Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Caldasia
versão impressa ISSN 0366-5232versão On-line ISSN 2357-3759
Caldasia v.32 n.1 Bogotá jan./jun. 2010
Comparación de la dieta y el uso de bromelias entre anuros en un afloramiento rocoso en el sudeste de Brasil
WESLEI PERTEL
ROGÉRIO L. TEIXEIRA
RODRIGO B. FERREIRA
Museu de Biologia Prof. Mello Leitão, Av. José Ruschi, 4, Centro, 29650-000, Santa Teresa, Espírito Santo, Brasil.
Museu de Biologia Prof. Mello Leitão, Av. José Ruschi, 4, Centro, 29650-000, Santa Teresa, Espírito Santo, Brasil. Correspondence: Department of Biology, Utah State University 5305 Old Main Hill, Logan, UT, USA. rbfherpeto@gmail.com
ABSTRACT
Anurans from an inselberg in southeastern Brazil were studied using a sample of sixty tank bromeliads Alcantharea sp. We found 153 tadpoles of S. arduous, 21 adults of S. arduous, 30 adults of T. miliaris, and two individuals of Scinax x-signatus, which were not considered in our analyses. Tadpoles of S. arduous were present in 35% of the analyzed plants. Adults of S. arduous (bromeligeneous) occurred in 25% of analyzed plants, while adults of T. miliaris (bromelicolous) occurred in 30%. Apparently the presence of toe pads in S. arduous allows them to occupy the center portion of bromeliads, while T. miliaris, which do not have pads on their toes, used the base of the plant axils for residency. The number of anuran species and the abundance of individuals found were low. This may be a result of the high altitude of our studied site or a restriction imposed by the saxicolous environment, such as high temperatures and low humidity during the day. Both species can be considered generalist feeders due to their wide variety of ingested prey. Formicidae was their main prey but was absent inside the bromeliads. Blattodea was very common inside the bromeliad axils and represented the most significant prey by weight in both frog species. We can conclude that both anurans forage inside and outside of bromeliads. The trophic niche breadth in S. arduous was larger than in T. miliaris. Even though both species are common inhabitants of the same environment, they demonstrated a marked spatial segregation in the bromeliads. Based on their diet, however, there may be disputes for territory outside of the bromeliads.
Key words. Scinax arduous, Thoropa miliaris, Hylidae, Cycloramphidae, bromeliads.
RESUMEN
Se estudiaron los anuros que usan las rosetas de 60 bromelias del género Alcantharea, en un afloramiento rocoso del sudeste de Brasil. Se encontraron 153 larvas y 21 adultos de Scinax arduous, 30 adultos de Thoropa miliaris, y dos individuos de Scinax x-signatus, éstos últimos no se incluyeron en los análisis. Las larvas de S. arduous se encontraron en el 35% de las plantas analizadas. Los adultos de S. arduous se presentaron en el 25% de las plantas estudiadas, mientras que los adultos de T. miliaris se encontraron en el 30% de las plantas. Aparentemente, la presencia de ventosas en las patas de S. arduous permite que los individuos de esta especie ocupen la porción central de las bromelias, en tanto que los individuos de T. miliaris, los que carecen de dichas ventosas en sus patas, ocupan las axilas inferiores de las hojas de las bromelias. El número de especies de anuros y la abundancia de individuos fue relativamente baja. Esto puede ser el resultado de la apreciable altitud del área estudiada o por las restricciones impuestas por el ambiente saxícola del área investigada, la cual se caracteriza por presentar elevadas temperaturas y baja humedad durante el día. Ambas especies pueden ser consideradas como consumidores generalistas. Los formícidos constituyeron la principal presa en número, aunque éstos no se registraron en las rosetas de las bromelias. Por su parte, los Blattodea fueron muy comunes dentro de las bromelias, los cuales representaron una presa más importante en peso para las dos especies de anuros. Se puede concluir que ambas especies de anuros forrajean en la parte interna como en la externa de las bromelias. El nicho trófico de S. arduous demostró ser más amplio. Aunque ambas especies de anuros son habitantes comunes del mismo ambiente, éstas presentan una marcada segregación espacial en las bromelias. No obstante, teniendo como base su dieta, las dos especies de anuros disputan territorio por fuera de las bromelias.
Palabras clave. Scinax arduous, Thoropa miliaris, Hylidae, Cycloramphidae, bromelias.
Recibido: 03/07/2009
Aceptado: 14/10/2009
INTRODUCTION
The major dimensions of niches are time, space, and food (Pianka 1994). Ecological differences in these three dimensions may reduce competition and facilitate the coexistence of a variety of species (Pianka 1975). In relation to feeding habits, anurans are considered opportunists and most of them act as ambush predators of small arthropods. Their diet should reflect their ability to be effective predators, the abundance of their potential prey, and also the microhabitat where they live (Duellman & Trueb 1994, Giaretta et al. 1998).
