Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232
Caldasia vol.36 no.1 Bogotá Jan./June 2014
https://doi.org/10.15446/caldasia.v36n1.43891
http://dx.doi.org/10.15446/caldasia.v36n1.43891
PAOLA M. GARCÍA-MENESES
PAUL M. RAMSAY
School of Geography, Earth and Environmental Sciences, Plymouth University, Plymouth, PL4 8AA, United Kingdom.
Marine Biology and Ecology Research Centre, Plymouth University, Plymouth, PL4 8AA, United Kingdom. pramsay@plymouth.ac.uk
ABSTRACT
High-altitude páramo grasslands are important for their biodiversity and the ecosystem services that they provide to Andean people, but they are sensitive to disturbances, such as fire. Understanding the ecological impacts of disturbance is critical for the effective management of páramos. Indicator species studies can provide a relatively efficient way to gain such understanding. Puya hamata is a flagship giant rosette plant and has potential as an indicator of recent páramo fire history. To determine population size structure, mortality, recruitment and growth rates of Puya hamata rosettes, all Puya plants in 400 m2 plots were surveyed in 2008 and again one year later. Sixteen plots were recorded in both years, containing exactly 1000 plants. Mortality was very low during this period (0.6%). Only 27 new plants were recruited. Three different size distribution patterns were observed in the plots: (1) low plant numbers across all size ranges; (2) a single dominant peak in numbers at a particular size; (3) two dominant peaks in numbers at distinct sizes. Estimated life span of Puya hamata was 28 years based on growth rates, and growth rate declined beyond the size at which most rosettes reproduce. To investigate the impact of different fire intensities on Puya hamata mortality, 400 m2 plots within a mosaic of unburned and burned patches of different fire intensities were surveyed one month after the fire. Fire mortality was low in the medium and high intensity plots, and fires selectively killed smaller plants rather than larger ones. No mortality was observed in the unburned and low intensity fire plots. It is proposed that Puya responds to burning with pulses of seedling recruitment during periods of open vegetation after fires and very little recruitment at other times. Therefore, surveys of Puya plants can reveal past fire events in their population size structure. The combination of sensitivity to fire at recruitment, low fire mortality rates afterwards, and a 28-year lifespan makes Puya hamata an ideal indicator species of recent fire history in páramos.
Key words. Bromeliaceae, burning, Ecuador , giant puya, mortality, páramo, population dynamics, seedling recruitment, semelparity.
RESUMEN
Los páramos son importantes por su biodiversidad y los servicios ecosistémicos que proporcionan a los pueblos andinos, pero son sensibles a los disturbios como las quemas. El entendimiento de los impactos ecológicos de los disturbios es crucial pare al manejo efectivo de los páramos. El estudio de especies indicadoras puede contribuir de manera eficiente a este entendimiento. Puya hamata es una roseta gigante, considerada como especie bandera que tiene el potencial de actuar como indicador de la historia reciente de quema dentro de los páramos. Para determinar la estructura de tamaño de la población, la mortalidad, reclutamiento y tasa de crecimiento de Puya hamata, se midieron todas las plantas de Puya dentro de cuadros de 400 m2 en 2008 y un año más tarde. Se registraron 16 parcelas en ambos años donde se encontraron exactamente 1000 plantas. La mortalidad fue bastante baja durante este periodo (0.6%). Se reclutaron solamente 27 plántulas. Se encontraron tres diferentes patrones de distribución en las parcelas monitoreadas: 1) bajo número de plantas de todos tamaños; 2) un solo pico dominante de un tamaño en particular; 3) dos picos dominantes de dos distintas categorías de tamaño. La duración estimada de vida de Puya hamata basada en la tasa de crecimiento, fue de 28 años la cual disminuyó al sobrepasar el tamaño en que la mayoría de las rosetas se reproducen. Para investigar el impacto de las quemas sobre la mortalidad de Puya hamata, se registraron, un mes después de la quema, parcelas de 400 m2 dentro de un mosaico de parches no quemados y quemados a diferentes intensidades. En los cuadros de baja y media intensidad de fuego, la mortalidad fue baja y los fuegos mataron selectivamente plantas pequeñas más que grandes. No se observó mortalidad en las parcelas sin quema y de baja intensidad. Se propone que Puya responde a las quemas con pulsos de reclutamiento de plantas durante periodos cuando la vegetación está abierta después de las quemas y muy bajo reclutamiento en otras ocasiones. Por lo tanto, el seguimiento de Puya puede revelar eventos de quemas pasadas dentro de su estructura poblacional. La combinación de la sensibilidad a las quemas al momento del reclutamiento, la baja tasa de mortalidad después de las quemas y su duración de vida hace a Puya hamata una especie ideal que funciona como indicador de la historia de fuegos recientes en los páramos.
Palabras clave. Bromeliaceae, dinámica poblacional, Ecuador, mortalidad, páramo, puya gigante, quemas, reclutamiento de plántulas, semelparidad.
