Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232On-line version ISSN 2357-3759
Caldasia vol.28 no.1 Bogotá June 2006
PHOTOSYNTHETIC EFFICIENCY OF PHYTOPLANKTON IN A TROPICAL MOUNTAIN LAKE
Eficiencia fotosintética del fitoplancton en un lago de montaña tropical
GABRIEL A. PINILLA-A.
JOHN CH. DONATO-R.
Departamento de Biología, Universidad Nacional de Colombia, Bogotá, Colombia. gapinillaa@unal.edu.co.
CARLOS A. RIVERA-R.
Unidad de Ecología y Sistemática, Departamento de Biología, Pontificia Universidad Javeriana, Cra. 7 No. 40-62, Bogotá, Colombia. crivera@javeriana.edu.co.
ABSTRACT
Minimum (F0), maximum (Fm), and variable (Fv = Fm - F0) fluorescence and photosynthetic efficiency (Fv/Fm) of phytoplankton were measured for several depths and under laboratory conditions during two seasons (June and October of 2000) in Lake Guatavita, Colombia. When the lake was stratified (June) the surface algae were photoinhibited and photosynthetic efficiency was very low, especially in the hypolimnion. In October, when the lake was circulating, efficiency was higher throughout the water column. Laboratory samples exhibited a gradual decline in efficiency over time, but with higher values in October. Significant differences between June and October samples were observed both in the laboratory and the field. Except for hypolimnetic samples for June, there were no significant differences between field observations and laboratory measures. When the lake was stratified, the concentration of chlorophyll-a was high in the hypolimnion, but the efficiency was low. During circulation, the concentration of chlorophyll-a was low but the efficiency was higher. The results demonstrate that the photosynthetic efficiency of the phytoplankton is independent of biomass and that in addition to biomass, other factors, such as availability of nutrients and light could affect photosynthetic efficiency.
Key words. Guatavita, photosynthetic efficiency, phytoplankton, tropical lake.
RESUMEN
Se midieron, bajo condiciones de laboratorio y a varias profundidades, la fluorescencia mínima (F0), máxima (Fm) y variable (Fv = Fm - F0) y la eficiencia fotosintética (Fv/Fm) del fitoplancton del lago Guatavita (Colombia), en los meses de junio y octubre de 2000. Cuando el lago estuvo estratificado (junio) las algas superficiales estuvieron fotoinhibidas y la eficiencia fotosintética fue muy baja, especialmente en el hipolimnio. En octubre, cuando el lago se mezcló, la eficiencia fue más alta en la columna del agua. Los ensayos del laboratorio exhibieron un descenso gradual en la eficiencia a lo largo del tiempo, pero con valores más altos en octubre. Se observaron diferencias significativas entre las eficiencias de junio y de octubre, tanto en laboratorio como en campo. A excepción de las eficiencias en el hipolimnio de junio, no hubo diferencias significativas entre las observaciones del campo y las mediciones de laboratorio. Cuando el lago estuvo estratificado, la concentración de la clorofila-a fue alta en el hipolimnio, pero la eficiencia fue baja. Durante la mezcla, la concentración de la clorofila-a fue baja pero la eficiencia fue más alta. Los resultados demuestran que la eficiencia fotosintética del fitoplancton es independiente de la biomasa y que además de ésta, otros factores tales como disponibilidad de nutrientes y la luz podrían afectar esta variable funcional.
Palabras clave. Eficiencia fotosintética, fitoplancton, Guatavita, lago tropical.
INTRODUCTION
Fluorescence has been employed as a technique to estimate photosynthetic efficiency of planktonic phytoplankton using the plant inhibitor DCMU 3-(3,r-diclorophenyl)-1, 1 dimethyl urea (Kiefer & Reynolds 1992). Under normal conditions and in the dark, the reaction center of photosystem II (called P680) is reduced, coenzyme Q is oxidized and P680 is said to be “open”. When the photosynthetic pigments are exposed to light and absorb photons, the energy of excitation is transferred to P680, causing the transfer of an electron, the oxidation of P680 and the reduction of coenzyme Q. In this state, the reaction center cannot absorb another photon, and is said to be “closed” until P680 is again reduced and coenzyme Q is oxidized. Fluorescence is produced while photosystem II is closed because energy from light absorbed by the pigments cannot be processed by P680 (Kolber & Falkowski 1993). DCMU blocks the flow of non-cyclic electrons of photosystem II by preventing the re-oxidation of coenzyme Q (Olson et al. 1996).
