Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232On-line version ISSN 2357-3759
Caldasia vol.28 no.2 Bogotá Dec. 2006
LECTIN PROSPECTING IN COLOMBIAN LABIATAE. A SYSTEMATIC-ECOLOGICAL APPROACH - II.
Prospección de lectinas en especies de labiadas colombianas. Un enfoque sistemático-ecológico- II.
GERARDO PÉREZ
NOHORA VEGA
JOSÉ LUIS FERNÁNDEZ-ALONSO
Chemistry Department, Biochemistry Laboratory, Universidad Nacional de Colombia, Bogotá. Colombia. jrperezg@unal.edu.co; navegac@unal.edu.co
Herbario Nacional Colombiano, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá, D.C., Colombia. jlfernandeza@unal.edu.co
ABSTRACT
This is the second study of lectin and mucilage detection in Labiatae nutlets from Colombia. It was carried out on 31 taxa belonging to 7 genera in which no previous studies have been carried out in this field. A differential response was observed in the group of genera and species studied in terms of mucilage presence as well as lectin activity which consistently increased following extract treatment with Pectinex. Lectin activity was detected in 28 species, being important (greater than 60% activity) in at least 75% of them. Genera such as Hyptis, Scutellaria, Aegiphila and Lepechinia, whilst not presenting mucilage, did present lectin activity, having high activity in most cases. By contrast, Salvia (in all sections studied) presented mucilage and important lectin activity.
Key words. Labiatae, lectin, mucilage, Aegiphila, Hyptis, Lepechinia, Ocimum, Salvia, Scutellaria, Stachys.
RESUMEN
Este es el segundo estudio sobre la presencia de mucílagos y lectinas en núculas de Labiadas colombianas. Se llevó a cabo en 31 taxones, pertenecientes a siete géneros, sobre los cuales no se disponía de información en este campo. Se observó una respuesta diferencial en los géneros y especies en lo relativo a la presencia de mucílago y a la actividad de lectina que se incrementó de manera consistente después de tratar los extractos con Pectinex. Se detectó actividad de lectina en 28 especies, siendo muy importante (mayor del 60%) en al menos 75% de ellas. Aunque los géneros Hyptis, Scutellaria, Aegiphila y Lepechinia no presentaron mucílago, su actividad de la lectina fue alta. Por el contrario Salvia (en todas las secciones estudiadas) presentó mucílago y una actividad importante de lectina.
Palabras clave. Labiatae, lectina, mucílago, Aegiphila, Hyptis,Lepechinia,Ocimum, Salvia, Scutellaria, Stachys.
INTRODUCTION
The Labiatae family, following traditional circumscription, has 23 genera and some 205 species in Colombia whose basic representation and diversity was described by Fernández-Alonso et al. (2003). Following mainly accepted criteria, according to which several genera provided with cymose inflorescences from the Verbenaceae family currently form part of the Labiatae family (Cantino & Sanders 1986; Cantino 1992a, 1992b; Olmstead et al. 1992; Cantino et al.1992; Harley et al. 2004; Ryding 1995; Steane et al. 1997; Wagstaff & Olmstead 1997; Wagstaff et al.1998), the new broadened circumscription for this family for Colombia has 32 genera and ca. 284 species. The 9 new “Verbenaceae” genera which are now dealt with within the Labiatae and which have a total of 79 native or naturalised species in Colombia are: 3 from the Teucrioideae subfamily: Aegiphila (42 spp.), Amasonia L. f. (4 spp.) and Clerodendrum L. (8 spp.); one from the Scutellarioideae subfamily: Holmskioldia Retz. (1 spp.); one from the Viticoideae subfamily: Vitex L. (15 spp.); and four more having uncertain location within the family: Callicarpa L. (2 spp.), Cornutia L. (4 spp.), Gmelina L. (1) and Tectona L. f. (1 spp.). Prospecting of this new group of genera has been begun with a high Andean species from the Aegiphila genus, this being the most diverse genus of Teucrioideae, having 42 taxa in Colombia (López-Palacios 1977, 1986). Four Colombian Labiatae genera have an important percentage of sampled species, Lepechinia with more than 50% (6 out of 10), Salvia with ca. 40% (32/83), Stachys with 33% (4/12) and Hyptis with ca. 31% (13/42).
Bird and Wingham’s pioneering work (1974, 1976, 1977, 1982) revealed the presence of lectins, mainly in Salvia L. species from the Salvia and Sclarea Benth. subgenera from Eurasia and North-America. By contrast, species from the neotropical Calosphace Benth. subgenus have been little explored, since only one work, which included 40 taxa belonging to 6 genera; can be found in the literature (Fernández-Alonso et al. 2003). Labiatae lectins are able to recognize the Tn antigen (GalNAcα-O-Ser/Thr) which is associated with a variety of carcinomas (Springer 1984) and is therefore potentially useful in detecting the antigen in transformed cells. Detailed structural studies of Labiatae lectins have been carried out, mainly on a few species from the Northern hemisphere’s temperate zone. The lectin from Salvia sclarea L. seeds was the first to be isolated and partially characterised (Piller et al. 1986) establishing its specific binding to native Tn red blood cells (RBCs) and enzyme-treated RBCs; several molecular features of the lectin have also been described (Medeiros et al. 2000) and competition binding studies with soluble synthetic glycopeptides have helped to define Tn structures’ density requirements. A lectin from Moluccella laevis L. has been isolated (Lis et al. 1988); the lectin binds strongly to Tn-bearing glycoproteins (Duk et al. 1992), Tn-bearing lymphocytes (Thurnher et al. 1993) and glycosphingolipids (Teneberg et al.1994). Wang et al. (2003a) recently found a lectin (Gleheda) in Glechoma hederacea L. leaves that readily interacts with O-glycans linked to asialo mucin or asialo fetuin in which Gal/GalNAc are terminally exposed. A potentially interesting development arises from the insecticidal properties shown by the lectin (Wang et al. 2003b). More recently, Vega and Pérez (2006) have described the isolation and molecular properties of Salvia bogotensis Benth. seed lectin which is the first one belonging to the Calosphace subgenus which has been studied; the protein specifically recognised the Tn antigen and showed some structural interesting features.
