed fruit plantations (such as those mentioned above).
Biomass change
Estimates of biomass stocks in our upland plots were relatively high with respect to estimates made for other forests in Colombia (459.5 - 487.6 ton.ha-1; Phillips et al., 2011). Although estimates of aboveground biomass accumulation in floodplains do not yet reach such high values (219.8 -295.4 ton.ha-1), it is expected to find that range of values in a few decades, given the high biomass accumulation rates estimated for floodplains (3.33 ton.ha-1.year-1 ± 5.15 SD). Studies of biomass dynamics in tropical primary forests have reported annual biomass increments per hectare on the order of 3 to 20 tons (Meister et al., 2012). Comparatively, values for floodplains would be within this range, but more towards the lower values. A direct comparison is not so straightforward when different allometric equations have been used for the studies. However, high values could be considered, compared to values reported by other studies of biomass dynamics in forests subject to fragmentation and edge effects (Nascimento and Laurance, 2004: -85 to 25 Ton.ha-1). On the other hand, the average rate of aboveground biomass accumulation in floodplains resulted slightly higher than that reported in ecological restoration plantations of tropical rainforest in Costa Rica, where 2.88 ± 1.35 Mg ha-1.year-1 and in nucleations, where 1.57 ± 0.96 is reported 9 years after planting (Holl and Zahawi, 2014). However, this average rate is lower than that reported in Colombian lowland moist secondary forests, where at 9 years of succession a maximum accumulation rate (including trees, lianas, palms and herbs > 1 cm DBH) equivalent to 4.4 Mg ha-1.year-1 (Sierra et al., 2012) is reached (Sierra et al., 2012). It has long been known that plant growth following disturbance is usually faster in areas of high soil fertility (Etter and Botero, 1990). In this study, patterns of biomass accumulation rates were found to be consistent with soil fertility estimates (i.e., CICE), as higher values were found in floodplains than in upland forests and a positive relationship between biomass accumulation rates and biomass accumulation index in different subplots of the floodplain. It has been highlighted that quantifying soil fertility is difficult, given high spatial and temporal variation (Vourlitis et al. 2017). Perhaps because of this, it has been reported in the literature that there are examples of positive, but also neutral or negative associations between productivity and soil fertility (Muller-Landau et al., 2021). Our study suggests the existence of a positive pattern; however, it would be important to generate soil sampling over time (e.g., when the forest is logged and at different stages of the ecological succession process) and perhaps with greater spatial amplitude.
The positive changes in biomass are given by most of the analyzed species, except for pioneer species such as Cecropia spp. consistent with a successional process in which pioneer species are being replaced by long-lived pioneers and shade tolerant species. The greatest accumulation of biomass was given by the dominant species, the snail (Anacardium excelsum), which plays a fundamental role due to its great abundance and the size of the trees. The snail has been characterized as a species dispersed mostly by bats and that has better growth in places with high light availability (Fournier and Vozzo, 2003). The annual diameter growth estimate for populations in Tolima was 1.40 cm (Lozano et al., 2012), higher than the estimate found in this study (0.022). The wood density is intermediate, but the species achieves large trees up to 3 m in diameter (Fournier and Vozzo, 2003), which makes it a candidate species for carbon accumulation in aboveground biomass, in addition to having a life span estimated at more than one hundred years (Lozano et al., 2012). The increase in biomass that has occurred in these ecosystems under a passive restoration strategy suggests that the generation of a protected area has had positive consequences in terms of carbon accumulation in the forests studied. This is relevant to mitigate the accumulation of greenhouse gases in the atmosphere, such as CO2, with positive consequences to avoid a very accelerated change in global temperature.
CONCLUSIONS
The dynamics of the flooded forests of El Paujil Reserve are characterized in this pilot study by a high temporal turnover, where Guadua angustifolia is a common species with a very high turnover of stems. On the other hand, the species displaying the highest mortality rates were the yarumos (Cecropia spp.), which lost individuals and the greatest amount of biomass, in agreement with the processes of ecological succession. The snail was the dominant species (Anacardium excelsum), which, like most of the other species, showed high growth rates, which offset the high mortality rates. This generates a substantial accumulation of biomass, especially in sub-quadrants of fertile soils and much faster than that of terra firme forests, which in a few decades could lead to values equivalent to those found in primary forests in the area (Rozendaal et al., 2019). Possibly, these patterns are mainly caused by the fertility of soils that are generated in flood zones, with CICE values almost twice that of terra firme zones. In addition, abundant animal-dispersed plant recruitment was detected in the flood plane. Therefore, for areas such as this, where several endangered animal species are present, such as spider monkeys and blue-billed curassows (ProAves, 2010), the protection of flooded forests can play a fundamental role given that, in these highly productive ecosystems, more animals can be maintained per unit area and can be key elements for the conservation of highly transformed landscapes.