Under normal conditions algae lose about 1% of the energy absorbed as fluorescence. If photosynthesis is inhibited, either because the reaction centers are closed or photosystem II is blocked by DCMU, fluorescence increases to about 3% (Kirk 1996). In the dark, when coenzyme Q is completely oxidized, fluorescence is at a minimum (F0). Under ambient light, fluorescence increases in proportion to the reduction of coenzyme Q, reaching its maximum (Fm) when all reaction centers are closed. Quantum efficiency refers to the maximum photosynthetic efficiency of the algae and indicates the flow capacity of non-cyclic electrons via photosystem II (Cullen et al. 1997, Magnusson 1997). Since Fm is related to the transfer of quanta to the photosystem II reaction centers, the ratio Fv/Fm is a measure of quantum efficiency of the photochemicals of photosystem II (Cullen et al. 1997). Fv and Fv/Fm permit an estimate of the rate of photosynthesis and indirectly the productivity of the phytoplankton. Cullen et al. (1986) demonstrated that the absorption of radioactive carbon is highly correlated with Fv. The measurement of fluorescence with DCMU has been utilized to estimate the amount of photosynthesis of natural populations of algae (Furuya & Li 1992), to evaluate photoinhibition, the response of some species to light intensity, or to herbicides (Neale & Priscu 1995, Koblizek et al. 1997, Moisan & Mitchell 1999, Sigiura et al. 1999, Komenda et al. 2000) and to establish the influence of cell size on the absorption of light (Finkel 2001). To date there have been no measurements of fluorescence in aquatic ecosystems in the Andean region of Colombia. Indeed, little is known about the functional aspects of phytoplankton communities in the region. In this study, the photosynthetic efficiency of the planktonic algae of Lake Guatavita was measured in vivo before and after inhibition of photosystem II by DCMU. The measurements were conducted during two different seasons and over a complete vertical profile. Specifically, the effect of stratification on photosynthetic efficiency was addressed. In addition, laboratory experiments were conducted to test if the algae in the surface layer of the lake were photoinhibited, as evidenced by having their reaction centers closed.
METHODS
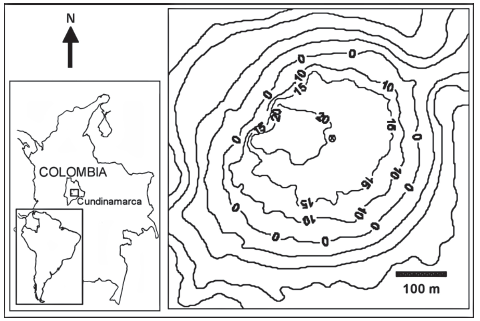
Study site. Lake Guatavita is a tectonic lake located (figure 1) in the Andean Alpine Zone of Colombia (Donato 1998) at an elevation of 2980 m in the Eastern Range of the Andes, in the town of Sesquile, Cundinamarca Province (4o 58’ 50.387’’ N; 73o 46’ 43.576’’ W). The maximum depth is 30 m. The lake is oligo-mesotrophic, and monomictic with a prolonged period of stratification. During the study period, annual rainfall was 1233 mm/year, the driest period was November-December and the rainiest period was from May to July. The greatest wind speed values were recorded between July and September (Rivera et al. 2005, Zapata 2001). The PAR radiation measurement during the week previous to the sample, presented an average of 803 μmol m-2 sec-1 (V.C. 39%) in June and 770 μmol m-2 sec-1 (V.C. 42%) in October (Rivera et al. 2005).