The presence of mucilage in Labiatae has been recently reviewed as well as the several hypothesis concerning its biological/ecological role which is still not clear (Ryding 2001; Fernández-Alonso et al. 2003). The available information indicates that mucilage in Labiatae is only present in the subfamily Nepetoideae (Ryding 1992b) where it occurs in 75% of the genera and species. Given the wide variety of habitats found in Colombia it seemed interesting to carry out a survey on mucilage presence in Colombian species and at the same time evaluate lectin presence and activity in the seeds.
Thirty-two accessions from 29 native Colombian Labiatae species from the Aegiphila Jacq., Hyptis Jacq., Lepechinia Willd., Ocimum L., Salvia, Scutellaria L. and Stachys L genera were analysed in this work. This second prospecting complements the information presented before (Fernández-Alonso et al. 2003) and taxa belonging to Aegiphila and Scutellaria are included for the first time. This extends analysis of mucilage and lectins to a considerable number of species about which no previous information has been presented concerning the presence of these types of compounds and also provides additional confirmatory evidence for some genera or species previously studied (Fernández-Alonso et al. 2003). Erythroagglutination assays were done for the first time for 34 species of Labiatae, including 4 species from the first prospecting.
MATERIALS AND METHODS
Collecting and preserving botanical samples and fruit
The same procedures described by Fernández-Alonso et al. (2003) have generally been followed in that referring to collection itineraries and dates, herbarium sample-taking protocols, collecting nutlets and live material for culturing.
Itineraries. The plants included in this study came from different itineraries mainly carried out on the eastern cordillera of Colombia and where the Andean cordillera divides into three in the South of Colombia. Species from the Hyptis genus were basically obtained in some Santander and Meta savannah formations; another group of species was obtained in Andean and sub-Andean cloud forests from the centre of the eastern cordillera (Boyacá, Cundinamarca, Santander) and Nariño including: Lepechinia betonicifolia, L. vulcanicola, Salvia sagittata (fig. 1) and S. tortuosa. Another group of recently described endemic species (Fernández-Alonso 1995a, 2003a), including Hyptis perbullata (fig. 1), Salvia chicamochae, Salvia sphacelioides subsp. pax-fluminensis (fig. 1) and S. xeropapillosa, were collected in dry inter-Andean Chicamocha and Soápaga river canyons (Boyacá-Santander). A total of 8 collection trips were made over a 3-year period.
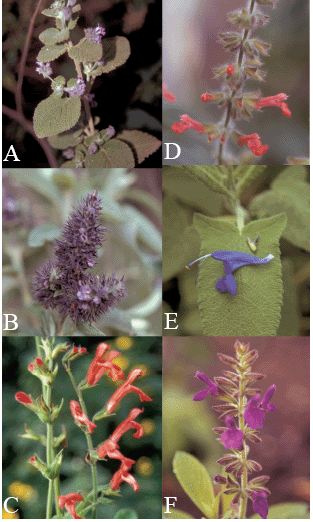
Figure 1. Some species of Colombian Labiatae analyzed. A- Hyptis perbullata. B- Lepechinia velutina. C- Salvia melaleuca subsp. totensis. D- Salvia rubescens subsp. dolychothryx. E- Salvia sagittata. E- Salvia sphacelioides subsp. pax-fluminensis. (Fotographs: J. L. Fernández-Alonso)
Samples: In all cases the following specimens were collected:
a) Collecting fruit (nutlets) for erythroagglutination and enzyme-linked lectinosorbent assays (ELLSA) and mucilage.
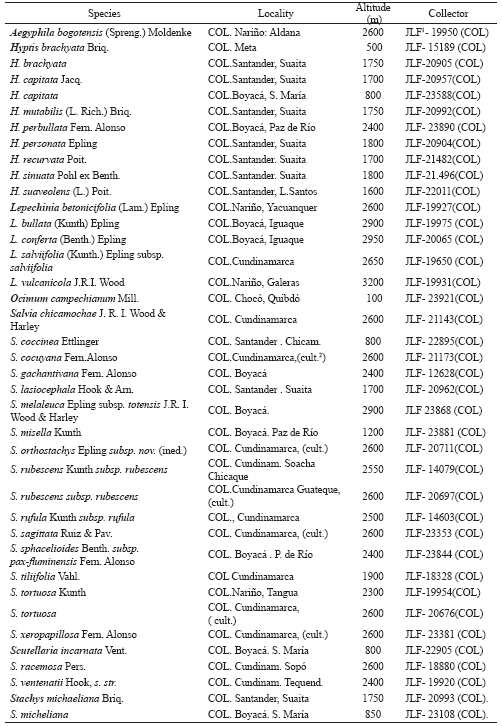
b) Collecting herbarium control samples (about 220 collections) which were included as vouchers (Table 1) and nutlet samples for the project’s sample file (currently containing 90 accessions);
c) Collecting live samples for culturing and follow-up in Bogotá’s Botanic Gardens, where around 50 taxa are being cultured. Control material, as well as fruit samples, were catalogued and deposited in the Colombian National Herbarium (COL) and a large part of these samples have been duplicated in the HUA, FMB, JBB, MEDEL herbaria, abbreviated according to Holmgren et al. (1990).
Table 1. Vouchers of species analyzed.
Some species from remote areas or those having difficult access in the country (Nariño, Santander, Boyacá) were cultured in Bogotá for obtaining nutlets. As a result of this study and other work on Labiatae taxonomy (Fernández-Alonso 2003a, 2005, 2006), an important collection of live reference plants has now been established in Bogotá, in greenhouse conditions in the Universidad Nacional de Colombia and outside in the José Celestino Mutis Botanical Garden in Bogotá.
A Labiatae nutlet reference collection has also been established with more than 400 accessions, some being stored in the nutlet/seed library in the Colombian National Herbarium and others in the Bogotá Botanical Garden’s seed bank.