Recibido: 26/02/2013
Aceptado: 03/04/2014
INTRODUCTION
High-altitude páramo grasslands are found at 3000–4800 m, above the limits of continuous forest in the Andes from Colombia and Venezuela to northern Perú (Hofstede et al. 2003) and most probably a narrow strip above the subalpine or high Andean rain forest to Bolivia (García & Beck 2006). An outlier of páramo is also present in Panamá and Costa Rica (Kapelle & Horn 2005). This ecosystem is sensitive to land-use changes, but is under significant pressure from local human populations for direct benefits, like agriculture, and indirect ecosystem services, such as water supply (Vásconez & Hofstede 2006). Páramo ecosystems are important for several reasons (Hofstede et al. 2003): a) their relatively high biological diversity and endemicity results in high conservation interest; b) they provide various ecosystem services to many Andean people, mostly in ecological zones at lower altitudes; c) ecotourism provides significant additional income to some rural communities; and d) as fragile ecosystems, they are particularly threatened by poor management and climate change.
One of the most common disturbances in the páramos is fire (Horn & Kappelle 2009). Several authors have considered that páramo below 4100–4300 m.a.s.l represents secondary vegetation in previously forested areas that has been shaped and maintained by anthropogenic fire (Ellenberg 1979, Lægaard 1992). However, Moscol & Cleef (2009a, b) were able to determine the upper forest line at about 3650 m in Páramo de El Angel and Guandera in northern Ecuador . Indeed there is a lot of "paramización" in Ecuador , especially in Central Ecuador, where the upper forest line is close to the 4000 m.a.s.l. The strongest argument against páramo as a man-made landscape is the numerous endemic plant species in it, which need natural open vegetation a habitat. Cochrane (2009) recognised that human activity seems to be responsible for the majority of current páramo fires.
Managing fire regimes is, therefore, a vital part of managing páramo grasslands for biodiversity conservation and sustainable use (Cochrane 2009). The fire regime often affects the recruitment, growth and mortality of plants (Keeley 2009). In part, the different responses of plants to fire depend on the location of critical tissues within the vegetation structure: fire temperatures vary considerably from ground to canopy within a tussock grass community (Ramsay & Oxley 1996). Some common páramo species like Hypericum or Vaccinium (Horn & Kappelle 2009) benefit from some degree of disturbance (especially for germination and establishment in otherwise dense vegetation; Grubb 1977) but too frequent or intense fires can result in significant ecological damage (Horn & Kappelle 2009). Grau et al., (2010) recognized the beneficial effects that fires can have when they clear areas for seedling recruitment and bad impacts when the frequency of fires is high that can kill whole populations. In many páramos, continued human visitor pressure and agricultural use suggests that preventing fires completely is unlikely, and a more pragmatic approach is to accept fire as inevitable, but attempt to manage the fire regime to minimise ecological damage. In order to determine appropriate fire regimes, landscape scale studies over long periods of time are needed. Unfortunately, it is practically impossible to conduct such studies at an ecosystem level across the whole range of biodiversity and ecosystem services. On the other hand, indicator species studies can provide an acceptable alternative to larger, more expensive approaches (Caro & Girling 2010, Ramsay 2014).
Puya is an indicator organism for the ecological effects of burning because it thrives in fire-dominated páramos and also with seldom burning (Lægaard 1992). It is also a flagship plant, often recognized by the public, and in some ways represents the páramo ecosystem in a wider sense (Vargas-Sierra 2013). By protecting flagship species, other species are also afforded protection via the "umbrella effect" (Heywood et al. 1995, Meffe & Carroll 1997, Simberloff 1998, Favreau et al. 2006, Caro & Girling 2010). Puya is a keystone species, too, because of its interactions with invertebrates like frogs (Miller 1988), hummingbirds (Woods & Ramsay 2001) and bears (Kattan et al. 2004). Such keystone species are important because they help to maintain the integrity of the overall structure and functioning of an ecosystem (Garibaldi & Turner 2004). For these reasons, Puya is a good study group to choose to analyze the fire regime and sustainable management of páramo grasslands. Other potential study giant Puya species concern e.g.P. weberbaueri from the Peruvian and Bolivian puna, P. goudotiana from Colombia and P. aristiguietae from Venezuela and adjacent Colombia . Puya hamata also extends in Colombian páramos.
Very little information has been published about population dynamics in Puya species. Augspurger (1985) investigated the demography of Puya dasylirioides in bogs of Costa Rica , and Miller & Silander (1991) studied the distribution of several Puya species in the Ecuadorian páramos. Other studies have been carried out with similar growth form plants in African mountains (e.g., Lobelia; Young 1984) and the Mexican highlands (e.g., Agave; Eguiarte et al. 1999).
Although much has been written about the effects of fire regime on the population structures of grassland plants in general (Dyer 2003, Gibson 2009), there is little published about their effects on giant ground rosette plants in mountain grasslands. The response of Puya to fires has been discussed by Lægaard (1992) and Miller & Silander (1991) but very little quantitative evidence was available to support their conclusions. Even in recent work on Puya, this issue has not been explained in detail (Grau et al. 2010).