Figure 1. Geographical location and depth map of the Lake Guatavita.
Measurement of fluorescence. Water samples were collected in the center of the lake from depths of 0, 1, 5, 10, 15, and 19 m with a Cole-Palmer horizontal alpha bottle during the months of June and October 2000. Three aliquots of 40 ml were collected for each depth, kept in the dark for 10 to 15 minutes before measuring minimum fluorescence (F0) with a Turner Designs model AU10 field fluorometer. Next, two or three drops of DCMU dissolved in ethanol were added, and after 60 seconds maximum fluorescence (Fm) was measured. The final concentration of DCMU was 10 µM (Neale et al. 1989). In both June and October, 10 l of water were collected from the first meter into a dark container. Samples were kept chilled and in the dark until return to the laboratory. In the laboratory, lake water was transferred to experimental tubes (25 x 115 mm, 40 ml). A total of 15 tubes, in groups of three, were used to measure minimum fluorescence (F0) and maximum fluorescence (Fm) over the course of five days. The tubes were incubated in the dark at ambient lake temperature (14 to 15 oC) in an incubator.
Physical and chemical characteristics. Vertical profiles of temperature, light (Photosynthetic Active Radiation - PAR, 400 to 700 nm), total dissolved solids, dissolved oxygen, pH, conductivity and redox potential were obtained with a Hydrolab® Sonde. Underwater irradiance measurements were corrected for dark current measured by fitting the radiometer with a light-tight plastic black cap, at in situ temperature. Samples were collected every meter for the measurement of ammonia (Nessler method), soluble reactive phosphorus (stannous chloride method), iron (phenanthroline method) and chlorophylla (acetone extraction and spectrophotometric analysis). Methods followed APHA-AWWA-WPCF (1998) in each case.
Numerical and statistical analysis. Light extinction coefficient was calculated by mean of exponential regression between deep and PAR radiation. The measurements of F0 and Fm, both in the field and in the laboratory, were used to calculate variable fluorescence (Fv = Fm-F0) and to calculate the photosynthetic efficiency (Fv/Fm). The photosynthetic efficiency, Fv/Fm, of the laboratory cultures of surface samples kept in the dark was compared with the Fv/Fm of samples from 5 m and deeper layers using the t-statistic (STATGRAPHICS Plus 2.1). The t-statistic was also used to compare the measurement of efficiency between samples. Chlorophylla concentration was estimated using the equations of Jeffrey and Humphrey (1975, cited in APHA 1998).
RESULTS
Physical and chemical conditions. Physical and chemical data are presented in Table 1 for Lake Guatavita for both seasons of observation. In June, three distinct layers were evident: a well-lit oxygenated epilimnion to a depth of approximately 10 m, a metalimnion centered on 13.5 m in which conditions change rapidly with depth, and below 15 m, an anoxic hypolimnion. In contrast, in October the water column was completely mixed with little difference in temperature, oxygen or conductivity from top to bottom. In both seasons, PAR was reduced below 5 m and measurable light don’t reached to bottom of the lake. However in June the photosynthetic radiation penetrated to greater depth. Light extinction coefficient (k) was lower in June (k= -0.52, p<0.01) than October (k= -3.9, p=0.01). In October, pH was somewhat more basic below 10 m.
The concentrations of ammonia, soluble reactive phosphorus and iron were influenced by the pattern of stratification and mixing (Table 1). In June, the concentrations were high in the hypolimnion in response to the higher solubility resulting from reducing conditions. In the surface layer, ammonia was nearly undetectable and iron and phosphorus were low, corresponding to the oxidizing conditions. In general, the metalimnion samples in June were more acid than adjacent layers. In October, these chemicals were uniformly distributed throughout the water column due to the vertical mixing, that eliminated any stratification conditions.
Table 1 Physical and chemical conditions at the Lake Guatavita during June (Jn) and October (Oc) of 2000.