Types of growth and types of habitat in those taxa studied
All taxa studied have been catalogued according to habit type (growth type) and habitat (altitude at which taxa are found) to enable correlation between these parameters with information resulting from mucilage and lectin assays. The biological/ecological function of mucilage is still not clear (even though various hypotheses have been suggested related to different aspects regarding germination) nor has a correlation been established between the presence of mucilage and determined environmental conditions. Detailed classical types of habit (shrubs, subshrubs, scandent shrubs, and perennial herbaceous and annual or biannual herbs) and habitat (tropical, subandean, andean and paramo) have been considered and described in a previous work (Fernández-Alonso et al. 2003). Two variants having general characteristics have been considered for each altitude range: humid or dry, according to the amount of rainfall, delimiting dry areas as being those having a rainfall of less than 800-1,500 mm/year, depending on altitude. When a plant lives in more than one altitude range its least habitual altitude is given in parentheses.
Lectin extraction
Extraction was done with 20 mM saline phosphate buffer, pH 7.2-7.4 (PBS) in a 1:10 (w/v) ratio, or 1:20 (w/v) ratio if a viscous solution appeared, seeds being left to soak in the solution for 2-3 h, at 4ºC; they were then macerated and agitated at 4ºC, for 16 h. The extract was centrifuged at 39,000xg for 15 min at 4ºC. The supernatant was used immediately or treated with Pectinex.
Pectinex treatment
Supernatant pH was adjusted to pH 4.7 with concentrated AcOH; 28 µl Pectinex Ultra SP-L (Novo) was then added per ml of extract. This was incubated at 28ºC for 4 h, being occasionally shaken. The pH was adjusted to 7.0 with diluted NaOH and lectin activity was determined.
Lectin detection and quantification assays
Two assays were done to assess the presence of lectin:
a) Erythroagglutination. The assays were done on human RBCs as described (Pérez 1984) and on T/Tn-exposed RBCs. A+ human RBCs were enzymatically treated to expose T or Tn determinants (Hirohashi et al. 1985). Crude extracts were used in all cases. If appropriate, the haemoagglutination titre was determined and expressed as the highest dilution where agglutination was still observed.
b) Lectin was also detected by ELLSA, according to the procedure described by Vega and Pérez (2006) for crude extracts treated with Pectinex and left un-treated. The plates were sensitised with asialo ovine submaxillary mucin (aOSM) isolate, using biotinylated Vicia villosa isolectin B4 as control (specific for Tn antigen) and streptavidin peroxidase as detection system.
Mucilage assay
Mucilage assay was performed using fresh nutlets, following Hedge’s recommendations (1970). A minimum 4 hour time was established for hydration (distilled water). Even though slight differences were detected in mucilage colour and degree of transparency, mucilage quantity was evaluated as being the following ratio: width of mucilage halo/seed width (smaller diameter), observed values varying between 0 and 3. Basic mucilage characteristics regarding colour, consistency and general appearance followed Hedge’s terminology (1970).
RESULTS AND DISCUSSION
Presence of mucilage
A- Subfamily Scutellarioideae and Teucrioideae.
None of the three species analysed in Scutellaria present mucilage (Table 2), agreed with Ryding (1992b) who pointed out the absence of mucilage in 10/10 species of Scutellaria. The three species analysed grew in humid or very humid environments, which could be related to the absence of mucilage. There is a complete absence of mucilage in Aegiphilla bogotensis (Spreng.) Moldenke (subfamily Teucrioideae), but the lack of data in the literature (Grubert 1974) prevents us making any inferences about this characteristic in this genus.
B- Subfamily Lamioideae.
Traces of mucilage were observed in the Stachys micheliana Briq. species (Table 2), suggesting that the presence of polysaccharide is infrequent in this genus, considering previous data in three Colombian species (Fernández-Alonso et al. 2003 ).
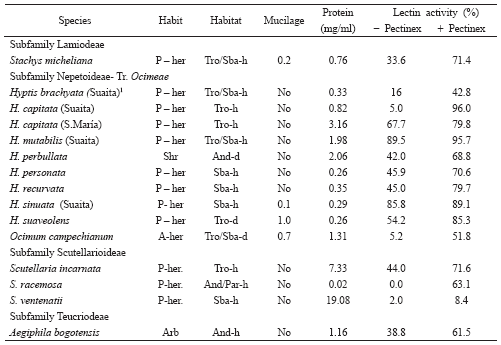
Table 2. Mucilage and lectin activity in species of Lamiodeae, Nepetoideae-Ocimeae, Scutellarioideae y Teucriodeae.
C- Subfamily Nepetoideae, tribe Ocimeae.
In that regarding the Ocimeae tribe, results obtained from analysing the Hyptis species (Table 2) revealed the absence of mucilage in 5 out of the 6 species whose seeds were collected from the field and three species cultured in greenhouses; this confirmed and extended the results obtained by Fernández-Alonso et al. (2003) in 6 additional species. The only specie from this genus where mucilage was detected was H. suaveolens (L.) Poit., from very large nutlets in which it was very conspicuous (1.0). This was in agreement with the data compiled by Grubert (1974) when prospecting myxospermy in seeds and qualitatively observed by Ryding (1992a, 1992b) who studied 23 species of Hyptis, finding that only 10 clearly exhibited myxocarpy (mucilage presence); this author found that H. recurvata Poit. and H. mutabilis (L. Rich.) Briq. nutlets did not present or are weakly mucilaginous.
The absence of myxocarpy in these species was confirmed by our semi-quantitative evaluation of the quantity of mucilage. The presence of mucilage in Ocimum campechianum Mill. was notable (0.7), (Table 2) confirming observations about this specie’s adhesiveness made by Grubert (1974) according to which mucilage has been detected in 14 species of Ocimum, indicating a particular characteristic for this genus. Ryding (1992a) has also pointed out the presence of mucilage in most of the 20 species studied (O. campechianum not being amongst them).
D- Subfamily Nepetoideae, tribe Mentheae.
Results in the Mentheae tribe revealed clear differences regarding the genus. Some previous work (Fernández-Alonso et al. 2003) showed the absence of mucilage in the Lepechinia genus; the results obtained in this work confirm this characteristic both in nutlets obtained from prospecting in the field and in nutlets obtained from plants cultivated in greenhouses (Table 3) and agree with the results obtained by Ryding (1992b) in 5 species from this genus. Colombian species of Lepechinia generally grow in high, humid areas of the Andean region with the exception of L. betonicifolia (Lam.) Epling which is usually found in subxerophytic environments.