Bromeliads are one of the habitats used by anurans. Most studies of anurans using bromeliads have been conducted in restingas (i.e. a group of vegetation types occupying sandy coastal plains) (Schineider & Teixeira 2001, Mesquita et al. 2004, Teixeira & Rödder 2007). Little effort has been made to study these ecological relationships in inselbergs. This term denotes solitary; usually monolithic mountains or groups of mountains that rise abruptly from surrounding plains (Bornhardt 1900). This ecosystem generally contains extreme conditions, which makes colonization for many anuran species difficult. Microclimate conditions on rocky surfaces can vary greatly throughout the day and seasons (Teixeira et al. 2006). Bromeliads in this habitat have an important role in maintaining diversity. These plants are capable of collecting and storing large amounts of rainwater, thereby offering shelter, humidity, food to the fauna, and a possible reproductive site for some species (vide Jiménes 1994, Vrcibradic & Rocha 1996, Dejean & Olmsted 1997, Lehtinen et al. 2004). In addition, bromeliads commonly occur on rocky walls where inclination can reach up to 90º, providing a stable shelter and security from predators.
In order to hide, reproduce, and feed inside the plant axils, anurans possess some morphological and behavioral adaptations (McDiarmid & Foster 1975, Altig & Johnston 1989, Hödl 1990, Lehtinen et al. 2004). Scinax arduous (Peixoto, 2002) is a species well adapted to live inside bromeliads. It is considered a bromeligenous species since it spends its entire life cycle inside bromeliads (Peixoto 1995). Its toe pads probably facilitate its success in spatially using these plants. Even with bromeliads being widespread throughout the New World (Benzing 1980), S. arduous has a limited geographic distribution since it is known only to its type locality, Santa Teresa in southeastern Brazil. In contrast, T. miliaris (Spix 1824) does not use bromeliads as a reproductive site and therefore, is classified as a bromelicolous species (Teixeira & Rödder 2007). Another difference between the species is that although T. miliaris is a widely distributed species along the Atlantic Rainforest, it is not adapted to climb due to the absence of a toe pad. It is a specialized species known for inhabiting rocky formations partly covered with vegetation and moistened by dripping water (Sazima 1971). All the life stages of T. miliaris can be found on the wet stones (Giaretta & Facure 2004).
During surveying at a saxicolous environment (inselbergs), we found S. arduous and T. miliaris sharing the same bromeliads. We, therefore, evaluated the diet of both ecologically different species, predicting S. arduous' diet is based on autochthones prey inside bromeliads while T. miliaris feeds on a variety of prey found outside of bromeliads. Additionally, we analyzed their spatial use of bromeliads, understanding that their morphology differences might influence their bromeliad occupancy. This is the first study to compare such ecological aspects between bromelicolous and bromeligenous anurans.
MATERIAL AND METHODS
Study Site
We studied anurans inhabiting the tank bromeliad Alcantharea sp. in a saxicolous habitat in Três Pontões (20o04' S, 41o02' W), located in the municipality of Afonso Cláudio, Espírito Santo State, southeastern Brazil. The rocky peak reaches 1 220 m of altitude. Vegetation is dominated by Bromeliaceae, Cactaceae, Orchidaceae, Velloziaceae, and Cyperaceae. However, the vegetation is rare and has a patchy spatial distribution with localized populations of Alcantharea sp. This bromeliad genus was the only one representing this family in the studied area and was very abundant, which was a determinant in choosing this area. This study site has been somewhat disturbed by tourism. Native plants like orchids and bromeliads have been taken from their natural habitat and traded for ornamental purposes. This activity might contribute to the spread the fauna that inhabits these bromeliads.
Afonso Cláudio, according to Köppen's Climate Classification, belongs to Aw Tropical type (Köppen 1936), with high temperatures, a rainy summer (December, January, February and March), and a dry winter (June, July, August and September). The average annual rainfall in the region of Afonso Cláudio is 1 150 - 1 300 mm, and the temperature varies from 7.3 to 27.80 C (Incaper 2009). Microclimates on the rock surfaces (inselberg studied here), however, can deviate from this pattern.
