Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232
Caldasia vol.38 no.1 Bogotá Jan./June 2016
https://doi.org/10.15446/caldasia.v38n1.57818
GLORIA ANDREA MURCIA
FAVIO GONZÁLEZ
MARTHA INÉS GARCÍA
JAIRO LASSO
Universidad Nacional de Colombia Sede Caribe, San Andrés, Providencia y Santa Catalina. Author for correspondence: gloriaandrea79@gmail.com
Corporación para el Desarrollo Sostenible CORALINA, San Andrés, Providencia y Santa Catalina.
Universidad Nacional de Colombia, Facultad de Ciencias, Instituto de Ciencias Naturales, Apartado 7495, Bogotá, Colombia.
Corporación para el Desarrollo Sostenible CORALINA, San Andrés, Providencia y Santa Catalina.
Universidad Nacional de Colombia Sede Caribe, San Andrés, Providencia y Santa Catalina.
Corporación para el Desarrollo Sostenible CORALINA, San Andrés, Providencia y Santa Catalina.
ABSTRACT
The concept of flag species has driven conservation projects for 50 years. Five species native to San Andrés Island are here proposed as flag species for conservation of the few mangrove remnants in the Island: Bontia daphnoides (Myoporaceae), Canella winterana (Canellaceae), Eustoma exaltatum (Gentianaceae), Rhabdadenia biflora (Apocynaceae), and Selenicereus grandiflorus (Cactaceae). Four of these species are documented here for the first time for the Flora of the Archipelago, and three of them represent the first reports for Flora of Colombia, two at a family level (Canellaceae and Myoporaceae).
Key words. Apocynaceae, Cactaceae, Canellaceae, Caribbean mangroves, Flora of Colombia, Flora of San Andrés Island, Gentianaceae, Myoporaceae.
RESUMEN
El concepto de especies bandera ha sido empleado en proyectos de conservación por 50 años. Se proponen aquí cinco especies nativas de la Isla de San Andrés como especies bandera para la conservación de los pocos remanentes de manglar en esta Isla: Bontia daphnoides (Myoporaceae), Canella winterana (Canellaceae), Eustoma exaltatum (Gentianaceae), Rhabdadenia biflora (Apocynaceae) y Selenicereus grandiflorus (Cactaceae). Cuatro de estas especies son documentadas aquí por primera vez para el Archipiélago, y tres representan los primeros reportes para la Flora de Colombia, dos de ellos (Canellaceae y Myoporaceae) a nivel de familia.
Palabras clave. Apocynaceae, Cactaceae, Canellaceae, Flora de Colombia, Flora de la Isla de San Andrés, Gentianaceae, manglares del Caribe, Myoporaceae.
Recibido: 06/09/2015
Aceptado: 28/04/2016
INTRODUCTION
The Colombian Archipelago of San Andrés, Old Providence, and Santa Catalina was declared a Biosphere Reserve, named Seaflower (UNESCO, 2000), and a Marine Protected Area (MAVDT, 2005), in order to protect the uniqueness of their ethnic communities and the global and regional importance as marine and coastal ecosystems, including mangroves.
San Andrés is an oceanic island in the Colombian Caribbean, formed by coral reef deposits during the Neogene. According to Vargas-Cuervo (2004), two geological calcareous units can be recognized, as follows: A Miocene-age limestone unit, known as the San Andrés Formation, which formed primarily the central portion of the island, where the highest elevations (to 87 m) of the island are found; and a Pleistocene-age, mainly coralline and limestone unit, known as the San Luís Formation, which consists mainly by emerged coral reef platform. Although San Andrés Island in on top of the Nicaragua/Hondura volcanic basement plate formed during the Miocene, there is no evidence of land connection between the island deposits and Central American mainland (Barriga et al. 1969).
The vascular flora of the Archipelago of San Andrés and Old Providence has been relatively well studied (Toro 1929, Proctor 1950, Barriga et al. 1969, González et al. 1995, Cabrera 2005). A total of 385 native species of vascular plants are reported in the Archipelago, 10 of which are lycophytes and monilophytes, 42 monocots, 8 magnoliids and 325 eudicots (González et al. 1995; Murcia 2009). The close distance of the Archipelago with Central America and the Antilles has facilitated that taxa from Honduras, Nicaragua, Jamaica or Hispaniola have reached the Archipelago but not the continental Colombia. According to Gentry (cited by González et al. 1995:11), the flora of the Archipelago is a "subset of those species that are shared by Yucatán and the Greater Antilles An interesting mix of mainland and Antillean flora, but more related to the latter and thus of great conservational significance".
Mangroves play a significant ecological role as they provide the primary sources of the food web and provide physical substrates and nursery grounds for a wide variety of the marine and estuarine fauna (Yáñez-Arancibia & Lara-Domínguez 1994). According to Tomlinson (1986), the following four zones can be recognized in a typical profile of the mangrove-associated flora: the seaward zone, the mesozone, the landward zone, and the terrestrial zone. The plants found on the first two zones exhibit most of the morphological and physiological adaptations (aerial, slilt roots, pneumatophores, vivipary, salt-tolerance, etc.), although the highest taxonomic diversity of mangroves occurs in the landward and the terrestrial zones. These four zones function as reciprocal (sea-to-land and land-to-sea) buffer zones that face specific impacts that can affect the mangrove, altogether strengthen the structure and maintaining the biotic productivity of these estuarine ecosystems (Oviedo et al. 2006). From the botanical viewpoint, these ecosystems are crucial to better understand the morphological, anatomical and physiological mechanisms for seed plant adaptation, their ecological roles, their biogeographic patterns and their taxonomic replacement between the Eastern hemisphere versus the Western hemisphere mangroves around the globe (Tomlinson 1986). According to Tomlinson (1986), mangrove elements can be characterized by: (a) fidelity to the mangrove environment; (b) a major role in the community structure; (c) morphological specialization, such as aerial, slilt-roots and vivipary; (d) ability to salt exclusion; and (e) taxonomic isolation from terrestrial relatives.