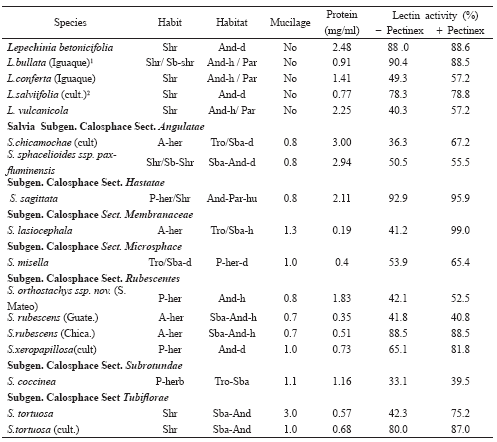
Table 3. Mucilage and lectin activity in species of Nepetoideae Tr. Mentheae.
1 In the case of previously studied taxons the new locality is specified.
2 Cultivated species
A-her : annual or biannual herbaceous ; P-her : perennial herbaceous ; Shr : Shrub 1-5 m; Sb-shr : Subshrub (having woody parts, being generally less than one metre in height); Sc-shr : Scandent shrub; Tro : Tropical area ranging from 0 – 1,000 m; Sba : Sub Andean area ranging from 1,000 – 2,200 m; And : Andean or High Andean area ranging from 2,200-3,300 m; Par : Paramo area ranging from 3,300 – 4,200 m; h : humid; d : dry.
The situation observed in Salvia was totally different, given that the presence of mucilage was constant and notable (0.7-3.0) (Table 3) in all species studied in this work (all belonging to the Calosphace genus). It should be pointed out that of the 36 accessions studied in total in this work and in our previous report, 33 had mucilage and it was lacking in just two species of Hastatae (Benth.) Epling section and in one from the Rubescentes (Epling) Epling section, without there seeming to have been correlation with the type of habitat from which they came (Fernández-Alonso et al. 2003). The predominance of Salvia species presenting myxocarpy have also been described by Oran (1997), who only found one out of 15 lacking mucilage and by Ryding (1992b) who pointed to the presence of mucilage en 70 out of 74 species. Apparently the absence of myxocarpy in Salvia is more frequent in Old World species (Subgenus Sclarea). Currently, in what is proposed as a possible dismembering of the Salvia genus, in response to the genus’ condition of being non-monophyletic as accepted today, it is possible that myxocarpy could be correlated to other morphological characteristics to better circumscribe new generic entities which are currently proposed as viable and which will raise the range of some of the Subgenera or Sections recognised today (Claben-Bockhoff et al. 2003, 2004; Walker et al. 2004).
An overall examination of the results obtained suggests that the lack of myxocarpy in Lepechinia, Hyptis, Stachys and Scutellaria genera is generalised and rarely species are found where mucilage is present. It is worth noting that, as far as we know, no studies have been done into the chemical structure or properties of these mucilages and given their abundance and ease of obtaining them in some species, particularly from the Salvia genus, it would be interesting to approach this field given the applications for this type of polysaccharide. The mucilage from some Hyptis (H. suaveolens) and Salvia (S. hispanica L.) species have been traditionally used as food in some communities, mainly in Mexico and Mesoamerica (Heinrich 1992; Cahill 2003).
Results as a whole do not show a clear correlation between the habitat, habit and the amount of mucilage found in the analyzed species.
Lectin presence
Given that lectin presence was evaluated by erythrocyte agglutination assays and ELLSA assay, analysing the results regarding the lectin presence and activity must take the following into account:
a) In those cases where erythrocyte haemolysis was observed (probable due to high polyphenol content in the extracts), erythroagglutination could not be evaluated but this does not in turn mean lectin absence.
b) Erythroagglutination assays and erythroagglutination titres were done with extracts which were not treated with Pectinex where observation of lectin activity was more difficult because of the high viscosity which some extracts presented.
The criteria used for evaluating whether there was recognition of the Tn antigen was based on the ELLSA assay given that it is more sensitive than erythroagglutination, meaning that negative results obtained with the latter do not necessarily mean the absence of lectins. On the other hand, the possibility of the presence of other determinants was eliminated in this assay, particularly the T antigen as this was absent from aOSM or asialo bovine submaxillary mucin (aBSM).
A- Lamioideae, Scutellarioideae and Teucrioideae subfamilies
The Scutellaria genus has ca. 22 taxa in Colombia, some still not having been taxonomically resolved; it is mainly concentrated in the cordilleras (Fernández-Alonso 1990; Harley & Paton 1998; Fernández-Alonso 2005) and in most cases one is dealing with infrequently found species in which it is difficult to find populations having a sufficient number of individuals allowing nutlets to be collected. It is interesting to note that Scutellaria ventenatii presented an absence of lectin even following treatment with Pectinex (Table 2) whilst the protein had high activity even without being treated with Pectinex in S. incarnata Vent.; the existence of anti-Tn lectins had not been described before in this genus.
Weak anti-Tn lectin activity (38.8%) was observed in the Aegiphila bogotensis species (Teucrioideae) which increased following treatment with Pectinex (Table 2); this activity was confirmed with the erythroagglutination assay (Table 4).
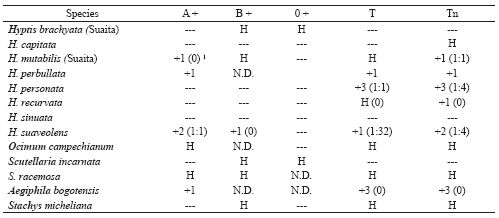
Table 4. Erythroagglutinating activity in species of Ocimeae, Lamiodeae and Scutellaroideae.
1 . Lectin titer is shown in parentheses.
+1 : Weak agglutination, +2 : Middle agglutination, +3 : Strong agglutination, +4 : Very strong agglutination
N.D. : Non- determined
H : Haemolysis
The Stachys genus has 12 species in Colombia (Epling 1934); 3 of them were analysed by ourselves (Fernández-Alonso et al. 2003) but only in an additional one (Stachys michelliana, a very frequent species in sub-Andean crops) did the extracts haemolyse RBC from different groups, no erythroagglutination activity thereby being detected in them (Table 4). However, activity was observed using ELLSA (33-64%) which appreciably increased with Pectinex digestion (Table 2). These results confirmed the presence of Tn-specific lectins in this genus concerning which there is no available information, except for one previous work (Fernández-Alonso et al. 2003).