Samples
The field study was conducted by two collectors over three days: November 11th, 16th, and December 1st 2007, from 09:00 to 16:00. Data collection, however, was occasionally interrupted by periods of heavy rain. We examined 60 randomly chosen bromeliads located between 900 and 1 000 m altitude. The bromeliads were cut near the ground and shaken upside down into a plastic vase (0.8 m diameter). This is not an invasive method to the plant because this bromeliad genus absorbs nutrients and water needed through its leaves. Its roots work mainly to hold the plant in the soil; whenever possible the bromeliads were replanted. The pH, dissolved oxygen, and percentage of oxygen saturation of the water inside the bromeliads were measured using digital field equipment. The water temperature was measured using a mercury stick thermometer (0.5oC precision). For each parameter, measurements were replicated six times per plant at different plant axils. We collected arthropods from 30 bromeliads. To determine the dominant arthropod groups inhabiting the bromeliads, we collected the organisms and put them in labeled plastic sacs containing 70% alcohol. Anurans that were found in the bromeliads were stomach flushed using the method of Solé et al. (2005), and had their SVL measured with veneer calipers (to the nearest 0.1 mm). In the lab, using a binocular microscope, stomach contents were identified to Order and when possible to Family level. The relative importance of every prey type was assessed based on its frequency, number, and wet mass weighed with a digital balance (0.0001 g precision). All the weights of the contents were taken after removing excess water with absorbent paper. The number of tadpoles and eggs in clutches were also counted.
Statistical Procedures
We compared the mean SVL of anurans between sexes using one-way analyses of variance (ANOVA). The sex was the independent variable and SVL the dependent variable. Before applying ANOVA we used the Levene's test to evaluate the homoscedasticity of variances and the Kolmogorov-Smirnov test to evaluate normality as not to violate ANOVA's assumptions (Zar 1998). Whenever necessary, data was log transformed (log10 x + 1) before applying the tests.
To test whether S. arduous or T. miliaris use prey items in the same proportion that they occur in the plants or if they preferentially feed on select prey items, we estimated the frogs electivity (D) based on percentages of prey item numbers in each prey category and calculated with the Jacobs method (1974):
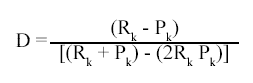
Where Rk = proportion of prey category -k- in stomach contents, and Pk = proportion of prey category -k- in the environment. D varies from +1 (complete preference for prey), through 0 (prey is taken in the same proportion found in the environment), to -1 (prey is present in the environment but absent in the diet). Agile insects were excluded because they were not adequately sampled in the bromeliads.
We calculated Simpson´s (1949) index to assess the trophic niche breadth (B) of both species using the equation:
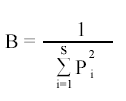
In this equation pi is the proportion of prey category in relation to all categories, and S is the richness of the prey categories.
RESULTS
Only three anuran species were found inside the bromeliads of the inselberg researched: Thoropa miliaris, Scinax arduous, and Scinax x-signatus. Of Scinax x-signatus, only two specimens were found, thus S. x-signatus is not included in the present comparison. This is the first record of S. arduous outside its type locality. This study extended its geographical distribution 50 km southward.
We collected 153 tadpoles, 21 adults of S. arduous and 30 adults of T. miliaris. Tadpoles occurred in 35% of the examined bromeliads, adults of S. arduous in 25%, and adult T. miliaris in 30%. The mean number of S. arduous eggs in the four clutches was 89.1 ± 9.8. The number of tadpoles per plant varied from one to 49. When high numbers of tadpoles were found in one bromeliad, the diversity of larval stages increased. The number of both S. arduous and T. miliaris adults varied between one and three individuals per plant. Adults of S. arduous (N=17; N%= 81) occupied the central portion of the plants, while adults of T. miliaris (N= 26; N%= 87) occupied the bases. In a bromeliad's base one specimen of T. miliaris was found being digested by the spider Cteniza sp. Latreille, 1829 (Mygalomorpha, Ctenizidae).
We caught 17 males and four females of S. arduous. The males measured 17.6 ± 2.9 mm in SVL (range 12.8 to 20.6 mm), and females averaged 24.6 ± 0.5 mm (range 24.1 to 25.0 mm). The mean SVL differed significantly between the sexes (ANOVA: F1, 19 = 14.3; p < 0.01). We caught 26 males and four females of T. miliaris. The males varied in SVL from 21.1 to 37.4 mm (mean = 29.4 ± 5.4 mm), and females from 50.4 to 51.6 mm (Mean= 51.0 ± 0.8 mm). The mean SVL differed significantly between the sexes (ANOVA: F1, 27 = 18.9; p < 0.01).
We examined potential anurans prey from 30 bromeliads. We found representatives of the following groups: Arachnida, Insecta, and Myriapoda (Table 1). The insects dominated due to high numbers of Coleoptera, Blattodea, and Odonata larvae.