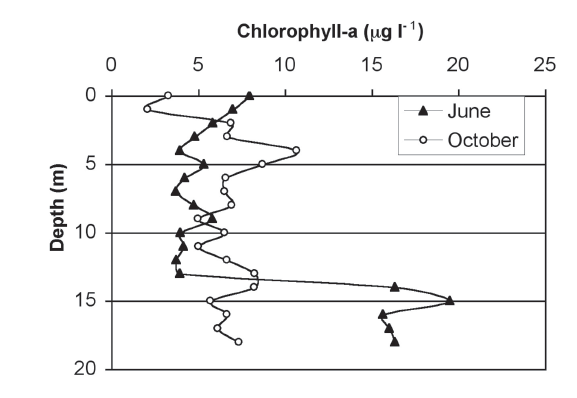
Chlorophylla. Figure 2 presents the concentration of chlorophyll observed throughout the water column for the two different months. It is apparent that the June stratification produced higher values in the metalimnion. The pattern of stratification had largely disappeared in October, although minor differences are evident.
Figure 2. Chlorophyll-a profiles in Lake Guatavita during the stratification (June) and mixing (October) periods of 2000.
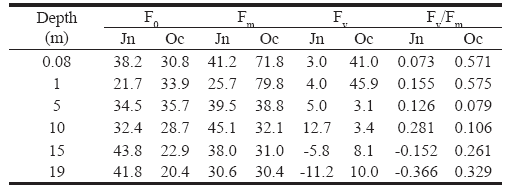
Efficiency of phytoplankton photosynthesis in the field. In June, Fv/Fm increased progressively with depth, up to 10 m depth, but below 15 m, the values were negative (Table 2). The pattern in October was the opposite: Fv/Fm was higher near the surface, decreased with depth to intermediate depth, and again increased near the bottom. In June, the highest efficiency occurred near the bottom of the epilimnion (10 m), where the light intensity was low (3 µmol m-2 sec-1), where more P680 were “open”. During the mixing period (October) a different pattern was evident: at 5 m, without light, efficiency was reduced (0.079, Table 2), but for deeper samples, the efficiency gradually increased with depth (Table 2).
Table 2. Means of minimum (F0), maximum (Fm), variable fluorescence (Fv = Fm - F0) and photosynthetic efficiency (Fv/Fm) of Lake Guatavita phytoplankton in June (Jn) and October (Oc) of 2000.
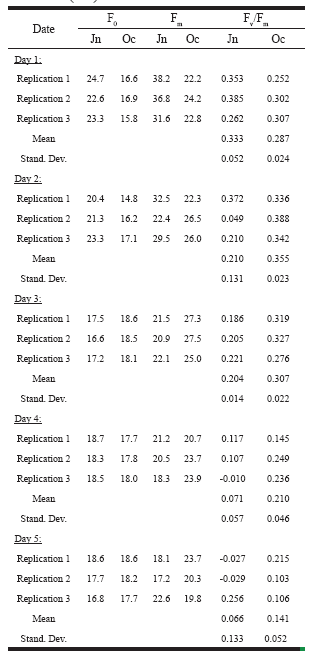
Efficiency of the phytoplankton in the laboratory. The results of the laboratory experiments are presented in Table 3. In the majority of cases, the variation among the replicates was low. In both cases, the efficiency decreased gradually over the course of the experiments.
Table 3. Minimum (F0) and maximum (Fm) fluorescence, and photosynthetic efficiency (Fv/Fm) of Lake Guatavita phytoplankton under laboratory conditions in June (Jn) and October (Oc) of 2000.
Comparison of field and laboratory measures of efficiency. A statistical comparison of the field (below 5 m depth) and laboratory measurements of Fv/Fm indicated significant differences (α = 0.95, p = 0.05) between the two measures for the June samples. This indicates that the efficiency of the deep algae in the lake was different than the surface samples used in the laboratory experiments; the laboratory measurements were significantly higher (α = 0.95, p = 0.026). For the October samples, no significant differences were observed between the hypolimnion samples in the field and the surface samples measured in the laboratory (α = 0.95, 0.19 < p < 0.97). On the other hand, the average values for field measurements in October were statistically higher than the field measurements in June (α = 0.95, p = 0.014). The average efficiencies observed in the laboratory also differed between the two months; in October, the laboratory results were higher than in June (α = 0.95, p = 0.03).