B- Subfamily Nepetoideae tribe Ocimeae.
Most Hyptis species only presented Tn erythrocyte agglutination (Table 4) and T erythrocyte agglutination was weak or non-existent with the exception of H. personata Epl. The case of H. suaveolens is worth noting as it showed the absence of specificity for erythrocytes with Tn antigen, confirming that described by Bird (1960) who found erythroagglutination activity for group A with this species. Titre determinations confirmed the previous results and allowed an idea to be obtained of the relative quantity of lectin; H. personata apparently showed greater specificity for the Tn group. The results obtained by ELLSA established the presence of Tn-specific lectins in the Hyptis genus since values indicating high lectin activity were even obtained in extracts which had not been treated with Pectinex, except H. capitata Jacq. (Suaita) and H. brachiata Briq. (Suaita) (Table 2). It is worth mentioning that Pectinex treatment (as well as increasing ELLSA values) led to detecting the presence of lectins in these two species which would otherwise have been catalogued as lacking lectin.
The set of results revealed the presence of Tn-specific lectin in H. capitata, H. personata and H. recurvata for the first time and, if the results of Fernández et al. (2003) are considered, from a total of 10 species, all had anti–Tn lectin. Except for the Bird’s work (1960) with Hyptis suaveolens, the extensive Hyptis genus (Epling, 1949) has remained unexplored regarding lectins and there is just the one report by Bird and Wingham (1982) which includes an undetermined Hyptis specie as possessing anti–Tn lectin. The absence of mucilage and high lectin activity observed suggested that this genus is a very convenient source for obtaining Tn antigen-specific lectins. Brazil is the centre of diversity for the Hyptis genus having more than 150 species and there is generally a good representation in the rest of the neotropical countries, 42 species being found in Colombia.
The Ocimum genus, which is mainly paleotropical but has an important number of naturalised species in America (Epling, 1935-37; Paton, 1992; Albuquerque et al., 1998), has at least 6 species in Colombia (unpublished data) of which just one, O. campechianum, is native to Colombia and was analysed during this work. Another four naturalised species in Colombia will soon be described (Fernández-Alonso et al., in preparation). Appreciable lectin activity was observed in the aforementioned species following digestion with Pectinex (Table 2), this being the first time that the presence of lectins able to recognise the Tn antigen has been described in this genus; this was confirmed with erythroagglutination assays (Table 4).
C- Subfamily Nepetoideae tribe Mentheae.
We currently have information about 3 genera and 40 species analysed from the Mentheae tribe which presents the greatest diversity in Colombia (111 species); 18 of these species belonging to the Lepechinia and Salvia genera are included in this work.
Lepechinia
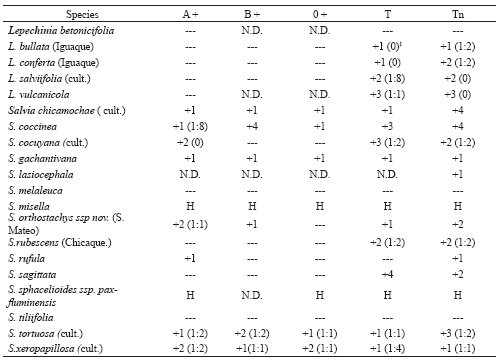
Five species from the ten taxa present in Colombia (species and subspecies) were analysed, representing 1/3 of those known in this neotropical genus (Wood 1998; Fernández-Alonso 2002). The erythroagglutination assay of the five taxa analysed revealed that 4 of them were able to agglutinate T- and Tn-erythrocytes (Table 5); L. betonicifolia surprisingly seemed to lack erythroagglutination activity which is why it was not included in titre assays confirming in the four taxa similar agglutination for T- and Tn-RBC. The ELLSA assay revealed that high Tn-specific lectin activity could be detected in all species (Table 3) and that treatment with Pectinex frequently increased observed activity, just like that observed in previous work (Fernández-Alonso et al. 2003). This is the first time that the presence of lectins able to recognise Tn antigen has been described in L. vulcanícola (57.2%) and L. betonicifolia (88.6%); appreciable variations have been found both in protein content and lectin activity in taxa corresponding to previously studied species (Fernández-Alonso et al. 2003), but originating in other localities. Ongoing work in our laboratory is currently dealing with isolating and characterising lectin from L. bullata (Kunth) Epling, leading to establishing some properties of lectins from the Lepechinia genus.
Table 5. Erythroagglutinating activity in species of Nepetoideae Tr. Mentheae.
1 . Lectin titer is shown in parentheses.
+1 : Weak agglutination, +2 : Middle agglutination, +3 : Strong agglutination, +4 : Very strong agglutination
N.D. : Non- determined
H : Haemolysis
Salvia
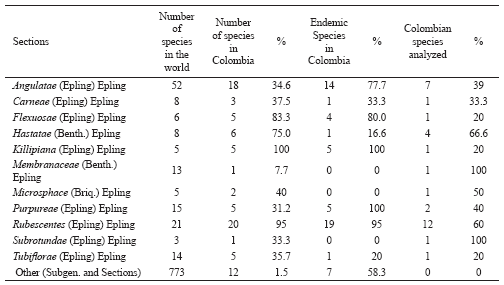
The number of Colombian taxa studied in this work rose to 32 belonging to 11 sections from a total of 80 native taxa belonging to 17 sections represented in Colombia (Fernández-Alonso 1995b, 1998, 2003a, 2006). Eight of these, belonging to 7 different sections, were analysed here for the first time (Annex 1). The first data is presented for species from the Membranaceae (Benth.) Epling., Microsphace (Briq.) Epling., Subrotundae (Epling) Epling and Tubiflorae (Epling.) Epling sections. The results are presented in Table 3 and Table 5.
Annex 1. Diversity of species in the Salvia Sections (Subgenus Calosphace) and percentage of species analyzed.