Mangroves are extremely important biomes in the Archipelago of San Andrés and Old Providence, as they protect shorelines from damaging storm and hurricane winds, waves, and floods. They also prevent erosion by stabilizing sediments with their tangled root systems, maintain water quality and clarity by filtering pollutants and trapping sediments originating from land, and provide one of the basic food chain resources for arboreal life and nearshore marine life through their leaves, wood, roots, and detrital materials. Furthermore, they contribute to maintain the local biodiversity, as they become breeding and feeding sites for resident birds and key feeding and resting areas for migratory birds.
Unfortunately, mangroves are some of the most fragile and threatened ecosystems on Earth. This is particularly true for the Caribbean mangroves of Colombia, and especially for the relicts left in San Andrés Island (González et al. 1995, Murcia 2009, Lópezet al. 2011). Studies on the dynamics of local mangroves in San Andrés Island have shown that they have been drastically affected by natural (storms and sea level rise) and human disturbances since the late Holocene (González et al. 2010).
The concept of "flag species", originally proposed in the 1970's, has been pivotal in the consolidation of the Conservation Biology. Several definitions have been given, but perhaps the most comprehensive one was provided by Heywood (1985), who described "flag species" as those "popular, charismatic species that serve as symbols and rallying points to stimulate conservation awareness and action". Further elaborations on conservation biology have added phylogeny as a crucial approach (e.g. Purvis et al. 2005), as phylogenetic analyses allow to focuss conservation of those lineages that represent early diversification events, regardless the number of extant species.
The goals of the present paper are: (a) to propose five angiosperm taxa as flag species for mangrove conservation in San Andrés Island, given that their are locally rare and mostly restricted to one mangrove relict in San Andrés Island, especifically to the landward and the terrestrial zones; (b) to contribute to the knowledge of the mangrove flora of San Andrés Island, recording for the first time these taxa: Bontia daphnoides (Myoporaceae), Canella winterana (Canellaceae), Eustoma exaltatum (Gentianaceae), Rhabdadenia biflora (Apocynaceae), and Selenicereus grandiflorus (Cactaceae); (c) to contribute to the knowlegde of the Flora of Colombia by documenting for the first time the presence of Canellaceae and Myoporaceae in the country; and (d) to emphasize the phylogenetic and biogeographic relevance of these taxa as crucial elements to evaluate the conservation status of local mangroves.
METHODS
The study is based on field observations and collections carried out in San Andrés Island and herbarium work centered mainly at COL, HUA, JAUM, MEDEL and NY. The field observations were carried out by some of us on the following time intervals since 1991: April and july 1991 (by FG), may and july 1992 (by FG); may 1993 (by FG); november 2009 (GAM); april 2010 (FG); december 2013 (by GAM); may 2015 (by GAM). For purposes of proper identifications and additional specimen search, virtual herbaria (http://plants.jstor.org/) were also examined, especially MO, P and US.
The field observations and collections were part of two monitoring periods carried out by the Environmental Authority of The Archipelago San Andrés, Old Providence and Santa Catalina, CORALINA since 1998 until 2013: 1) mangrove monitoring using the methodology described by Schaeffer-Novelli and Cintrón-Molero (1986), and Del Valle Arango and Gómez-Restrepo (1996); 2) coastal vegetation monitoring with 10 x 10 m quadrants on Landscape Units (sand beach, coralline rock and transitional mangrove area); this area was determined through to the minimal sample area, similar to the methodology used in other Caribbean islands, such as the ecological characterization of Múcura and Tintipán Islands (Flórez& Etter 2003).
RESULTS AND DISCUSSION
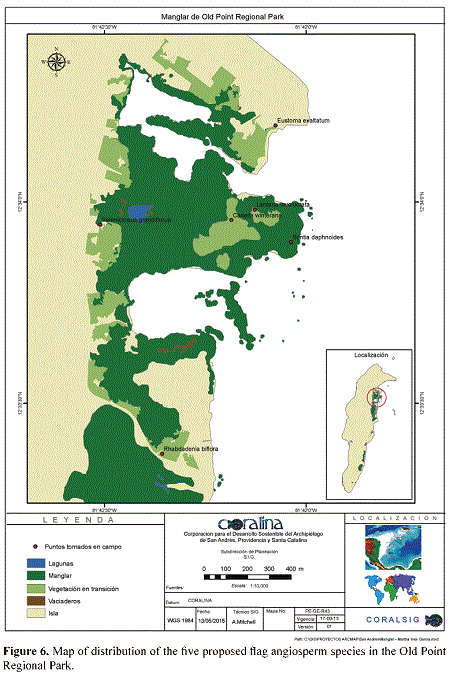
Six mangrove relicts are still found in San Andrés, five of which are located in the East side of the Island; these are from north to south (areas following Lópezet al. 2011): (1) Old Point mangrove, located at the NE end between the Hooker Bay and Bahía Honda. It occupies approximately 54,95 Ha, being the largest mangrove of the Archipelago. The Old Point mangrove was declared Regional Natural Park in 2001 by CORALINA. (2) Mount Pleasant magrove, located at the Coconut Bay. It occupies an area of about 50,01 Ha. (3) Salt Creek mangrove, which covers about 3,6 Ha. (4) Sound Bay mangrove, located at the southeast side, occupies approximately 16,24 Ha. (5) Smith Channel mangrove, located at the SE end of the Island, covers 18,13 Ha. The sixth mangrove, located in The Cove, is the only one found at the West side of the Island, and covers ca 1,99 Ha.