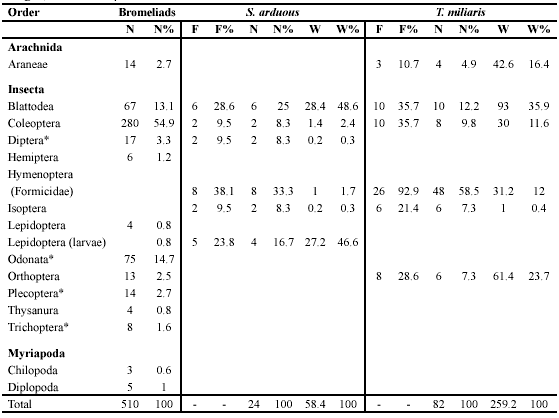
The stomach contents of 21 individuals of S. arduous were analyzed. All of them had ingested at least one type of prey, with only six types found overall (Table 1). Formicidae, were the most frequent prey item, although they are not found inside bromeliads. Blattodea represented the greatest wet weight. We analyzed the stomach contents of 29 individuals of T. miliaris, of which 28 had ingested at least one type of prey. For this species, the diet results were similar to those of S. arduous with only six types of prey (Table 1). Formicidae were also the most frequent and in greatest number and Blattodea dominated in wet weight.
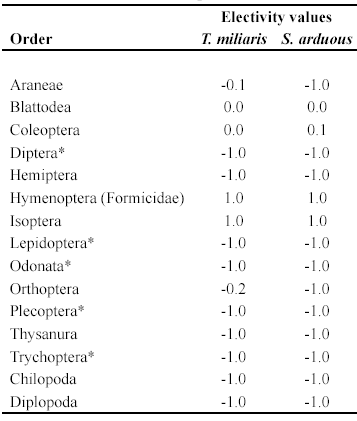
Of the arthropods found in the bromeliads, Coleoptera were the only prey category contained in the diet of S. arduous with a weak positive electivity value (D = 0.1). Blattodea were ingested by both species in the same proportions that they were found in the bromeliads (D = 0.0). Orthoptera had a weak negative electivity value in T. miliaris (D = -0.2). Aquatic larvae, although present in high numbers, were not included in the diet of either species (D = -1, all). All calculated values are presented in Table 2. The trophic niche breadth was not equal for both species, being larger in S. arduous (B = 4 500) than in T. miliaris (B = 2 631).
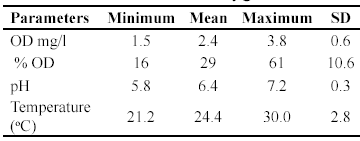
Values of dissolved oxygen of bromeliad's water varied from 1.5-3.8 mg/l, and the percentage of dissolved oxygen varied between 16 and 61% (Table 3). The pH was slightly acidic, ranging from 5.8-7.2, and temperature fluctuated from 21.2- 30.0oC.
DISCUSSION
Our study site presented one of the lowest anurans richness when compared to other studies on the amphibian-bromeliad communities from Atlantic Rainforest in the State of Espirito Santo (Teixeira et al. 1997, Schineider et al. . 2000, Schineider & Teixeira 2001, Teixeira et al. 2002, Mesquita et al. 2004, Pertel et al. 2006, Teixeira et al. 2006, Teixeira & Rödder 2007). Although comparisons among those cited studies might not be accurate, their richness varies from one to six anurans species. The lowest rate of richness, only one species, was found by Teixeira et al. (1997), studying restinga, which has very similar abiotic conditions to inselbergs. With this exception, all others studies collected more species than our studied site and Teixeira et al. (2006), which found only two species. Both studies were conducted in inselbergs above 850 m of sea level, which possess severe conditions for many anuran species (Teixeira & Rodder 2007). Even situated in a rainforest, inselbergs form edaphically and microclimatically -xeric islands- (e.g. in Cote d'Ivoire, for details see Szarzynski 1993). The open exposed rocks are subject to high degrees of insolation in combination with high evaporation rates. The temperatures on the rock surface regularly reach 60º C at noon, while relative air humidity falls below 30% (Porembski et al. 1998). These aspects indicate that inselbergs are probably the driest study environment compared to the Atlantic Rainforest ecosystems previously studied. Therefore, restrictions imposed by microclimate conditions may be the limiting factors for anuran colonization at our study site. Additionally the vegetation of inselbergs is relatively sparse, offering few shelters to vertebrates and is restricted to species adapted to live on rocks or in bromeliads (Teixeira et al. 2006).