Salvia sect. Angulatae and Hastatae
Representation of the Angulatae (Epling) Epling section in Colombia has recently increased in several new species and subspecies (Fernández-Alonso 2003a) opening up new perspectives respecting characterising lectins in this group which has shown important activity in the taxa analysed to date. 55% activity was presented in S. sphacelioides, related to the S. bogotensis complex, having similar values to those observed in other species from this group. Similar values were obtained in nutlets obtained from cultivated S. chicamochae plants to those found before in wild plants. Lectin from S. bogotensis has been recently characterised in this group (Vega & Pérez 2006).
Salvia sect. Membranacea and Microsphace
The Membraneacea (13 spp, 1 in Colombia) and Microsphace (5 spp., two in Colombia) sections group small herbaceous, annual or perennial plants usually growing in altered or disturbed environments such as banks and landslides near roads or river banks and undercut banks. They are generally aromatic and have very small bluish or pinkish flowers. Salvia lasiocephala Hook. and Arn. presented high lectin activity (99%) following treatment with Pectinex, being weak before such treatment was applied (41%) probably due to the important quantity of mucilage present in the nutlets. By contrast, there was little increase in S. misella Kunth following treatment, rising from 53% to 65% activity. Even though Salvia lasiocephala seeds are very small, it is an easily cultivated annual species producing numerous spikes of flowers when grown in good fertiliser and humidity conditions and, as such, enables obtaining an easy supply of nutlets for extraction.
Salvia sect. Rubescentes
This is an almost endemic section in Colombia (Fernández-Alonso 2003b) already having had important prospecting; data is included here concerning four of the species, basically confirming the situation observed in the first prospecting. Greater lectin activity was observed in nutlets obtained from cultivated S. xeropapillosa (S. orthostachys complex) plants than in those obtained from wild plants (first prospecting). This could have been due to the presence of some degree of introgression / intro-regression in culture conditions between S. xeropapillosa and other species from the same complex presenting important activity (S. orthostachys Epl. or S. gachantivana Fern. Alonso) which were cultivated in the same plots of land. Higher activity (88%) was observed in S. rubescens subsp. rubescens in the variety from the Chicaque (Cundinamarca) which lies above 2,200 m. On the contrary, low activity (40%) was presented in a variety which grows in low regions (Guateque, less than 1,500 m, on the slopes of the Orinoco river basin).
Salvia sect. Subrotundae and Tubiflorae
The Subrotundae (3 spp, 1 in Colombia) and Tubiflorae (14 spp., 1 in Colombia) sections, in which the first Colombian species were studied, both presented lectin activity. There was discrete activity in S. coccinea Ettlinger without Pectinex (33%) and an increase with the treatment could only just be shown. By contrast, activity was greater in S. tortuosa Kunth in both nutlets obtained in the field and those obtained in culture conditions, ranging from 75% to 87% and increasing with Pectinex treatment.
Regarding erythroagglutination ability, the results (Table 5) revealed that S. melaleuca Epling and S. tillifolia Vahl did not agglutinate RBCs from any blood type; S. misella and S. sphacelioides subsp. pax-fluminensis haemolysed erythrocytes and the rest of the species analysed agglutinated erythrocytes carrying the Tn antigen; an important number of species recognised A or T determinants, frequently having lower titres, implying that one must be very careful when defining anti-Tn specificity.
Comparing these results with those obtained in ELLSA assay (Table 3), it can be seen that the presence of lectins recognising the Tn antigen could be detected in all the species, including those producing negative results for erythroagglutination. The high levels of lectin activity observed in S. sagittata (95.9%), S. lasiocephala (99.0%), S. rubescens (Chicaque) (88.5%) and S. tortuosa (87.0%) should be highlighted as they were identified as anti–Tn-lectin-rich sources. The set of results in Salvia show the presence of lectins in another 6 neotropical species from the Calosphace subgenus for the first time and extends evidence regarding the presence of lectins able to recognise Tn antigen to 32 species.
Except for the work of Fernández-Alonso et al. (2003), previous work has concentrated on qualitatively describing the presence of mucilage (or lack of it) or lectins recognising the Tn antigen without complementing these data with erythroagglutination titer assays. Semi-quantitative data is presented here about mucilage and erythroagglutination ability as well as determinations of lectin activity by ELLSA giving a better idea of lectin levels and using Pectinex treatment to reveal lectin in species which otherwise would have been considered as lacking this type of protein.
Given the diversity of native Labiatae species in Colombia (including the genera recently ascribed to the former Verbenaceae) and considering the appreciable quantity of exotic Labiatae which are currently being cultivated or found to be naturalised in Colombia, it is planned to continue short- and medium- term prospecting. An ongoing study deals with mucilage and lectins in around 30 species belonging to different genera; most of them are Old World plants, having occasionally been naturalised or cultivated in Colombia.
ACKNOWLEDGMENTS
We would like to thank the Universidad Nacional de Colombia (DIB) and COLCIENCIAS for financing this project’s fieldwork and laboratory work. We would also like to thank the Universidad Nacional’s Chemistry Department and the Institute of Natural Sciences for the facilities provided for carrying out the present study, Francisco Sánchez and Gustavo Morales from the José Celestino Mutis Botanical Gardens in Bogotá for the help received in culturing and preserving the live Labiatae collection for more than 8 years in their installations, Wilson Piedrahita from the Faculty of Agronomy and Fany Mena from this University’s maintenance section for having made space available in the greenhouse for propagating some species, directors of the Universidad de Nariño and colleagues Ayda Patiño and Bernardo Ramírez for having facilitated fieldwork in the Nariño department and Carlos Aedo from the Botanical Garden in Madrid for his collaboration in obtaining the literature.