DISCUSSION
The maximum photosynthetic efficiency as measured by Fv/Fm has been estimated at approximately 0.8 (Magnuson 1997). In Lake Guatavita, the highest values were observed at 10 m in June (Fv/Fm = 0.28) and at 1 meter in October (Fv/Fm = 0.571). During the period of stratification, the algal community had a low efficiency, possibly as a result of unfavorable environmental conditions. In the surface layers, ample light was present but nutrient concentrations were low, whereas below 10 m, where nitrogen and phosphorus were more abundant, light was absent and the water was nearly anoxic. In October, efficiency was higher than in June, especially near the surface. At depth, the efficiency was higher than in June, suggesting a more efficient utilization of light and possibly nutrients.
Low light, low redox potential and low oxygen in the hypolimnion during the period of stratification could contribute to the negative efficiencies observed in June in deeper water. The thermocline would inhibit the vertical transport of nutrient rich water to the surface of the lake. The negative values of Fv/Fm observed in the deeper layers can be due to the dissolved organic matter, which can interfere with the measurements of fluorescence (Neale 1987, Vodacek et al. 1997). For this reason the Fv/Fm may appear low or even negative. It is evident that the maximum fluorescence is much reduced during the period of stratification. It may be that the algae in the hypolimnion possess few reaction centers or that the reaction centers are blocked or inactive. Otherwise, the low Fv/Fm could actually be due to the accumulation of pigment degradation products in the surface waters (which are not discriminated by the Jeffrey and Humphrey equations). All those interferences could be addressed in future studies.
Independently of mentioned aspects above, the algae present in the surface layer of the lake had a lower Fv/Fm in June, but a higher value in October. Low light (that is, a minor possibility of photoinhibition) and higher k may explain the higher Fv/Fm of the phytoplankton in the surface during October. On the other hand, the lower Fv/Fm observed in June suggests photoinhibition in the surface layer or, like it was mentioned, the accumulation of pigment degradation products.
During June, the efficiency was lower in deeper water owing to the poor conditions for photosynthesis during the period of stratification. In contrast, during the period of mixing (October) efficiency increased below 5 m. At his point is important to consider that the depth/time trends of chlorophyll and Fv/Fm are subject to day-to-day variations depending on light history. However, the greater availability of nutrients (especially ammonia) recycled from the sediment may have been improved the phytoplankton photosynthesis at that depth.
The values of Fv/Fm observed in the laboratory for the two different sampling dates, clearly decreased with time. This could indicate “dark adaptation”, or a gradual loss of the P680 reaction centers (Kolber & Falkowski 1993), or that the population was dying off in the experimental tubes. Statistical analysis indicated that the efficiency was higher for the October samples, suggesting better environmental conditions, especially for nutrients.
The comparison of the laboratory data with the deep-water field samples indicates that the laboratory samples from the surface had a higher Fv/Fm than the deep-water algae. This may be the result of the algae kept in the dark cease to be light saturated, leaving more reaction centers open and giving rise to an increase in F0. These results confirm that the surface algae were photoinhibited in June; it is probable that this condition could be aggravated by the lack of nutrients in that month that inhibited the algal growth.
In October there were no significant differences between the laboratory conditions and samples from deep in the water column, likely reflecting the well-mixed water column. Accordingly, there was no evidence of photoinhibition of the surface populations during October, in agreement with the lack of difference between laboratory cultures and in situ results. In October, the highest efficiency was observed in the surface, which demonstrates that surface photoinhibition is not a constant feature of Lake Guatavita. During the period of mixing there was sufficient light but also favorable physical conditions and adequate nutrient supply.