According to selective pressures in the environment, some anuran species developed strategies allowing them to take advantage of the use of microhabitats (Juncá & Borges 2002, Teixeira & Rödder 2007). Thoropa miliaris appears to be successful in colonizing saxicolous habitats occupying 30% of our evaluated bromeliads, whereas Teixeira et al. (2006) encountered them in 56.7% of the plants. Besides, T. miliaris represented 83.8% of all anuran fauna encountered in that inselberg area by Teixeira et al. (2006). Its habit of using bromeliads regularly as diurnal shelter is essential to protecting themselves from the extreme external abiotic conditions. The other species, Scinax arduous, was also shown to adapt to living in inselberg bromeliads since they occupied 20% of our evaluated plants. Although both species are well adapted to living in bromeliads and sometimes sharing the same plant, they do not compete for reproductive sites (reproductive mode, S. arduous = 6 and T. miliaris = 9 sensu Haddad & Prado 2005). Moreover, both species appear to have different strategies for exploiting the available habitat. Scinax arduous occupies the central portion of the plants where water fills the axils; this being likely a product of adhesive pads at the tips of their digits. In contrast, T. miliaris, which lacks the pads, occupies the base and the bromeliad axils where little or no water is stored. As we expected, there is space partitioning occurring inside the bromeliads between these species, therefore, competition does not occur.
Although we have no data about seasonal changes in the analyzed habitat, we detected strong variations in the abiotic factors in the bromeliad Alcantharea sp. through this short sampling period. The low rate of dissolved oxygen, the slightly low pH in the stored water inside the bromeliad axils, and the possible variation of these rates may influence the survival of tadpoles. Additionally, rates may vary strongly during a daily cycle due to temperature fluctuations. Conditions may become more stable and suitable during the rainy season than during the dry season due to a more consistent water supply.
Predators inside bromeliads are an additional concern to tadpoles as well as adults. Tank bromeliads are broadly used by invertebrates, and some of them, such as Arachnida (spiders and scorpions), Blattodeae, and Chilopoda may prey upon, or compete with, anuran species (McCormick & Polis 1982, Teixeira et al. 2006). Arachnids are known to predate on amphibians (e.g., Lycosidae: Kwet 1999, 2001, Ctenidae: Hödl 1993, Pisauridae: Schiesari et al. 1995). The population decreases due to Arachnida predation may be relatively large in the humid tropics (Hödl 1993). In this study, we witnessed the predation of an adult individual of T. miliaris by a large spider. Though Thoropa sp. may be spider victims, they are also spider predators which may account for 16.4% of gut contents. Therefore the definition of who is predator and who is prey may be relative to the individual sizes involved in the dispute. Araneae was absent in the S. arduous diet, possibly due to the larger size of Araneae in bromeliads and its location generally in the axils bases.
Both anurans studied here showed higher numbers of males than females. This result has been previously reported for anurans present at reproductive sites (Ferreira et al. 2007, Ferreira & Teixeira 2009). Siqueira et al. (2006) found T. miliaris males were more abundant then females in three of four studied sites. This indicates there is a high degree of competition to access mates. Another explanation that is more consistent with our study is that males of both species maintain themselves in the inselbergs habitat to defend their reproductive territory against intraspecific intruders, since bromeliad is a reproductive site to S. arduous, and rock substrate to T. miliaris. Even so, we suggest further investigation to define why this difference between sexes exists. The fact that males were smaller than females is a characteristic finding in most anurans, representing a compromise between fecundity and risk of predation (Duellman & Trueb 1994).
In this study, we sampled potential prey available in 30 bromeliads and compared our results with their actual diet. The examined bromeliads showed few arthropod groups inhabiting them, with dominance of Coleoptera, aquatic insect larvae, and Blattodea. Prey availability is thought to be one of the most important factors determining frog diet (Labanick 1976). The diet composition of both anuran species, however, did not consistently reflect the prey availability inside the bromeliads. Electivity values for both anurans were higher for colonial arthropods, considered aloctonous prey, such as Isoptera and Formicidae. Although they feed on authochtonous prey, such as Blattodea, Araneae, and Coleoptera, the results suggest both anurans frequently forage outside of the bromeliad. We expected this to be consistent for the bromeliculous species, T. miliaris, but not for the bromeligenous S. arduous. Another unexpected result was that T. miliaris fed on more autochtonous prey than S. arduous, therefore, our data suggests both bromeligenous and bromelicolous species dispute for feeding territory outside of the bromeliads. The size differences of both frogs is probably the primary factor involved in those different electivity values. Since they are a larger species, Thoropa miliaris might have more facilities to eat autochtonous prey than the smaller frog S. arduous, which based its alimentary preferences on searching exogenous items. In addition, foraging preferences of S. arduous might also capture prey outside of bromeliads because prey is more exposed and abundant.