LITERATURE CITED
1. Albuquerque, U. P. & L.c. Andrade. 1998. El género Ocimum (Lamiaceae) en el nordeste del Brasil. Anales Jard. Bot. Madrid 56: 43-64. [ Links ]
2. Bird, G.W.G. 1960. Anti-A agglutinins from the seeds of Hyptis suaveolens Poit. Brit J. Haemat. 6: 151-153. [ Links ]
3. Bird, G.W.G. & J. Wingham. 1974. Hemagglutinins from Salvia. Vox-Sang. 26: 163-166. [ Links ]
4. Bird, G.W.G. & J. Wingham. 1976. More Salvia agglutinins. Vox-Sang. 30: 217-219. [ Links ]
5. Bird, G.W.G. & J. Wingham. 1977. Yet more Salvia agglutinins. Vox-Sang. 32: 121-122. [ Links ]
6. Bird, G.W.G. & J. Wingham. 1982. More Tn-specific lectins from seeds of Labiatae genus: Hyptis sp. Chan, Salvia lyrata and Marrubium velutinum. Clin. Lab. Haematol. 4: 403-404. [ Links ]
7. Cahill, J. P. 2003. Ethnobotany of Chia, Salvia hispanica L. (Lamiaceae). Econ. Bot. 57: 604-618. [ Links ]
8. Cantino, P.D. 1992a. Evidence for a polyphyletic origin of the Labiatae. Ann. Missouri Bot. Gard. 79: 361-379. [ Links ]
9. Cantino, P.D. 1992b. Toward a phylogenetic classification of the Labiatae. pp In: R.M. Harley & T.Reynolds (eds.). Advances in Labiatae Science. Royal Botanic Gardens. Kew. [ Links ]
10. Cantino, P.D.& R.V. SANDERS. 1986. Subfamilial classification of Labiatae. Syst. Bot. 11: 163-185. [ Links ]
11. Cantino, P.D. R.M. Harley & S.J.Wagstaff. 1992. Genera of Labiatae: Status and Classification. pp. 511-522. In: R.M. Harley & T. Reynolds (eds). Advances in Labiatae Science. Royal Botanic Gardens. Kew. [ Links ]
12. Claben-Bockhoff, R., P. Wester & E. Tweraser. 2003. The staminal lever mechanism in Salvia -A review. Plant. Biol. 5: 33-41. [ Links ]
13 .Claben-Bockhoff, R., T. Speck, E. Tweraser, P. Wester, S. Thimm & M. Reith. 2004. The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptative radiation?. Organisms Diver. Evol. 4: 189-205. [ Links ]
14. Duk, M., D. Mitra, E. Lisowska, E.A. Kabat, N. Sharon & H. Lis. 1992. Immunochemical studies on the combining site of the A+N blood type specific Moluccella laevis lectin. Carbohydr. Res. 236: 245-258. [ Links ]
15. Epling, C. 1934. Preliminary revision of American Stachys. Rep. Spec. Nov. Reg. Veg. Beih. 80: 1-75. [ Links ]
16. Epling, C., 1935-37. Synopsis of the South American Labiatae. Rep. Spec. Nov. Reg. Veg. Beih. 85: 1-341. [ Links ]
17. Epling, C. 1949. Revisión del género Hyptis (Labiatae). Rev. Mus. La Plata, secc. Bot. 7: 153-497. [ Links ]
18. Fernández-Alonso, J. L. 1990. Notas sobre Scutellaria (Labiatae) en Colombia y Ecuador. Anales Jard. Bot. Madrid 47: 105-123. [ Links ]
19. Fernández-Alonso, J. L.,1995a. Estudios en Labiatae de Colombia I. Novedades en los géneros Salvia e Hyptis. Rev. Acad. Colomb. Cienc. 19: 469-480. [ Links ]
20. Fernández-Alonso, J. L. 1995b. Estudios en Labiatae de Colombia II. Novedades en Salvia sect. Longipes Epling. Anales Jard. Bot. Madrid 53: 41-46. [ Links ]
21. Fernández-Alonso, J. L.1998 . Estudios en Salvia (Labiatae) de Colombia. p. 296. Resumenes VII Congreso Latinoamericano de Botánica. Ciudad de Mexico. [ Links ]
22. Fernández-Alonso, J. L. 2003a. Estudios en Labiatae de Colombia IV. Novedades en Salvia y sinopsis de las Secciones Angulatae y Purpureae. Caldasia 25: 235-281. [ Links ]
23. Fernández-Alonso, J. L. 2003b. Algunos patrones de distribución y endemismo en plantas vasculares de los páramos de Colombia. pp. 213-240. In: Congreso Mundial de Páramos, Memorias tomo I Paipa 2002. Ministerio de Medio Ambiente. [ Links ]
24. Fernández-Alonso, J. L. 2005. “Estudios en Labiatae de Colombia V. Nuevo nombre para Scutellaria leptosiphon Epling, planta redescubierta en la Cordillera Oriental de Colombia. Rev. Acad. Colomb. Cienc. 29: 319-324.