It is important to acknowledge that páramo fires are variable in intensity and effects (Ramsay 2001). The intensity of vegetation fires, in general, relates to the amount of fuel and fire spread: lower intensity fires happen when fires move rapidly through drier fuels, up slopes, and with the wind (Bond & Van Wilgen 1996). Higher intensity fires are produced when the fuel is slower to burn, and the fire moves down slope and/or against the wind. Such factors vary in combination across the landscape, changing the physical combustion process (Keeley 2009) and resulting in quite different outcomes from place to place and time to time. Therefore, it is unwise to assume that burning represents one single, consistent form of disturbanceit can have very different impacts, according to local circumstances at the time of the fire (Ramsay 2001).
This study aims to describe recruitment, growth, mortality rates and rosette size structure of Puya hamata L.B. Smith populations within a burned páramo landscape mosaic. In addition, the impact of a fire and different burning intensities within the burned area, on Puya hamata mortality is reported.
Materials and Methods
Study species
Puya hamata has been recognized as a plant which generally benefits from páramo burning (Lægaard 1992). It is a common species of the high-altitude páramo grasslands in parts of Ecuador and Colombia . It forms large rosettes, which can reach more than 2 m in diameter. At the end of its life, the plant produces a single, 4 m-tall inflorescence containing a succession of hummingbird-pollinated flowers (García-Meneses & Ramsay 2012). Flowering lasts over 100 days, the ripening of fruits takes a further two months, seed capsules open only when all fruits have matured, and the dispersal of the seeds inside occurs over six months or longer (García-Meneses 2012).
The study was carried out in the páramo grasslands of El Ángel and Volcán Chiles, in northern Ecuador near the border with Colombia . Part of the area belongs to the Reserva Ecológica El Ángel, and the rest forms land managed by the community of La Esperanza for agriculture, conservation and ecotourism. This páramo area has been promoted by its inclusion as one of 14 intervention sites for Proyecto Páramo Andino in Venezuela, Colombia, Ecuador and Perú (Proyecto Páramo Andino 2012), selected to conserve ecosystems and ecosystem services, with a variety of land use and human cultural diversity. Fires are common throughout these páramos, despite policies to prevent them, and the landscape consists of a mosaic of patches in different stages of recovery after burning (Moscol Olivera & Cleef 2009b).
The vegetation was dominated by tussock grasses (e.g., Calamagrostis, Festuca), giant rosette plants (Espeletia, Puya), small shrubs (e.g., Hypericum, Loricaria, Brachyotum) and herbs (e.g., Geranium, Castilleja); a comprehensive species list for this area is provided by Balslev (2001).
Population size structure, mortality, recruitment and growth rates
To determine the population size structure, mortality, recruitment and growth rates of Puya hamata rosettes, 20 permanent plots, 20 × 20 m in area, were established in randomly chosen locations at an altitudinal range of approximately 3400–3700 m (Appendix 1). In July–August 2008, the coordinates (to the nearest 10 cm) and diameter of all Puya hamata plants in these 20 plots were recorded. The same plots were recorded again in July–August 2009. Unfortunately, four plots could not be re-surveyed because their marker posts were stolen.
Fire impact on Puya hamata mortality
On 3 August 2009, a fire burned the páramo in the south-west part of the Páramo de El Ángel and, owing to the topography and wind conditions on the day; a mosaic of unburned and burned patches of different fire intensities was created in one area. Normally, a fire burns until it meets barriers that prevent further spread (streams, cliffs, etc.) or if rains. In this case, the local fire brigade, reserve rangers, and ecologists attempted to control the spread of the fire, and this resulted in fire boundaries that were not associated with the usual barriers. Fire was prevented from spreading to some areas that would otherwise have burned.
This known fire provided an opportunity to investigate Puya hamata mortality rates according to fire intensity. Within an altitudinal range of 3600–3700 m, four fire intensity "treatments" were determined by observing the fire during its course and preventing its spread in some places (based on form of combustion and time spread) (Fig. 1). One month after the fire, these areas were revisited and a single plot of 20 m x 20 m was established randomly in each.