Unfortunately, the seaward zones of these mangroves have been severely fragmented and isolated from the landward and the terrestrial zones by the road construction around the Island. This fragmentation has caused a negative effect especially the mesozone, which the most affected by the impacts of human activities that threaten the mangrove. CORALINA and the Departmental Government have carried out actions to recover mangroves in Old Point; thus, it is critical to have a better knowledge of the native plants associated with mangrove in order to take appropriate conservation and management.
The overall floristic composition of the mangroves of San Andrés Island is similar in terms of genera and number of species per family to continental mangroves of the Caribbean. The seaward zone of these mangroves is formed by Rhizophora mangle L. (Rhizophoraceae; locally called "red mangle"), Avicennia germinans (L.) Stearn (Acanthaceae s.l., formerly in the Avicenniaceae; "black berries" or "black mangrove"), and two Combretaceae, Conocarpus erectus L. ("black mangrove", "wild mangrove", or "mangle botoncillo"), and Laguncularia racemosa C. F. Gaertn. ("white mangrove" or "mangle blanco"). The terrestrial and peripheral zones are dominated by Abrus praecatorius L., Canavalia maritima Thouars, Clitoria ternatea L., Crotalaria verrucosa L., Dalbergia brownei (Jacq.) Schinz, Gliricidia sepium (Jacq.)Kunth, and Lonchocarpus pentaphyllus Kunth (Fabaceae); Acalypha sp., Dalechampia scandens L., and Hippomane mancinella L. (Euphorbiaceae); Talipariti tiliaceum (L.)Fryxell and Thespesia populnea (L.)Correa (Malvaceae); Chrysobalanus icaco L. (Chrysobalanaceae); Coccoloba uvifera L. (Polygonaceae); and Terminalia catappa L. (Combretaceae). The presence of these elements in the local mangroves corresponds to the floristic composition given by Tomlinson (1986) on a cosmopolitan basis, and by Cortés & Rangel (pers. comm.) for mangroves of the continental Caribbean of Colombia. The later work reports that the most specious families are Fabaceae (with 7 genera and 7 species), Apocynaceae (4/4), Bignoniaceae (3/3), Combretaceae (3/3), Euphorbiaceae (3/3), and Mimosaceae (3/3).
A number of plant species native to the Island´s mangroves can be at risk of local extinction, e. g. the fern Acrostichum aureum L. (Pteridaceae), the monocots Tillandsia dasyliriifolia Baker (Bromeliaceae), and Brassavola nodosa Lindl. (Orchidaceae), the magnoliid Annona glabra L. (Annonaceae), and the eudicots Hippomane mancinella (Euphorbiaceae), Lantana involucrata L. (Verbenaceae), Crossopetalum rhacoma Hitchc. (Celastraceae), Suriana maritima L. (Surianaceae, locally called "cedro playero" or "lavender", almost locally extinct in the Archipelago, as it is currently restricted to a small population in San Luís and in Johnny Cay), and Talipariti tiliaceum and Thespesia populnea (Malvaceae). However, we have focussed on the following five species that, in addition, are locally rare and are new records for the Archipelago and for the Flora of Colombia.
Bontia daphnoides L. (Myoporaceae R. Brown) Figs. 1, 6
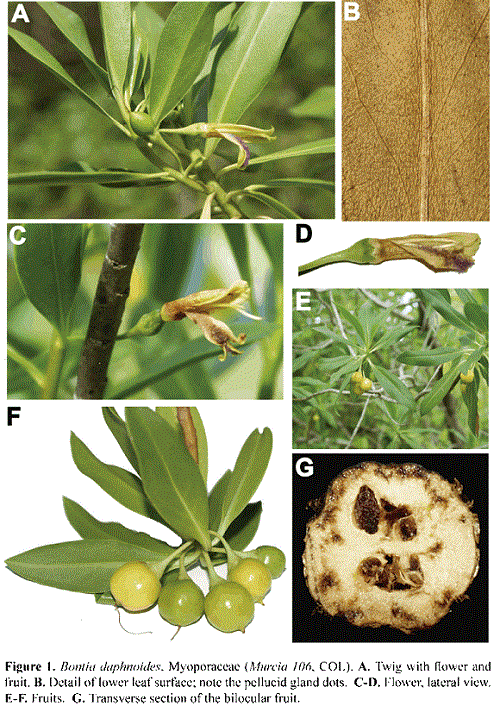
The traditional circumscription of the Myoporaceae includes seven genera and approximately 250 species (Heywood et al. 2007). Most members of the family (Eremophila, Myoporum, and Pentacoelium, plus three undescribed genera) are found primarily in Australia, New Zealand and the Pacific islands Mauritius and Rodrigues (Heywood et al. 2007). The genus Bontia, however, has a disjunct distribution from the remaining members of the family, as its single species is confined to the Caribbean. Bontia was described by Kelchner et al. (2000:136) as "the extreme outlier of a primarily southern- hemisphere Old World family, the Myoporaceae" and that its distribution "is due to dispersal from an Australian ancestral lineage."