25. Fernández-Alonso, J. L. 2006. “Estudios en Labiatae de Colombia VII. Salvia yukoyukparum, nueva especie y primer representante de la Sección Tomentellae en Colombia. Novon (submitted)
26. Fernández-Alonso, J.L., N. Vega, J.J. Filgueira & G. Pérez. 2003. Lectin prospecting in Colombian Labiatae. A systematic-ecological approach. Biochem. System. Ecol. 31: 617-633. [ Links ]
27. Grubert, M. 1974. Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 8: 315-551. [ Links ]
28. Harley, R. M. & A. Paton. 1998. Notes on New Word Scutellaria. Kew Bull. 54: 221-225. [ Links ]
29. Harley, R. M., S. Atkins, A. L. Budantsev, P. D. Cantino, B. J. Conn, R. Grayer, M. M. Harley, R. De Kok, T. Krestovskaja, R. Morales, A. J. Paton, O. Ryding & T. Upson 2004. Labiatae. In J. W. Kadereit (ed.), K. Kubitzki (ed. in chief). The Families and Genera of Vascular Plants VII. Flowering Plants. Dicotyledons: Lamiales (except Acanthaceae including Avicenniaceae): 167-275. Springer. Berlin. [ Links ]
30. Hedge, I.C. 1970. Observations on the mucilage of Salvia fruits. Not. Royal. Bot. Gard. Edinburgh 30: 79-95. [ Links ]
31. Heinrich, M. 1992. Economic botany of American Labiatae. In: R.M. Harley & T. Reynolds (eds.) Advances in Labiatae Science. Royal Botanic Gardens, Kew, pp. 475-488. [ Links ]
32. Hirohashi, S., H. Clausen, T. Yamada, Y. Shimosato & S-I. H. Hakomori. 1985. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC Lu-35 and 81 expressed in cancer of blood group O or B individuals. Its identification as Tn antigen. Proc. Natl. Acad. Sci. U.S.A. 82: 7039-7043. [ Links ]
33. Holmgren, P.K., N.H. Holmgren & L.C. Barnett. 1990. (eds.). Index Herbariorum, I. New York Botanical Garden. [ Links ]
34. Lopez-Palacios, S. 1977. Flora de Venezuela - Verbenaceae. Universidad de los Andes. Mérida, pp 654. [ Links ]
35. Lopez-Palacios, S. 1986. Lista preliminar de las Verbenaceae existentes en Colombia con algunos de sus usos y nombres vulgares. Caldasia 15: 155-176. [ Links ]
36. Lis, H., H. Latter, R. Adar, & N. Sharon. 1988. Isolation of two blood type A and N specific isolectins from Moluccella laevis seeds. FEBS Letters, 233: 191-195. [ Links ]
37. Medeiros, A., S. Bianchi, J. Calvete, H. Balter, S. Bay, A. Robles, D. Cantacuzene, D.M. Nimtz, P. Alzari & E. Osinaga. 2000. Biochemical and functional characterization of the Tn-specific lectin from Salvia sclarea seeds. Eur.J.Biochem. 267: 1434-1440. [ Links ]
38. Olmstead, R. G., K.M. Scott & J.D. Palmer. 1992. A chloroplast DNA phylogeny for the Asteridae: implications for the Lamiales. pp. 19-25. In: R.M. Harley & T. Reynolds (eds.). Advances in Labiatae Science. Royal Botanic Gardens. Kew [ Links ]
39. Oran, S.A., 1997. Nutlet anatomy of the genus Salvia L. in Jordan. Flora Mediterranea. 7: 27-40. [ Links ]
40. Paton, A. 1992. A synopsis of Ocimum L. (Labiatae) in Africa. Kew Bull. 47: 403-435 [ Links ]
41.Pérez, G. 1984. Isolation and characterization of a lectin from the seeds of Erythrina edulis. Phytochemistry 23: 1229-1232. [ Links ]
42. Piller, V., F. Piller & J. Cartron.1986. Isolation and characterization of N-acetylgalactosamine specific lectin from Salvia sclarea seeds. J. Biol. Chem. 261: 14069-14075. [ Links ]
43. Ryding, O. 1992a. Pericarp structure and phylogeny within Lamiaceae subfamily Nepetoideae tribe Ocimeae. Nord.J. Bot. 12: 273-298. [ Links ]
44. Ryding, O., 1992b. The distribution and evolution of myxocarpy in Lamiaceae. pp. 85-96. In: R.M. Harley & T. Reynolds (eds.), Advances in Labiatae Science. Royal Botanic Gardens, Kew. [ Links ]
45. Ryding, O. 1995. Pericarp structure and phylogeny of the Lamiaceae-Verbenaceae. Plant Syst. Evol. 198: 101-141. [ Links ]
46. Ryding, O. 2001. Myxocarpy in the Nepetoideae (Lamiaceae) with notes on myxodiaspory in general. Syst. Geogr. Pl. 71: 503-514. [ Links ]
47. Springer, G.F. 1984. T and Tn, general carcinoma autoantigens. Science 244: 1198-1206. [ Links ]
48. Steane, D.A., R.W. Scotland, D.J. Mabberley, S.J. Wagstaff, P.A. Reeves & R.G. Olmstead. 1997. Phylogenetic relationships of Clerodendrum s.l. (Lamiaceae) inferred from chloroplast DNA. Syst. Bot. 22: 229-243. [ Links ]
49. Teneberg, S.I. Leonardsson, M.J. Angstrom, S. Ehrlich-Rogozinski & N. Sharon. 1994. Characterization of the specificity of binding of Moluccella laevis lectin to glycosphingolipids. Glycoconj. J. 11: 418-423. [ Links ]
50. Thurnher, M.,H.clausen, N. Sharon & E.G. Berger. 1993. Use of O-glycosylation-defective human lymphoid cell lines and flow cytometry to delineate the specificity of Moluccella laevis lectin and monoclonal antibody 5F4 for the Tn antigen ( GalNAc α1-O-Ser/Thr). Immunol.Lett. 36: 239-244.
51. Vega, N. & G. Pérez, G. 2006. Isolation and characterization of a Salvia bogotensis seed lectin specific for the Tn antigen. Phytochemistry 67: 347-355.
52. Wagstaff, S.J. & R.G. Olmstead. 1997. Plylogeny of Labiatae and Verbenaceae inferred from rbcL sequences. Syst. Bot. 22: 165-179. [ Links ]
53. Wagstaff, S.J., L. Hickerson, R. Spangler, P.A. Reeves & R.G. Olmstead. 1998. Plylogeny in Labiatae s.l., inferred from cpDNA sequences. Plant Syst. Evol. 209: 265-274. [ Links ]
54. Walker, J.B., K.J. Systma, J. Treutlein & M. Wink. 2004. Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation and ecological specializations of Salvia and Tribe Mentheae. Amer. J. Bot. 91: 1115-1125. [ Links ]
55. Wang, W., W.J. Peumans, P. Rougé, C. Rossi, P. Proost, J. Chen & E.J.M. Van Damme. 2003a. Leaves of the Lamiaceae species Glechoma hederacea (ground ivy) contain a lectin that is structurally and evolutionary related to the legume lectins. Plant J. 33: 293-304. [ Links ]
56. Wang, W.,B. Hause, W.J. Peumans, G. Smagghe, A. Mackie, R. Fraser & E.J.M. Van Damme. 2003b. The Tn antigen-specific lectin from ground ivy is an insecticidal protein with an unusual physiology. Plant Physiol. 132: 1322-1334. [ Links ]
57. Wood, J.R.I. 1989. The genus Lepechinia (Labiatae) in Colombia. Kew Bull. 43: 291-301. [ Links ]
Recibido: 06/06/2006
Aceptado: 08/11/2006