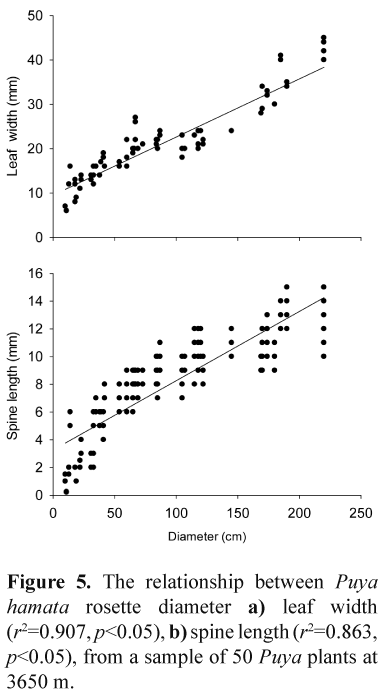
In the case of the control plot, or plants that escaped the fire in the burned plots, the diameters of all Puya plants was measured. However, most of the plants in the high intensity burned plots were badly damaged by the fire (and the diameters of the plants were not measured before burning). To estimate the original sizes of damaged Puya plants at the time of the fire, leaf width and spine length were measured for 50 Puya hamata rosettes across a wide range of diameters. For each plant, two horizontally-orientated leaves were sampled at random. For each leaf, leaf width near the base was recorded, and the lengths of two leaf spines were measured, from the point where the spines change direction from backward- to forward-pointing. Leaf width and spine length was calibrated against rosette diameter. Based on the calibrations, the original diameters of fire-damaged Puya rosettes were estimated from leaf width measurements on the burned plants.
Statistical analysis
A manual G-test was calculated to compare mortality rates between the different plots. As well as correlations to determine the relationship between rosette diameters, leaf width and spine length.
RESULTS
Population size structure, mortality, recruitment and growth rates of Puya hamata
In total, 1310 Puya rosettes, from 20 plots, were measured in 2008representing a density of 0.14 m-2. Only 16 plots were recorded in both years, and they contained exactly 1000 plants in 2008. Of these 1000 plants, 0.6% had died by the following year. Bears usually consume large sized plants with high concentration of sugar in their rosettes. One large rosette ( 1.6 m diameter) had been eaten, most likely by a spectacled bear (Tremarctos ornatus). The remaining five mortalities were for rosettes 0.1–0.5 m diameter. In these same 16 plots, only 27 new plants were recruited over the year. Mean rosette diameter of reproducing Puya hamata plants was 2.01 m (min=1.3 m, s=0.33 m, n=63).
The size distribution of Puya hamata rosettes varied from plot to plot (Fig. 2). Broadly, three different size distribution patterns were observed:
a single dominant peak in numbers at a particular size
two dominant peaks in numbers at distinct sizes
low plant numbers across all size ranges
After one year, the size distributions had shifted a little for each plot, reflecting the growth of Puya rosettes and their movement into larger categories (Fig. 3).
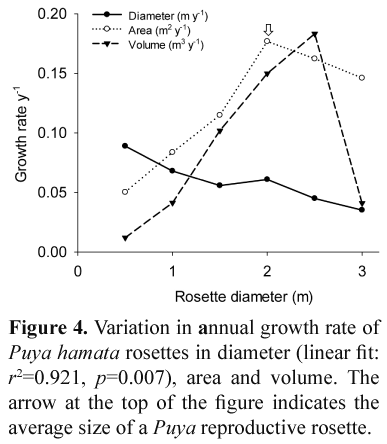
The mean annual diameter growth rate of Puya hamata rosettes was 0.081 m y-1 (s=0.032, n=1000). Life span of Puya hamata was 28 years based on growth rates. Clonal growth or vegetative propagation was not recorded in Puya hamata plants. Annual growth rate declined as rosette diameter increased (Fig. 4), but this does not mean larger plants grew more slowly. The increase in overall plant biomass associated with a change in diameter of 1 cm is much greater for large plants than for small ones. If annual growth rate in rosette area (in plan view: view of an object as projected on a horizontal plane) or volume (assuming the rosette is a perfect hemisphere) is plotted, the true pattern of biomass accumulation becomes clearer. However, the growth rate declines significantly close to the average size of a reproductive Puya hamata rosette (approximately 2 m).
Both Puya hamata leaf width and spine length were closely related to rosette diameter, with the best predictor being leaf width (Fig. 5). The original rosette diameters of Puya plants damaged by fire were estimated using measurements of leaf width, which was also the easier of the two estimates to measure in the field.
The total number and size distributions of Puya plants differed between the control and burned plots before the observed fire (Fig. 6). The fire killed plants only in the high and medium fire intensity fire plots, but mortality rates were not significantly different between any of the plots (Gadj= 9.893, df= 3, p=0.019). Medium and high intensity fires selectively killed smaller plants rather than larger ones (Gadj= 93.44, df=3, p<0.0001 and Gadj= 7.63, df=3, p=0.054 respectively). Low intensity fire and control plots did not experience any mortality within one month of the date of burning (Table 1).
DISCUSSION
Even though the growth rate of Puya hamata varied according to rosette size, there was little variation in the size of reproductive rosettes (mean diameter of 2.01 m). A few studies have suggested more variation in growth rate and reproductive rosette size in other Puya species. Miller (1988) found that Puya clava-herculis showed considerable variation in growth rates between size categories, and reported that rosettes in well drained, low elevation sites grew faster and flowered at larger sizes than rosettes from a higher elevation site. Augspurger's (1985) study of Puya dasylirioides found to a minimum critical size ( 40 cm diameter) before flowering in a Costa Rican páramo. From the current study, Puya hamata appears to be more consistent in flowering rosette size in the Páramo of El Angel.