The phylogenetic relationships of Myoporaceae have been in dispute since Olmstead et al. (2001; see also Weber 2004), among others researchers, who suggested the inclusion of the family in the Scrophulariaceae. Although closely related to Scrophulariaceae, various authors (e.g. Kelchner et al. 2000, Theisen & Fischer 2004, Chinnock 2007, Heywood et al. 2007, Acevedo-Rodríguez & Strong 2008) recognize the Myoporaceae as a distinct, monophyletic family.
Bontia daphnoides is a small tree to 5 m tall, with pellucid gland dots that correspond to resiniferous cavities in both vegetative and reproductive organs. The leaves are spirally arranged, exstipulate, with a short petiole to 3 mm in length; the leaf blade is narrowly-elliptic, to 10 x 2.5 cm, with a cuneate base that runs decurrent almost to the base of the petiole, glabrous on both sides but the upper side warty, margin slightly undulate, venation pinnately veined, midvein impressed above, prominent below, secondary veins 10-12 ascending pairs, tertiary veins obscurely reticulate, apex obtuse to acute. The flowers are axillary, solitary; the peduncle is 1-1.5 cm long; the calyx is formed by five free, imbricate, broadly triangular sepals, to 3 x 3 mm each, with ciliate margin and acuminate to caudate apex; the corolla is sympetalous, monosymmetric, to 2 cm long including the ca. 1 cm long tube and the five lobes that form an upper and a lower lip, each to ca. 1 cm long, reflexed at anthesis, the corolla surface is yellow suffused with green, and has pellucid gland dots externally, and a carpet of villous, long, multicellular, uniseriate, lilac trichomes that densely cover the inner side of the lips, especially that of the abaxial lip; the five stamens, fused to the corolla tube, are slightly extrorse; the ovary is superior, bicarpelar and bilocular. The fruit is a subglobose schizocarpic capsule to 1.2 x 1 cm, with the sepals and the filiform, 1-1.5 cm long style persistent, and with few (< 5) seeds per locule.
Bontia daphnoides is here reported for the first time for the Flora of Colombia. The species was collected, in flower and fruit, on December 12th, 2013 (G. A. Murcia 106, COL), scattered among red mangrove (Rhizophora mangle L., Rhizophoraceae) and sea grape (Coccoloba uvifera (L.) L., Polygonaceae) trees, at the inner part of the mangrove forest of Old Point Regional Park; it has not been recorded in other mangroves of the Archipelago. In Cuba, the species has also been reported as a mangrove element (Oviedo et al. 2006); according to Nellis (1994), it has a considerable potential to be used in shoreline stabilization.
Canella winterana (L.) Gaertn. (Canellaceae Mart.). Figs. 2, 6
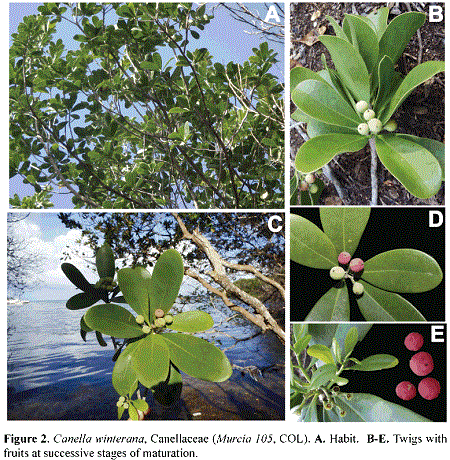
The Canellaceae consists of six genera and 23 species (Müller et al. 2015) disjunctly distributed in eastern Africa, Madagascar, the Caribbean, Central America, and northern and eastern South America. Although Canella winterana is a common element of the West Indies (Asprey & Robbins 1953), the species is here reported for the first time for the Flora of Colombia. Given that the species is sister to the remaining member of the family, its presence in San Andrés Island provides a significant value to increase the phylogenetic diversity in the Island, as the Canellaceae is one of the few magnoliid families in San Andrés Island, along with the Annonaceae (2 spp.), the Lauraceae (4 spp.) and the Piperaceae (2 spp.) (González et al. 1995). Along with its sister order (Piperales), Canellales could date back to 143.18-125-90 Myr, one of the oldest evolutionary history among Angiosperms; at a family level, the Canellaceae date back to 52.34-11.33 Myr (Massoni et al. 2015). Interesting, these authors also estimate that these two orders have higher speciation rates but higher extinction rates with respect to other magnoliids, including the orders Magnoliales and Laurales.
Although Canella winterana is widely distributed in the Caribbean (Tomlinson 2001, Salazar & Nixon 2008), and its presence in Colombia was incidentally anticipated by Gilg (1925), this is the first documented record of the species in the country. The species is a tree of approximately 5-6 m tall and 20 cm DAP, with aromatic bark and leaves, which have a bitter taste when chewed. The wood lacks growth rings. The leaves, spirally arranged, lack stipules and are shortly petioled (to 5 mm long); the leaf blade is narrowly obovate, 10-11 x 2.7-3.4 cm, glabrous on both sides, with minute but numerous round glands visible especially on the abaxial surface; the base is cuneate and decurrent almost to the proximal end of the petiole; the pinnate venation is formed by a midvein that is impressed above and prominulous below, and by 8-11 pairs of secondary veins; the tertiary veins are inconspicuous and obscurely reticulate. The flowers are formed in terminal corymbiform inflorescences bearing deciduous bracts and bracteoles; each flower possesses three sepals, five reddish petals, ten connate stamens with extrorse anthers, and a superior, unilocular ovary with 4 to 6 ovules attached to parietal placentas. The fruits are globose, red berries with 4-6 shining, black seeds.