It is well known that during periods of stratification the highest concentrations of chlorophyll-a may occur in the metalimnion, precisely where conditions are least favorable. Sharples et al. (2001) observed a chlorophyll maximum in the metalimnion zone of the English Channel where nutrients, especially nitrogen, were more available, similar to the conditions in Lake Guatavita during the period of stratification. In Lake Guatavita, photosynthetic efficiency was lowest, even negative, in the metalimnion. It might be expected that higher biomass of algae would result in higher fluorescence and therefore more efficiency. However, the results indicate that the metalimnetic phytoplankton is not efficient during stratification perhaps due to adverse physical and chemical conditions.
Chlorophylla is high in the metalimnion but is it is possible that this reflects the accumulation of degradation products (e.g. phaeopigments). During the period of mixing, the highest efficiency is observed in the surface because of more adequate light but also because of the availability of a sufficient supply of nutrients, especially ammonia, from deeper in the lake. This higher efficiency is in spite of the fact that chlorophylla concentration is low in the surface layer. The algae in the surface layer, although less numerous, capitalize on the more favorable conditions and attain a higher photosynthetic efficiency. These results confirm the fact that photosynthetic efficiency is relatively independent of the algal biomass (Neale 1987). Much the same is observed with productivity: for the same algal biomass, productivity may be high or low depending on environmental conditions and the physiological state of the algae (Capblancq & Catalan 1994). For example, Carpenter et al. (1998) found that the biomass and the primary productivity may react differently to factors such as grazing, phosphorus supply, or dissolved organic matter. Thus, photosynthetic efficiency as evaluated by fluorescence is more related to productivity than to biomass. Although good correlations can be found between Fv/Fm and photosynthesis for specific cases, the relationship is complex and no conclusion about photosynthetic efficiency in a given assemblage should be made without some parallel measurements of photosynthesis. Therefore, it will be necessary to make in the future this type of measurements to know if the Fv/Fm changes are associated to low light (“alpha”) or light-saturated (“Pmax”) photosynthesis.
ACKNOWLEDGMENTS
This study was supported by Colciencias (No. 12031320399), the Javeriana University (No. 481) and the Jorge Tadeo Lozano University. We thank to Richard R. Petersen of Portland State University for English translation and suggestions on the manuscript. We also thank to Angela María Zapata for her help in the field.
LITERATURE CITED
1. American Public Health Association (APHA), American WaterWorks Association (AWWA) & Water Pollution Control Federation (WPCF). 1998. Standard methods for examination of water and sewage and wastewater. Nueva York.
2. Capblancq, J. & J. Catalan. 1994. Phytoplankton: Which, and how much? pp. 9-31 in: R. Margalef (ed.). Limnology now. A paradigm of planetary problems. Elsevier. [ Links ]
3. Carpenter, S.R., Cole, J.J., Kitchell, J.F. & M. Pace. 1998. Impact of dissolved organic carbon, phosphorus, and grazing on phytoplankton biomass and production in experimental lakes. Limnology and Oceanography 43(1):73-80. [ Links ]
4. Cullen, J.J., Zhu, M. & D. Pierson. 1986. A technique to assess the harmful effects of sampling and containment for determination of primary production. Limnology and Oceanography 31(6):1364-1373. [ Links ]
5. Cullen, J.J., Ciotti, A.M., Davis, R.F. & M.R. Lewis 1997. Optical detection and assessment of algal blooms. Limnology and Oceanography 42(5):1223-1239. [ Links ]
6. Donato-R., J.Ch. 1998. Los sistemas acuáticos de Colombia: síntesis y revisión. Págs. 31-47 en: E. Guerrero (ed.). Una aproximación a los humedales de Colombia. Fondo FEN Colombia, UICN, Bogotá. [ Links ]
7. Finkel, Z.V. 2001. Light absorption and size scaling of light-limited metabolism in marine diatoms. Limnology and Oceanography 46(1):86-94. [ Links ]
8. Furuya, K. & W.K. Li. 1992. Evaluation of photosynthetic capacity in phytoplankton by flow cytometric analysis of DCMU-enhanced chlorophyll fluorescence. Marine Ecology Progress Series 88:279-287. [ Links ]
9. Kiefer, D.A. & R.A. Reynolds. 1992. Advances in understanding phytoplankton fluorescence and photosynthesis. pp. 155-174 in: P.G. Falkowski & A.D. Woodhead (eds.). Primary Productivity and Biogeochemical Cycles in the Sea. Plenum Press, New York. [ Links ]
10. Kirk, J.T. 1996. Light and photosynthesis in aquatic ecosystems. Cambridge Univ. Press, Bristol. [ Links ]
11. Koblizek, M., Marek, M., Komenda, J., & L. Nedbal. 1997. Light adaptation in the cyanobacterium Synechococcus sp. PCC 7942 measured by the dual-modulation fluorometer. Journal of Luminescence 72-74:589-590. [ Links ]
12. Kolber, Z. & P.G. Falkowski. 1993. Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnology and Oceanography 38(8):1646-1665. [ Links ]
13. Komenda, J., Koblizek, M. & O. Prasil. 2000. Characterization of processes responsible for the distinct effect of herbicides DCMU and BNT on photosystem II photoinactivation in cells of the cyanobacterium Synechococcus sp. PCC 7942. Photosynthesis Research 63(2):135–144.