Based on the electivity values, our study agrees with Siqueira et al. (2006) for defining T. miliaris as having opportunistic foraging habits and being a generalist feeder. In both studies, ants were the most frequent and numerous items in the diet which can wrongly indicate specialization to T. miliaris. However, it can simply be a result of its thicker facial skin that provides more resistance to ant bites and stings, allowing them to feed on these insects for longer periods. Another aspect to contrast the specialization is that in terms of weight Blattodea and Orthoptera were the main diet items in our study. Siqueira et al. (2006) found ants were most important volumetrically in T. miliaris diet of two studied sites, whereas Coleoptera and Orthoptera were the primary volume in two of their other studied areas. Concentrating their diet on relatively large prey is advantageous for frogs and is expected for animals that swallow their prey whole (Siqueira et al. 2006). Studying a population of T. miliaris inhabiting a rocky seashore, Sazima (1971) found three species of marine invertebrates in its diet (one of which was consumed by 18% of the frogs). Later Feio et al. (2006) considered this population as a distinct Thoropa species. Although literature investigating S. arduous diet does not exist for comparison, we suggest this species is also generalist feeder due to its wide variety of consumed prey. This pattern has occurred for other Scinax studied (see Munõz-Guerreiro et al. 2007). Thoropa miliaris and S. arduous seem to be sit-and-wait foragers based on their variety of prey and the similarity of prey numbers in each frog. The niche breadth of T. miliaris was much narrower than that of S. arduous. This is probably because T. miliaris only uses the habitat horizontally while S. arduous can use it also vertically, which facilitates access to vertical habitats and thus allows it to feed on different prey types, such as Diptera.
Post-metamorphic frogs in general are carnivorous, although some can be folivorous (Das 1996) or frugivorous (Silva & Britto-Pereira 2006). Several studies have reported frogs eating plant materials but considered it as accidental ingestion (Brandão et al. 2003, Solé & Pelz 2007). This conclusion was also made by Siqueira et al. (2006), after they found flowers, seeds, and leaves in the stomachs of T. miliaris at four different sites. Our study only found animal prey in the T. miliaris analyzed. Although studies have detected the genus Scinax eating plant materials (Solé & Pelz 2007), these items were absent in our study of S. arduous. These different results might be due to the steepness of inselberg habitats, which makes it difficult for plant material to remain on the rock, reducing the probability of these materials being ingested by frogs.
Our studies thus indicate that inselbergs, especially above 850 m of altitude are hard areas to colonize for anurans. Both bromelicolous (T. miliaris) and bromeligenous (S. arduous) species cohabiting bromeliads during the day and strongly distinguish their use strategies, showing a significant segregation inside of those plants. We also found that night competition for food seems to take place outside of bromeliads.
ACKNOWLEDGEMENTS
To Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for releasing the samples (Proc. No. 11172-1/2007) and also the NGO Idea Wild for donation of equipment to conduct this study.
LITERATURE CITED
1. ALTIG, R. & G.F. JOHNSTON. 1989. Guilds of anuran larvae: Relationships among developmental modes, morphologies, and habitats. Herpetological Monographs 3: 81-109. [ Links ]
2. BENZING, D.H. 1980. The biology of the bromeliads. Mad River Press Inc, Eureka. [ Links ]
3. BORNHARDT, W. 1900. Zur Obeifleichengestaltung iunid Geologie Deuitsch- Ostafr ikas. Reimer, Berlin. [ Links ]
4. BRANDÃO, R.A., A. GARDA, V. BRAZ & B. FONSECA. 2003. Observations on the ecology of Pseudis bolbodactyla (Anura, Pseudidae) in central Brazil. Phyllomedusa 2: 3-8. [ Links ]