Canella winterana was collected in a transitional mangrove forest, in an area of difficult access, as it is surrounded by black mangrove (Avicennia germinans (L.) Stearn, Acanthaceae), Rhizophora mangle and white mangrove (Laguncularia racemosa C. F. Gaertn., Combretaceae). The species was collected in the mesozone of Old Point Mangrove Park, San Andrés Island, on December 11th 2013 (G. A. Murcia 105, COL).
Selenicereus grandiflorus (L.) Britton & Rose (Cactaceae Juss.). Figs. 3, 6
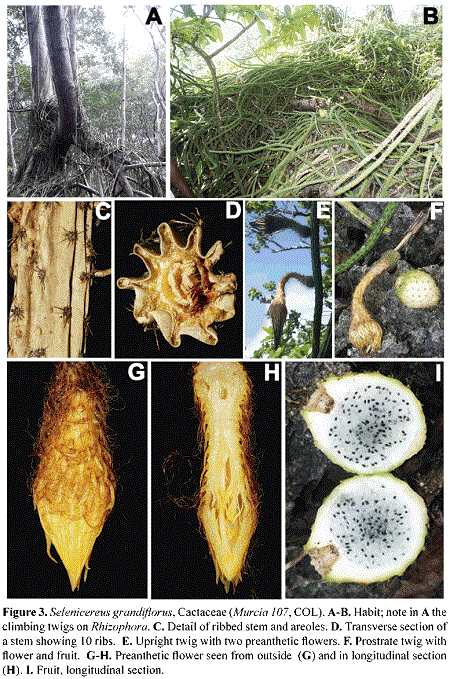
Selenicereus (A. Berger) Britton & Rose (subfamily Cactoideae, tribe Hylocereae) is a taxonomically complex genus of night-flowering cacti (Hunt 1989). The estimated number of species in the genus varies between 11 (Mabberley 2008) to 28 (Anderson 2001). The name of the genus stands after Selena, the Greek moon goddess (Anderson 2001), and refers to the nocturnal anthesis that occurs in most of its species. Additionally, the species of Selenicereus can be distinguished by the mostly epiphytic habit, the often slender, prostrate to climbing stems (thus, considered a vine cactus; Plume et al. 2013), the actinomorphic perianth with ray-like parts, the flowers to 40 cm long, and the pericarpels and floral tubes covered with scales, hairs, and spines. The fruits are globose or subglobose, sometimes oblong, fleshy, red to green, with persistent spiny areoles.
Selenicereus grandiflorus is a lithophytic to epiphytic cactus that often climbs on tree trunks with clinging roots. The stems reach 2.5 m long and 2.5 cm in diameter and have 4-8 longitudinal ribs. The scattered areoles are formed by 6-14 spines, each 0.6-1 cm long. The flowers, to 20 cm long, are formed by numerous spirally arranged outer tepals, linear, to 3 x 0.4 cm, pink to white externally, white internally, followed by spirals of inner narrowly oblong tepals, to 3 x 0.7 cm, white, and by numerous slender stamens; the inferior ovary is ellipsoid and surrounded by a hypanthium densely covered with scales, long, filiform hairs, and short spines. The fruits are subglobose, to 7 x 6 cm, white to green, scattered with punctiform areoles externally; the endocarp is hyaline and the seeds are black. The species is distributed in E Mexico and widely distributed in the Caribbean, and it has long been used "for treating dropsy and as a cardiac remedy as it contains substances similar to digitalis for treatment of heart problems" (Anderson 2001:62).
Until now, only three species of Cactaceae had been reported to occur in the Archipelago of San Andrés and Providencia (González et al. 1995). These are Acanthocereus tetragonus (L.) Hummelinck, Pereskia bleo (Kunth) A. P. De Candolle, and Opuntia cf. caracassana Salm-Dyck, probably all of them introduced to the Archipelago. Selenicereus grandiflorus is the fourth species of the family and the only vine cactus in the Island. It was collected in May Cliff, San Andrés Island, on December 12th, 2013 (G. A. Murcia 107, COL). We have also seen this species in Old Point and Smith Channel mangroves forest, growing on roots and trunks of Rhizophora mangle. In Jamaica, Puerto Rico and the Virgin Islands, this vine cactus is commonly found in forests and coastal scrub (Acevedo-Rodríguez & Angell 2003); in Cuba, it is also associated with mangrove and their ecotones (Oviedo et al. 2006).
The inclusion of Selenicereus grandiflorus in a phylogenetic analysis by Plume et al. (2013) was critical to demonstrate the paraphyly of the genus with respect to Hylocereus; furthermore, it was crucial to suggest that S. pteranthus (Link ex A. Dietr.) Britton & Rose is likely a hybrid species between S. grandiflorus and S. hamatus Britton & Rose.
Eustoma exaltatum (L.) Salisb. (Gentianaceae) Figs. 4. 6
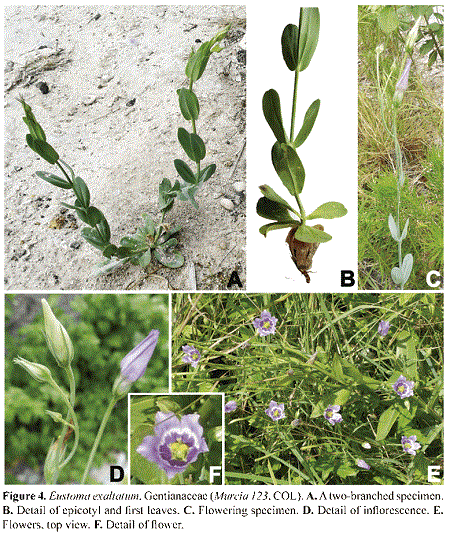
Eustoma Salisb. is a small (2 to 3 species) genus of the tribe Chironieae, subtribe Chironiinae (Mason 1998, Turner 2014).