14. Magnusson G. 1997. Diurnal measurements of Fv/Fm used to improve productivity estimates in macroalgae. Marine Biology 130(2):203-208. [ Links ]
15. Moisan, T.A. & Mitchell, B.G. 1999. Photophysiological acclimation of Phaeocystis antartica Karsten under light limitation. Limnology and Oceanography 44(2):247-258. [ Links ]
16. Neale, P.J. 1987. Algal photoinhibition and photosynthesis in the aquatic environment. pp. 35- 65 in: D.J. Kyle, C.B. Osmond & C.J. Arntzen (eds.) Photoinhibition. Amsterdam, Elsevier. [ Links ]
17. Neale, P.J., Cullen, J.J. & C.M. Yentsch. 1989. Bio-optical inferences from chlorophyll a fluorescence: What kind of fluorescence is measured in flow cytometry? Limnology and Oceanography 34(5):1739-1748. [ Links ]
18. Neale, P.J. & J.C. Priscu. 1995. The photosynthetic apparatus of phytoplankton from a perennially ice-covered Antarctic lake: Acclimation to an extreme shade environment. Plant and Cell Physiology 36(2):253-263. [ Links ]
19. Olson, R.J., Chekalyuk, A.M. & H.M. Sosik. 1996. Phytoplankton photosynthetic characteristics from fluorescence induction assays of individual cells. Limnology and Oceanography 41(6):1253-1263. [ Links ]
20. Rivera-R. C., Solano, D., Zapata-A., A. & J. Donato-R. 2005. Phytoplankton diversity in a tropical high mountain lake. Verhandlungen der Internationale Vereinigung für Limnologie 29(1):418-421. [ Links ]
21. Sharples, J., Moore, C.M., Rippeth, T.P., Holligan, P.M., Hydes, D.J., Fisher, N.R. & J.H. Simpson. 2001. Phytoplankton distribution and survival in the thermocline. Limnology and Oceanography 46(3):486-496. [ Links ]
22. Sigiura, M., Minagawam J. & Y. Inoue. 1999. Properties of Chlamydomonas photosystem. II Core complex with a His-Tag at the C-terminus of the D2 protein. Plant and Cell Physiology 40:311-318. [ Links ]
23. Vodacek, A., Blough, N.V., Degrandpre, M.D., Peltzer, E.T. & R. K. Nelson. 1997. Seasonal variation of CDOM and DOC in the Middle Atlantic Bight: Terrestrial inputs and photooxidation. Limnology and Oceanography 42(4):674-686. [ Links ]
24. Zapata-A., A.M. 2001. Variaciones diarias y mensuales de la producción primaria en un lago andino (Lago de Guatavita – Cundinamarca). Tesis de Maestría. Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá, Colombia.
Recibido: 15/08/2005
Aceptado: 21/03/2006