5. DAS, I. 1996. Folivory and seasonal changes in diet in Rana hexadactyla (Anura, Ranidae). Journal of Zoology 238: 785-794. [ Links ]
6. DEJEAN, A. & I. OLMSTED. 1997. Ecological studies on Aechmea bracteata (Swartz) (Bromeliaceae). Journal of Natural History 31: 1313-1334. [ Links ]
7. DUELLMAN, W.E. & L. TRUEB. 1994. Biology of the Amphibians. The Johns Hopkins University Press, Baltimore. [ Links ]
8. FEIO, R.N., M.F. NAPOLI & U. CARAMASCHI. 2006. Considerações taxonômicas sobre Thoropa miliaris (Spix, 1824), com revalidação e redescrição de Thoropa taophora (Miranda-Ribeiro, 1923) (Amphibia, Anura, Leptodactylidae). Arquivos do Museu Nacional Rio de Janeiro 64: 41-60. [ Links ]
9. FERREIRA, R.B, R.B. DANTAS & R.L. TEIXEIRA. 2007. Reproduction and ontogenetic diet shifts in Leptodactylus natalensis (Anura, Leptodactylidae) from southeastern Brazil. Boletim do Museu de Biologia Mello Leitão 22: 45-55. [ Links ]
10. FERREIRA, R.B. & R. L. TEIXEIRA. 2009. Feeding pattern and use of reproductive habitat of the Striped toad Rhinella crucifer (Anura: Bufonidae) from Southeastern Brazil. Acta Herpetologica 4(2): 125-134. [ Links ]
11. GIARETTA, A., M.S. ARAÚJO, H.F. MEDEIROS & K.G. FACURE. 1998. Food habits and ontogenetic diet shifts of the litter dwelling frog Proceratophrys boiei (Wied). Revista Brasileira de Zoologia 15: 385- 388. [ Links ]
12. GIARETTA, A.A. & K.G. FACURE. 2004. Reproductive ecology and behavior of Thoropa miliaris (Spix, 1824) (Anura: Leptodactylidae, Telmatobiinae). Biota Neotropica 4: 1- 10. [ Links ]
13. HADDAD, C.F.B. & C.P.A. PRADO. 2005. Reproductive modes in frogs and their unexpected diversity in the Atlantic Forest of Brazil. BioScience 55: 207-217. [ Links ]
14. HÖDL, W. 1990. Reproductive diversity in Amazonian lowland frogs. Fortschritte der Zoologie 38: 41- 60. [ Links ]
15. HÖDL, W. 1993. Amazonien aus der Froschperspektive. Zur Biologie der Frösche und Kröten des Amazonastieflandes pp. 499-545. In Amerika- Zur Entdeckung- Kulturpflanzen- Lebensraum Regenwald. Kataloge des OÖ. Landesmuseums, Neue Folge. [ Links ]
16. INCAPER, 2009. Sistema de Informação Agrometeorológica/série histórica/INMET. Downloaded on: 14 January 2008. [ Links ]
17. JACOBS, J. 1974. Quantitative measurement of food selection: a modification of the forage ration and Ivlev's electivity index. Oecologia 14: 413- 417. [ Links ]
18. JIMÉNES, C.E. 1994. Utilization of Puya dasyliriodes (Bromeliaceae: Pitcaunoidea) as foraging site by Bolitoglossa subpalmata (Plethodontiidae: Bolitoglossinii). Revista de Biologia Tropical 42(3): 703-710. [ Links ]
19. JUNCÁ, F.A. & C.L.S. BORGES. 2002. Fauna associada a bromélias terrícolas da Serra da Jibóia, Bahia. Sitientibus Série Ciências Biológicas 2(1/2): 73- 81. [ Links ]
20. KÖPPEN, W. 1936. Das Geographische System der Klimatologie. Berlin. [ Links ]
21. KWET, A. 1999. Pfeiffrösche und andere Anuren im Araukarienwaldschutzgebiet Pró-Mata. Elaphe 7: 92- 100. [ Links ]
22. KWET, A. (ed) 2001. Frösche im Brasilianischen Araukarienwald. Natur und Tier Verlag Press, Münster. [ Links ]
23. LABANICK, G.M. 1976. Prey availability, consumption and selection in the cricket frog, Acris crepitans (Amphibia, Anura, Hylidae). Journal of Herpetology 10:293-298. [ Links ]
24. LEHTINEN, R.M., M.J. LANNOO & R.J. WASSERSUG. 2004. Phytotelm-breeding anurans: past, present and future research. Special Publications of Museum of Zoology, University of Michigan 193: 1- 9. [ Links ]
25. MCDIARMID, R.W. & M.S. FOSTER. 1975. Unusual sites for two Neotropical tadpoles. Journal of Herpetology 9: 264- 265. [ Links ]
26. MCCORMICK, S. & G.A. POLIS. 1982. Arthropods that prey on vertebrates. Biological Review 57: 29- 58. [ Links ]
27. MESQUITA, D.M., G.C. COSTA & M.G. ZATZ. 2004. Ecological aspects of the casque-headed frog Aparasphenodon brunoi (Anura, Hylidae) in a Restinga habitat in southeastern Brazil. Phyllomedusa 3(1): 51- 59. [ Links ]
28. MUÑOZ-GUERREIRO, J., V.H. SERRANO & M.P. RAMÍREZ-PINILLA. 2007. Microhabitat use, diet and time of activity of four sympatric Neotropical hylid frogs (Anura: Hylidae). Caldasia 29(2): 413-425. [ Links ]