Based on growth rates calculated in this study, the mean time that Puya hamata took to reach the mean size of a reproductive plant (approximately 2 m diameter) was 28 years. This matched to the 27–28 year time proposed for this species by Miller (1988). The much larger Puya raimondii is estimated to grow for at least 100 in the field (Benzing 2000) to 120 (Ruiz 1978) years before reproduction, but few other estimates for Puya reproductive maturity have been published (Hornung-Leoni & Sosa 2006). It is worth pointing out that Puya hamata growth rates and life span at other altitudes are likely to be different to the results provided here for plants at approximately 3400–3700 m as it has been shown in other species like Puya clava-herculis (Miller 1988).
Very little recruitment of Puya hamata seedlings was observed in our study. It was only observed in five plots (P1, P7, P8, P9 and P13). These plots had already seen some recruitment before the investigation began, and additional seedlings appeared during the study. All five plots with recruitment of seedlings were characterised by short, sparse, tussock grass vegetation where light and warmth reached the ground more readily during the day. Puya hamata needs temperatures over 14 °C to germinate and prefers full light to shaded conditions (García-Meneses 2012). Open areasmost common after firesprovide conditions for germination and promote recruitment. By contrast, Miller and Silander (1991) suggested that Puya clava-herculis seedlings are associated with tussock edges and survive poorly in open areas and in vegetation dominated by cushion and mat plants. There is no evidence that this is the case for Puya hamata, where recruitment seems to be associated with more open areas, including cushion and mat vegetation (García-Meneses 2012). Moscol Olivera & Cleef (2009b) found some páramo bogs in the same region to be almost completely dominated by Puya hamata as a result of abundant seedling recruitment.
The size structure of Puya hamata populations varied from place to place and these differences might be related to fire history. Three size structure patterns were found, 1) Plots with low plant number across all size ranges (with some stochasticity): this pattern results from the absence of fires during life time of the Puya plants, and resulting low but constant recruitment rates (illustrated in Fig. 7, t0); 2) Plots with a single dominant peak at a particular size: this pattern results from a single fire within the last 30 years that opened up the vegetation. Recruitment was low before the fire, but then, for several years after the fire, there was higher recruitment, before it returned to low levels again (Fig. 7 t5, t15); 3) Plots with two dominant peaks at distinct sizes: the pattern is caused by two fires during 30 years. There are two periods of higher recruitment but otherwise low recruitment levels are present (Fig. 7, t28).
In grassy ecosystems, fires frequently provide safe sites for seedlings, and fire-stimulated seedling recruitment is common (Enright & Lamont 1989, Bond & Keeley 2005). Perturbations at large scales can lead to a population structure with distinct cohorts and also to very uneven size and age distributions (Smith & Young 1982, Smith & Young 1987, Ramsay 1998). Puya genus has shown a high adaptation of new environments during its evolutionary processes on the Andes (Jabaily & Sytsma 2010). Disturbances is another factor that it is been used for colonization by Puyas (Grau et al. 2010). It seems that Puya hamata population size structure reflects fire regimes in more than just the density of individuals. The number of recruitment pulses indicates the number of fire events during the last 30 years or so, and the sizes of the Puya rosettes can indicates when these fires happened (if the growth rate of Puya is known for the species in that place).
Mortality of Puya hamata plants was low at all sizes in the revisited plots from 2008 to 2009. However, the smallest plants were the most vulnerable. It is well known that small plants have higher probabilities of death (Gatsuk et al. 1980, Rogers 1985, Fenner & Thompson 2005, Doak & Morris 2010). Only one large plant died in this period of time, mostly likely due to damage by a spectacled bear (Tremarctos ornatus) which inhabit the páramos and are probably are the only predators of Puya capable of dealing with the spiny leaves to reach sugar rich tissues in the centre of the plant (Kattan et al. 2004).
The low mortality at all life stages of Puya hamata is typical of semelparous plants in general (Young & Augspurger 1991). For semelparity to be a viable strategy, the risks of mortality before reproduction must be low or iteroparity is more successful (Stearns 1977, Young 1984, Young 1990). Nevertheless, our understanding of mortality rates in different Puya species is poor and more research is needed.
Interestingly, the growth rate of Puya hamata declines significantly once rosettes reach about 2–2.5 m in diameter. This corresponds very closely to the average size at which rosettes produce an inflorescence. In semelparity, there is a trade-off between growth rate and the risk of mortality before reproduction has taken place. At some point, the risks of mortality outweigh the benefits of additional time for growth, and reproduction should take place. It appears that this tipping threshold is reached at 2–2.5 m for Puya hamata at the altitudinal range of our study. Comparisons of growth rates, mortality rates and size at reproduction would make interesting studies for different Puya species across a range of lifespans and altitudes.