Eustoma exaltatum is a short-lived perennial herb to 60 cm tall. The leaves are simple, opposite, ovate, oblong or lanceolate, 2-9 x 0.7-3.5 cm, sessile and often with clasping bases. The inflorescences are panicles. The flowers are supported by long (to 7 cm) pedicels; the lobes of the deeply cleft calyx are 1-1.5 cm long, usually longer than the corolla tube; the corolla is bell-shaped, 2-3 cm long, and the corolla lobes are oblong to obovate, 1.5-2.8 x 0.5-1.5 cm, glabrous, purple to lavender, pink to white; the 5(6) stamens are inserted on the corolla tube, and possess versatile, yellow anthers, coiled after pollen release; the styles are slender, upright, to 5 mm long, glabrous, and the bilobed stigmas are glandular and pubescent. The capsules are ellipsoid, 2-valvately dehiscent, glabrous, and contains numerous, minute, globoid seeds with reticulate seed coat.
Eustoma exaltatum is native to the southern United States, Mexico, Central America, and the West Indies (Villareal 1998, Mansion & Zeltner 2004, Turner 2014); this is the first report of the genus for Flora of Colombia. The species was collected on May 12th, 2015 (G. A. Murcia 123, COL), in an area strongly disturbed by the construction of a dock in the buffer zone of Old Point Regional Park, where the greatest abundance of the specie was found (Murcia 2009). Thus, it is important to take actions to protect the place where some individuals still remain.
Rhabdadenia biflora (Jacq.) Müll.Arg. (Apocynaceae) Figs. 5, 6
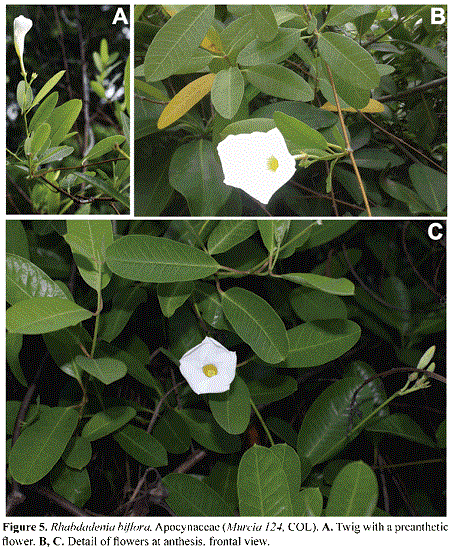
Rhabdadenia Müll. Arg. (subfamily Apocynoideae tribe Echiteae) is a small genus of three species from subtropical and tropical America. R. biflora is a glabrous liana, with milky sap, opposite leaves with petioles to 1.5 cm long and laminas oblong to elliptic, to 4-10 x l-5 cm, with an obtuse to acute and decurrent base, and an acuminate to mucronate apex. The inflorescences are axillary dichasia with a peduncle to 7 cm long; the pentamerous flowers are supported by a pedicel to 1.5 cm long; the calyx reaches to 1 cm long, and the white, funnel-shaped corolla to 7 cm long, with a tube to 2 cm long, with retrorse fimbriae in the inner surface of the corolla, at the level of the insertion of the included stamens; the anthers reach 5 mm long, are tighly appresed to the stigma, and are villous at their apices; the fruits are straight, slender, paired follicles to 12 cm long, with narrow, plumose seeds.
Rhabdadenia biflora is widely distributed in the Caribbean region. The species grows in fresh- or salt –water swamps, usually on the back mangrove zone. The wind dispersed seeds, such as those exhibited by this species, are atypical in mangrove plants (Tomlinson, 1986). According to Morales (2009), there is a previous collection of this species in the island of San Andrés, made by Proctor in 1948. However, we could not locate this specimen for the present study. We found Rhabdadenia biflora only in the mangrove of Old Point, where it was collected on May 12th, 2015 (G. A. Murcia 124, COL), climbing on twigs of Rhizophora mangle. In Cuba, Jamaica, the Cayman Islands, Puerto Rico and the Virgin Islands, the species is also an element associated with mangroves (Asprey & Robbins 1953, Woodroffe 1982, Acevedo-Rodríguez & Angell 2003, Oviedo et al. 2006).
From the phylogenetic viewpoint, Rhabdadenia biflora, as the representative of its genus, is one of the most unstable species in all analyses presented by Straub et al. (2014). Thus, the phylogenetic and biogeographic relationships of the genus are still uncertain; the three main competing hypotheses presented by these authors are that the genus is either (a) sister to the "Asian clade" of the Apocyneae; (b) sister to the genus Periploca; or (c) sister to the (Anodenon + (Aganosma + Epigynum)) clade. In addition, Rhabdadenia is atypical in having monads instead of the typical pollinia in the family (Straub et al. 2014). The inclusion of Rhabdadenia in the phylogenetic analysis presented by Straub et al. (2014) allowed the authors to suspect a long branch effect of Rhabdadenia, which might cause critical artifacts in the phylogenetic reconstruction. Nevertheless, if hypothesis (a) results to have stronger support in future analyses, the sister-group relationship between Rhabdadenia and the Old World Periplocoideae would suggest a complex biogeographic scenario for this particular subclade.