29. PEIXOTO, O.L. 1995. Associação de anuros a bromeliáceas na Mata Atlântica. Revista da Universidade Federal Rural do Rio de Janeiro 17: 75- 83. [ Links ]
30. SILVA, H.R. & M.C. BRITTO-PEREIRA. 2006. How much fruit do fruit-eating frogs eat? An inverstigation on the diet of Xenohyla truncata (Lissamphibia: Anura: Hylidae). Journal of Zoology 270: 692-698. [ Links ]
31. PERTEL, W., R.L. TEIXEIRA & D. RÖDDER. 2006. Anurans inhabiting soil Bromeliads in Santa Teresa, southeastern Brazil. Amphibia 5(2): 16-19. [ Links ]
32. PIANKA, E.R. 1975. Niche relations of desert lizards pp. 292-314. In M. Cody & J. Diamond (eds.) Ecology and Evolution of Communities. Harvard University Press, Cambridge. [ Links ]
33. PIANKA, E.R. 1994. Biodiversity of Australian desert lizards pp. 259-281. In C. I. Peng & C. H. Chou (eds.) Biodiversity and Terrestrial Ecosystems. Academica Sinica Monograph Series, Taipei. [ Links ]
34. POREMBSKI, G.M., R. OHLEMULLER & W. BARTHLOTT. 1998. Diversity and Ecology of Saxicolous Vegetation Mats on Inselbergs in the Brazilian Atlantic Rainforest. Diversity and Distributions 4(3): 107-119. [ Links ]
35. SAZIMA, I. 1971. The occurrence of marine invertebrates in the stomach contents of the frog Thoropa miliaris. Ciencia e Cultura 23: 647-648. [ Links ]
36. SCHIESARI, L.C., F.A. JUNCÁ & A.G. MATTOS. 1995. Hylodes phyllodes. Predation. Herpetology Review 26: 30- 31. [ Links ]
37. SCHINEIDER, J.A.P., R.L. TEIXEIRA & G.I. ALMEIDA. 2000. Aspectos de comunidades de anfíbios bromelícolas em região de Mata Atlântica do Espírito Santo, sudeste do Brasil. Revista Nordestina Zoologia 2(1): 57-62. [ Links ]
38. SCHINEIDER, J.A.P. & R.L. TEIXEIRA. 2001. Relacionamento entre anfíbios anuros e bromélias da restinga de Regência, Linhares, Espírito Santo, Brasil. Iheringia 91: 41- 48. [ Links ]
39. SIMPSON, E.S. 1949. Measurement of diversity. Nature 163: 688. [ Links ]
40. SIQUEIRA, C.C., M. VAN SLUYS, C.V. ARIANI & C.F.D. ROCHA. 2006. Feeding ecology of Thoropa miliaris (Anura, Cycloramphidae) in four areas of Atlantic rain forest, Southeastern Brazil. Journal of Herpetology 40(4): 520-525. [ Links ]
41. SOLÉ, M., O. BECKMANN, B. PELZ, A. KWET & W. ENGELS. 2005. Stomach-flushing for diet analysis in anurans: an improved protocol evaluated in a case study in Araucaria forests, southern Brazil. Studies on Neotropical Fauna and Environment 40: 23-28. [ Links ]
42. SOLÉ, M. & B. PELZ. 2007. Do male tree frogs feed during the breeding season? Stomach flushing of five syntopic hylid species in Rio Grande do Sul, Brazil. Journal of Natural History 41: 2757-2763. [ Links ]
43. SZARZYNSKI, J. 1993. Inselberge i1w tropischeni Regenwiiald. Gelhindekli7natologische Uniters uichtingen hil Ta- Nationalpar-k (Rep. C6te d'Ivoi7e, Elfe7nbeinki/ste). Unpublished M.Sc. thesis, University of Bonn, Germany. [ Links ]
44. TEIXEIRA, R.L., C. ZAMPROGNO, G.I. ALMEIDA & J.A.P. SCHINEIDER. 1997. Tópicos ecológicos de Phyllodytes luteolus (Amphibia, Hylidae) da restinga de Guriri, São Mateus-ES. Revista Brasileira Biologia 57(4): 647-654. [ Links ]
45. TEIXEIRA, R.L., J.A.P. SCHINEIDER & G.I. ALMEIDA. 2002. The occurrence of amphibians in bromeliads from a southeastern Brazilian restinga habitat, with special reference to Aparasphenodon brunoi (Anura, Hylidae). Brazilian Journal of Biology 62: 263- 268. [ Links ]
46. TEIXEIRA, R.L., P.S.M. MILI & D. RÖDDER. 2006. Ecology of anurans inhabiting bromeliads in a saxicolous habitat of Southeastern Brazil. Salamandra 42: 155- 163. [ Links ]
47. TEIXEIRA, R.L. & D. RÖDDER. 2007. A rapid assessment of an anuran community inhabiting tank bromeliads in saxicolous habitat of southeastern Brazil. Amphibia 6(1): 46- 53. [ Links ]
48. VRCIBRADIC, D. & C.F.D. ROCHA. 1996. Ecological differences in tropical sympatric skinks (Mabuya macrorhyncha and Mabuya agilis) in South-eastern Brazil. Journal of Herpetology 30: 60-67. [ Links ]
49. ZAR, J.H. 1998. Biostatistical analysis. Prentice-Hall Press, New Jersey. [ Links ]