With respect to páramo burning, one month after the fire, Puya mortality was only observed in plots which burned at medium and high fire intensities; all plants survived in the control plot and in the plot subjected to low fire intensity. It is possible that more plants died later, but additional monitoring would be needed to determine this. Such work on mortality rates in burned and unburned areas is essential to understand the impact of different intensities of fire in plant populations. Too often, burning is assumed to be homogeneous and the results of a single study can be extrapolated, perhaps incorrectly, to a much wider range of fire scenarios.
Clearly, Puya hamata is able to survive burning. In tropical alpine habitats, the "basal rosette" growth form (Ramsay & Oxley 1997) is common, including Lobelia, Agave, Aloe, Draba, Senecio and Puya. Like other basal rosette species, Puya hamata plants insulate the meristem from cold temperatures at night (Smith & Young 1987) and hot temperatures during fires (Ramsay & Oxley 1996, Simon et al. 2009). Other Andean Puya species are also able to survive fire with mantles of persistent insulating basal leaves (Benzing 2000). Even if a fire burns away almost all leaves from a Puya plant, recovery is relatively rapid from the protected meristem. Such resistance to fire disturbance is a characteristic of many rosette plants (McIntyre et al. 1995), including a wide range of species in the family Bromeliaceae (Benzing 2000), such as Cryptanthus, Dyckia and Encholirium (native of Brazil ), Ayensua and Brocchinia (from highland habitats in Guyana ), and Hechtia (México).
The combination of sensitivity to fire at recruitment, low fire mortality rates afterwards, and a 28-year lifespan makes Puya hamata an ideal indicator species of recent fire history in páramo grasslands where it lives. Puya hamata population density and size structure in a particular place shows recruitment pulses related to past fires during the plants' lifespan. Potentially, other species of the widely distributed Puya genus could be used in a similar way, if their fire responses are similar, but this would require further investigation. Since Puya is a well-known páramo plant, easily recognized by the public, it could also act as a flagship plant, linking the plant itself with broader aspects of páramo ecology and management.
ACKNOWLEDGEMENTS
CONACYT, México, provided the financial support for this study. The work was carried out with permission from the Ecuadorian Ministry of Environment (001-IC-FLO-DPAC). We are grateful to Felipe Campos and Miguel Montenegro ( Ministerio del Ambiente, Ecuador ) for their help with the permits. Susana León, Hugo Navarrete and Carmen Torres Tapia (all at the Pontificia Universidad Católica del Ecuador, Quito) and David Suarez (Corporación Randi-Randi) provided institutional support for the work. Carlos Molina and Wilson Enríquez (Reserva Ecológica El Ángel) arranged accommodation at the reserve guardpost at El Voladero and Antonio Martinez, provided help with transport and logistics. Marta Montalvo and María Eugenia Ramos Montalvo assisted with arrangements in the town of El Ángel. Field assistance was given by Andrea Bustos, Nelly Muñoz, Carlos A. Rodríguez, Santiago E. Yerovi Echeverría, Jorge A. Castillo Castro, Isabel Jones, and Erik Valentín Silva Rodríguez. Special thanks to Salomón Ramírez Contla, Alejandra Moscoso Estrella, Mayra Ninazunta Anaguano, Margarita Miño Ron, Alejandra Domínguez Álvarez, Saul R. Castañeda Contreras, provided with help and support in the field.
LITERATURE CITED
1. Augspurger, C.K. 1985. Demography and life history variation of Puya dasylirioides, a long-lived rosette in tropical subalpine bogs. Oikos 45: 341-352. [ Links ]
2. Balslev, H. 2001. Vascular plants on Volcán Chiles and Páramo del Ángel, Ecuador -a preliminary list. In: P. M. Ramsay (ed.), The Ecology of Volcán Chiles : high-altitude ecosystems on the Ecuador-Colombia border: 1-26. Pebble and Shell Publications, Plymouth. [ Links ]
3. Benzing, D.H. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge. 690 pp. [ Links ]