CONCLUSION
We have reported the presence of five angiosperm species that can be considered flag species to protect the mangroves of the Island of San Andrés, Colombia. All the species are rare and in risk of local extinction. The species are primarily found in the landward and the terrestrial zones of the Old Point mangrove. Two of these species are new records, at a family level, for the Flora of Colombia: Bontia daphnoides (Myoporaceae) and Canella winterana (Canellaceae). All the species possess also significant interest in terms of phylogenetic reconstructions and biogeography for their respective lineages, as well as atypical morphologies for mangrove plants.
ACKNOWLEDGEMENTS
The authors express their appreciation to the Universidad Nacional de Colombia, Sedes Caribe and Bogotá. They specially thank Elizabeth Taylor, Adriana Santos and Godfray Peterson, for their logistic and technical support. They also thank Denisse Cortés and Dr. O. Rangel-Ch. (Universidad Nacional de Colombia, Instituto de Ciencias Naturales) for providing unpublished floristic data on continental mangroves in Colombia.
LITERATURE CITED
1. Acevedo-Rodríguez, P. & B. Angell. 2003. Bejucos y plantas trepadoras de Puerto Rico e Islas Vírgenes. Smithsonian Institution, Washington. [ Links ]
2. Acevedo-Rodríguez, P. & M.T. Strong. 2008. Floristic richness and affinities in the West Indies. Botanical Review 74:5-36. [ Links ]
3. Anderson, E.F. 2001. The cactus family. Timber Press, Oregon. [ Links ]
4. Asprey, G.F. & R.G. Robbins. 1953. The vegetation of Jamaica. Ecological Monographs 23(4): 359-412. [ Links ]
5. Barriga, E., J. Hernández, I. Jaramillo, R. Jaramillo, L.E. Mora-Osejo, P. Pinto & P. Ruiz. 1969. La Isla de San Andrés. Contribución al conocimiento de su ecología, flora, fauna y pesca. Dirección de Divulgación Cultural, Universidad Nacional de Colombia, Bogotá [ Links ].
6. Cabrera, I. 2005. Las plantas y sus usos en las islas de Providencia y Santa Catalina. Programa Editorial Universidad del Valle, Cali. [ Links ]
7. Chinnock, R.J. 2007. Eremophila and allied genera: A monograph of the plant family Myoporaceae. Rosenberg, Dural Delivery Centre, New SouthWales. [ Links ]
8. Del Valle-Arango, J.I. & M. Gomez- Restrepo. 1996. Guía de campo para el estudio de la dinámica y estructura de los manglares en Colombia. Proyecto MINAMBIENTE Manglares de Colombia PD 171/91 (Fase 1) rev. 2, OIMT. Santa Fe de Bogotá D. C. Informe Técnico, 7, 106p. [ Links ]
9. Flórez, C. & A. Etter. 2003. Caracterización ecológica de las islas Múcura y Tintipán, archipiélago de San Bernardo, Colombia. Rev. Acad. Colomb. Cienc. 27: 343-356. [ Links ]
10. Gilg, E. 1925. Canellaceae. Pp. 323-328. In: A. Engler & K. Prantl. (eds.), Die naturlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig. [ Links ]
11. González, C., L.E. Urrego, J.I. Martínez, J. Polanía& Y. Yokoyama. 2010. Mangrove dynamics in the southwestern Caribbean since the 'Little Ice Age': A history of human and natural disturbances. The Holocene 20(6): 849-861. [ Links ]
12. González, F., J.N. Díaz & P. Lowy. 1995. Flora Ilustrada de San Andrés y Providencia. Universidad Nacional de Colombia-Sena, Bogotá [ Links ].
13. Heywood, V.H. 1985. Global biodiversity assessment. Cambridge University Press, Cambridge. [ Links ]
14. Heywood, V.H., R.K. Brummitt, A. Culham & O. Seberg. 2007. Flowering plant families of the world. Royal Botanic Gardens, Kew. [ Links ]
15. Hunt, D. 1989. Notes on Selenicereus (A. Berger) Britton and Rose and Aporocactus Lemaire (Cactaceae-Hylocereinae).Bradleya 7: 89-96. [ Links ]
16. Kelchner, S.A., R.J. Bayer, R.J. Chinnock, M.D. Crisp & J.G. West. 2000. Data partition analysis of noncoding sequence evolution in Bontia and Myoporaceae. American Journal of Botany 87: 136. [ Links ]
17. López, A., M. García, P.C. Sierra, M. Hernández, I. Machacón, J. Lasso, O. Bent, A. Mitchell, A. Pacheco, C. Segura, S. Nieto & J. Espriella. 2011. Ordenamiento ambiental de los manglares del Archipiélago de San Andrés, Providencia y Santa Catalina. [ Links ]
18. Mabberley, D.J. 2008. Mabberley´s plant-book. A portable dictionary of plants, their classification and uses. Third edition. Cambridge University Press, Cambridge. [ Links ]
19. Mansion, G. & L. Zeltner. 2004. Phylogenetic relationships within the New World endemic Zeltnera (Gentianaceae-Chironiinae) inferred from molecular and karyological data. American Journal of Botany 91: 2069-2086. [ Links ]
20. Mason, C.T. 1998. Gentianaceae, Gentian Family. Journal of the Arizona-Nevada Academy of Science 30: 84-95. [ Links ]
21. Massoni, J., T.L.P. Couvreur & H. Sauquet. 2015. Five major shifts of diversification through the long evolutionary history of Magnoliidae (angiosperms). BMC Evolutionary Biology 15: 1-14. DOI 10.1186/s12862-015-0320-6 [ Links ]
22. MAVDT, 2005. Declaratoria del Area Marina Protegida Seaflower Resolución 107 del 27 de Enero 2005 Ministerio de Ambiente, Vivienda y Desarrollo Territorial, Colombia. 6 pp. [ Links ]
23. Morales, J.F. 2009. Estudios en las Apocynaceae neotropicales XXXVII: Monografía del género Rhabdadenia (Apocynoideae: Echiteae). Journal of Botanical Research Institute of Texas 3: 541-564. [ Links ]
24. Müller, S., K. Salomo, J. Salazar, J. Naumann, M. A. Jaramillo, C. Neinhuis, T. S. Field & S. Wanke. 2015. Intercontinental long distance dispersal of Canellaceae from the New to the Old World revealed by a nuclear single copy gene and chloroplast loci. Molecular Phylogenetics and Evolution 84: 205-219. [ Links ]
25. Murcia, G.A. 2009. Caracterización de la vegetación vascular terrestre del borde costero de la Isla de San Andrés, Caribe Colombiano. Tesis de pregrado en Biología, Facultad de Ciencias, Universidad del Tolima. Ibagué [ Links ].