4. Bond, W.J. & J.E. Keeley 2005. Fire as a global herbivore': the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20: 387-394. [ Links ]
5. Bond, W.J. & B.W. Van Wilgen 1996. Fire and Plants (population and community biology series 14). Chapman & Hall, London. 264 pp. [ Links ]
6. Caro, T.M. & S. Girling 2010. Conservation by Proxy: indicator, umbrella, keystone, flagship, and other surrogate species. Island Press, Washington, DC. 374 pp. [ Links ]
7. Cochrane, M.A. 2009. Tropical Fire Ecology: climate change, land use, and ecosystem dynamics. Springer-Praxis, United Kingdom . 645 pp. [ Links ]
8. Doak, D.F. & W.F. Morris 2010. Demographic compensation and tipping points in climate-induced range shifts. Nature 467: 959-962. [ Links ]
9. Dyer, A.R. 2003. Burning and grazing management in a California grassland: growth, mortality, and recruitment of Nassella pulchra. Restoration Ecology 11: 291-296. [ Links ]
10. Eguiarte, L.E., J. Larson-Guerra, J. Nunez-Farfan, A. Martinez-Palacios, K.S. Del Prado & H.T. Arita 1999. Phylogenetic diversity and conservation: examples at different scales and a population level proposal for Agave victoriae-reginae in the Mexican Chihuahuan desert. Revista Chilena de Historia Natural 72: 475-492. [ Links ]
11. Ellenberg, H. 1979. Man's influence on tropical mountain ecosystems in South America: the second Tansley lecture. Journal of Ecology 67: 401-416. [ Links ]
12. Enright, N.J. & B.B. Lamont 1989. Seed banks, fire season, safe sites and seedling recruitment in five co-occurring Banksia species. Journal of Ecology 77: 1111-1122. [ Links ]
13. Favreau, J.M., C.A. Drew, G.R. Hess, M.J. Rubino, F.H. Koch & K.A. Eschelbach 2006. Recommendations for assessing the effectiveness of surrogate species approaches. Biodiversity and Conservation 15: 3949-3969. [ Links ]
14. Fenner, M. & K. Thompson 2005. The Ecology of Seeds. Cambridge University Press, Cambridge. 250 pp. [ Links ]
15. García-Meneses, P.M. 2012. Lanscape-scale Population Dynamics: field observations and modelling of Puya hamata, a flagship plant from the Andes. PhD, Plymouth University. Plymouth. [ Links ]
16. García-Meneses, P.M. & P.M. Ramsay 2012. Pollinator response to within-patch spatial context determines reproductive output of a giant rosette plant. Basic and Applied Ecology 13: 516-523. [ Links ]
17. García, E.E. & S.G. Beck 2006. Puna. In: M. Moraes, B. Ollgaard, L. P. Kvist, F. Borchsenius & H. Balslev (eds), Botánica Económica de los Andes Centrales: 51–76. Universidad Mayor de San Andrés, La Paz. [ Links ]
18. Garibaldi, A. & N. Turner 2004. Cultural keystone species: implications for ecological conservation and restoration. Ecology and Society 9(3): 1-20. [ Links ]
19. Gatsuk, L.E., O.V. Smirnova, L.I. Vorontzova, L.B. Zaugolnova & L.A. Zhukova 1980. Age states of plants of various growth forms: a review. Journal of Ecology 68: 675-696. [ Links ]
20. Gibson, D.J. 2009. Grasses and Grassland Ecology. Oxford University Press, Oxford. 305 pp. [ Links ]
21. Grau, A., S.E.G. Romero & E. Ardoz 2010. Puyas andinas. Ciencia Hoy 20: 8-15. [ Links ]
22. Grubb, P.J. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107-145. [ Links ]
23. Heywood, V.H., R.T. Watson & United Nations Environment Programme 1995. Global biodiversity assessment. Cambridge University Press, Cambridge. 1140 pp. [ Links ]
24. Hofstede, R., P. Segarra & P. Mena-Vásconez 2003. Los Páramos del Mundo. Proyecto Atlas Mundial de los Páramos. Global Peatland Initiative/NC-IUCN/Ecociencia, Quito. 299 pp. [ Links ]
25. Horn, S.P. & M. Kappelle 2009. Fire in the páramo ecosystems of Central and South America. In: M. A. Cochrane (ed.), Tropical Fire Ecology: 505-539. Springer-Praxis, Reino Unido. [ Links ]
26. Hornung-Leoni, C. & V. Sosa 2006. Morphological variation in Puya (Bromeliaceae): an allometric study. Plant Systematics and Evolution 256: 35-53. [ Links ]
27. Jabaily, R.S. & K.J. Sytsma 2010. Phylogenetics of Puya (Bromeliaceae): Placement, major lineages, and evolution of Chilean species. American Journal of Botany 97: 337-356. [ Links ]
28. Kapelle, M. & S.P. Horn 2005. Hacia una breve descripción del concepto Páramo. In: I. N. D. B. -Inbio (ed.), Páramos de Costa Rica: 29-36. Inbio, San José [ Links ].
29. Kattan, G., O.L. Hernández, I. Goldstein, V. Rojas, O. Murillo, C. Gómez, H. Restrepo & F. Cuesta 2004. Range fragmentation in the spectacled bear Tremarctos ornatus in the northern Andes. Oryx 38: 155-163. [ Links ]
30. Keeley, J. 2009. Fire intensity, fire severity and burn severity: a brief review and suggested usage. International Journal of Wildland Fire 18: 116-126. [ Links ]
31. Laegaard, S. 1992. Influence of fire in the grass páramo vegetation of Ecuador . In: H. Balslev & J. L. Luteyn (eds), Páramo: an Andean ecosystem under human influence. Academic Press, London. [ Links ]
32. McIntyre, S., S. Lavorel & R.M. Tremont 1995. Plant life-history attributes: their relationship to disturbance response in herbaceous vegetation. Journal of Ecology 83: 31-44. [ Links ]
33. Meffe, G.K. & C.R. Carroll 1997. Principles of Conservation Biology. Sinauer, Sunderland. 729 p. [ Links ]
34. Miller, G.A. 1988. The Population Biology and Physical Ecology of Species of Puya (Bromeliaceae) in the Ecuadorian Andes. PhD, University of Conneticut. Conneticut. [ Links ]
35. Miller, G.A. & J.A. Silander 1991. Control of the distribution of giant rosette species of Puya (Bromeliaceae) in the Ecuadorian páramos. Biotropica 23: 124-133. [ Links ]
36. Moscol Olivera, M.C. & A.M. Cleef 2009a. Vegetation composition and altitudinal distribution of Andean rain forests in El Angel and Guandera reserves, northern Ecuador . Phytocoenologia 39(2): 175 – 204. [ Links ]
37. Moscol Olivera, M.C. & A.M. Cleef 2009b. A phytosociological study of the paramo along two altitudinal transects in El Carchi province, northern Ecuador . Phytocoenologia 39: 79-107. [ Links ]
38. Proyecto Páramo Andino. 2012. Proyecto Páramo Andino: trabajando por la vida en las alturas (Online). http://www.condesan.org/ppa/node/1022. (Accessed 13th June 2012). [ Links ]
39. Ramsay, P.M. 1998. Landscape mosaic in High Andes: the role of fire in páramo communities. In: P. Kovar (ed.), Nature and Culture in Landscape Ecology (Experiences for the 3rd Millenium): 192-200. Charles University, Prague. [ Links ]
40. Ramsay, P.M. 2001. Páramo vegetation recovery in the first two years after the fire on Volcan Chiles, Ecuador . In: P. M. Ramsay (ed.), The Ecology of Volcán Chiles : high-altitude ecosystems on the Ecuador-Colombia border: 65-73 pp. Pebble and Shell, Plymouth. Reino Unido. [ Links ]
41. Ramsay, P.M. 2014. Giant rosette plant morphology as an indicator of recent fire history in Andean páramo grasslands. Ecological Indicators. In press. [ Links ]
42. Ramsay, P.M. & E.R.B. Oxley 1996. Fire temperatures and postfire plant community dynamics in Ecuadorian grass paramo. Vegetatio 124: 129-144. [ Links ]
43. Ramsay, P.M. & E.R.B. Oxley 1997. The growth form composition of plant communities in the Ecuadorian paramos. Plant Ecology 131: 173-192. [ Links ]
44. Rogers, G.F. 1985. Mortality of burned Cereus giganteus. Ecology 66: 630-632. [ Links ]
45. Ruiz, H.M. 1978. A propósito de la mayor de las bromeliaceas. Boletín de la Sociedad Geográfica de Lima 97: 35-41. [ Links ]
46. Simberloff, D. 1998. Flagships, umbrellas and keystones: is single-species management passé in the landscape era? Biological Conservation 83: 247-257. [ Links ]
47. Simon, M.F., R. Grether, L.P. de Queiroz, C. Skema, R.T. Pennington & C.E. Hughes 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences 106: 20359-20364. [ Links ]
48. Smith, A.P. & T.P. Young 1982. The cost of reproduction in Senecio keniodendron, a giant rosette species of Mt Kenya . Oecologia 55: 243-247. [ Links ]
49. Smith, A.P. & T.P. Young 1987. Tropical alpine plant ecology. Annual Review of Ecology and Systematics 18: 137-158. [ Links ]
50. Stearns, S.C. 1977. The evolution of life history traits: a critique of the theory and a review of the data. Annual Review of Ecology and Systematics 8: 145-171. [ Links ]
51. Vargas-Sierra, N. 2013. Evaluación del estado actual de la población de Puya raimondii harms en la comunidad de p'isqu mayu-municipio vacas, cochabamba. Maestria, Universidad Mayor de San Simon. Cochabamba. [ Links ]
52. Vásconez & R. Hofstede 2006. Los páramos ecuatorianos. In: R. M. Moraes, B. Øllgaard, L. P. Kvist, B. F. & H. Balslev (eds), Botánica Económica de los Andes Centrales: 91-109. Universidad Mayor de San Andrés, La Paz. [ Links ]
53. Woods, S. & P.M. Ramsay 2001. Variability in nectar supply: implications for high-altitude hummingbirds. In: P. M. Ramsay (ed.), The Ecology of Volcán Chiles : high-altitude ecosystems on the Ecuador-Colombia border: 209-217. Pebble and Shell, Plymouth. Reino Unido. [ Links ]
54. Young, T. 1990. Evolution of semelparity in Mount Kenya lobelias. Evolutionary Ecology 4: 157-171. [ Links ]
55. Young, T.P. 1984. The comparative demography of semelparous Lobelia telekii and iteroparous Lobelia keniensis on Mount Kenya. Journal of Ecology 72: 637-650. [ Links ]
56. Young, T.P. & C.K. Augspurger 1991. Ecology and evolution of long-lived semelparous plants. Trends in Ecology & Evolution 6: 285-289. [ Links ]