26. Nellis, D. 1994. Seashore plants of South Florida and the Caribbean. Pineapple Press, Inc., Sarasota, Forida. [ Links ]
27. Olmstead, R.G., C.W. dePamphilis, A.D. Wolfe, N.D. Young, W.J. Elisens & P.A. Reeves. 2001. Disintegration of the Scrophulariaceae. American Journal of Botany 88: 348-361. [ Links ]
28. Oviedo, R., L. Menéndez & J.M. Guzmán. 2006. Flora asociada a manglares y sus ecotonos. Ecosistema de manglar en el Archipiélago Cubano, estudios y experiencias enfocados a su gestión. La Habana. Editorial Academia, pp. 44-57. [ Links ]
29. Plume, O., S.C.K. Straub, N. Tel-Zur, A. Cisneros, B. Schneider & J.J. Doyle. 2013. Testing a hypothesis of intergeneric allopolyploidy in vine cacti (Cactaceae: Hylocereeae). Systematic Botany 38: 737-751. [ Links ]
30. Proctor, G. 1950. Results of the Catherwood Chaplin West Indies Expedition, Part. I. Plants of Cayo Largo (Cuba), San Andrés and Providencia. Proceedings of the Academy of Natural Sciences of Philadelphia 102:27-42. [ Links ]
31. Purvis, A., J.L. Gittleman & T. Brooks (eds.). 2005. Phylogeny and Conservation. Cambridge University Press, Cambridge, UK. [ Links ]
32. Salazar, J. & K. Nixon. 2008. New discoveries in the Canellaceae in the Antilles: How phylogeny can support taxonomy. Botanical Review 74: 103-111. [ Links ]
33. Schaeffer-Novelli, Y. & G. Cintrón-Molero. 1986. Guía para estudo de áreas de manguezal: estrutura, função e flora. Caribbean Ecological Research. [ Links ]
34. Straub, S.C.K., M.J. Moore, P.S. Soltis, D.E. Soltis, A. Liston & T. Livshultz. 2014. Phylogenetic signal detection from an ancient rapid radiation: Effects of noise reduction, long-branch attraction, and model selection in crown clade Apocynaceae. Molecular Phylogenetics and Evolution 80: 169-185. [ Links ]
35. Theisen, I. & E. Fischer. 2004. Myoporaceae. Pp. 289-292, In: J. Kadereit (ed.), The Families and Genera of Vascular Plants. VII. Flowering Plants:Lamiales (except Acanthaceae including Avicenniaceae). Springer, Berlin. [ Links ]
36. Tomlinson, P.B. 1986. The botany of mangroves. Cambridge University Press, Cambridge. [ Links ]
37. Tomlinson, P.B. 2001. The biology of trees native to tropical Florida.Harvard University Press, Allston, Massachussets. [ Links ]
38. Toro, R. 1929. Una contribución a nuestro conocimiento de la flora silvestre y cultivada de San Andrés. Revista de la Sociedad Colombiana de Ciencias Naturales 6(103): 201-207. [ Links ]
39. Turner, B.L. 2014. Taxonomic overview of Eustoma (Gentianaceae). Phytologia 96 (1) 7-11. [ Links ]
40. UNESCO, 2000. UNESCO's Man and the Biosphere (MAB) Program declared Seaflower Biosphere Reserve, as a member of the World Network of Biosphere Reserves. [ Links ]
41. Vargas-Cuervo, G. 2004. Geología y aspectos geográficos de la Isla de San Andrés, Colombia. Geología Colombiana 29: 71-87. [ Links ]
42. Villarreal, J. 1998. Flora del Bajio e de regiones adyacentes: Familia Gentianaceae. Fascículo 65. Instituto de Ecología A.C. Pátzcuaro, Michoacán. [ Links ]
43. Weber, A. 2004. Gesneriaceae and Scrophulariaceae: Robert Brown and now. Telopea 10:543-571. [ Links ]
44. Woodroffe, C.D. 1982. Geomorphology and development of mangrove swamps, Grand Cayman Island, West Indies. Bulletin of Marine Science 32: 381-398. [ Links ]
45. Yánez-Arancibia, A. & A.L. Lara-Domínguez. 1994. Los manglares de América Latina en la encrucijada. Faro 1: 3-7. [ Links ]














