INTRODUCTION
Detailed studies of bird plumage characteristics and careful descriptions of patterns and mechanisms of molt processes have helped understand and clarify the temporal and spatial dynamics in the life cycles of a variety of bird species from temperate zones (e.g., Jenni and Winkler 1994, Pyle 1997, 2008), which has lead to better comprehension of the ecology and evolution of this key stage within the life cycle of these animals. Unfortunately, few research articles evaluate these aspects in Neotropical birds, which hinders their discussion in functional, evolutionary and biogeographical contexts.
Molt duration is one of the variables with enough information for comparative purposes, especially in European birds. Among the data presented by Ginn and Melville (1983) on the molt patterns of 239 species, duration of molt for primary feathers in Passerines, excluding Corvidae, show that these birds take an average of 65 days (IC95 % = 61-68 days). Silveira and Marini (2012) estimated that seven species of Brazilian passerines take about 126 days to undergo the same process. Similarly, Johnson et al. (2012) described a duration range of 98-301 days for 27 Amazonian passerines, showing that these species take between two to four times more to replace flight feathers than the duration recorded in most species from temperate zones. This difference in molt duration could support the hypothesis of the slow-pace of life for tropical birds (Wikelski et al. 2003), as a mean to organize their life cycle (Wingfield 2008). Given that molt duration is an important variable in understanding the evolution of the organization of annual cycles in birds, since it must be carefully regulated and tuned with other energetically important events of the life cycle, like migration (de la Hera et al. 2011), and reproduction (Dawson et al. 2000, Rohwer and Wang 2010), knowing the duration and related variables (e.g. intensity) could greatly enhance our chances of comparing different life history strategies.
The time needed to replace primary feathers has been used as a standard for evaluating molt duration in Passerines, given that the replacement of these feathers generally encompasses the period of molting all other feathers (Mallet-Rodrigues and Noronha 2001), although several exceptions exist (e.g.Wolf 1977). Duration has usually been established through linear regression between time and molt score (Pimm 1976), and requires considerable field effort. However, given that the time it takes to replace feathers is the result of the interaction of at least three other variables, such as rate of feather growth (Grubb 1989, 2006, Yosef and Grubb 1992), molt intensity (number of flight feathers growing simultaneously), and feather length (Rohwer and Wang 2010), these could be measured independently and used to calculate the duration of primary feather replacement. One of the advantages of this method is the feasibility in which these variables can be obtained from few samples, given that little variability has been shown between individuals (Grubb 2006).
This work presents and compares estimates of molt duration in two species of Neotropical Passerines, the Blue-black Grassquit (Volatinia jacarina Linnaeus, 1766) and Gray Seedeater (Sporophila intermedia Cabanis, 1851), using the two most common methods by Pimm (1976) and Rohwer and Wang (2010). We emphasized benefits and drawbacks with these methods for studying tropical species, which could contribute to a more efficient and complete study of this key aspect of the avian life cycle. Also, studying the birds molt cycle may permit to compare them and to understand the ecological and evolutionary factors involved in the molt duration and characteristics. By describing the molt duration and details of tropical species in detail, it will be possible to elaborate comprehensive comparisons among the variations between temperate and tropical species life-cycles.
MATERIAL AND METHODS
The study took place at the Centro Universitario Regional del Norte (CURDN) at Universidad del Tolima, municipality of Armero-Guayabal, northern of the department of Tolima, Colombia (05°00’North, 74°54’West; 280 m a.s.l.), in a Tropical dry forest. The region has a mean annual temperature of 28 °C and precipitation of 1791 mm, distributed in a bimodal regime, with rainy seasons from Mar to May and Oct to Dec. The field work phase was carried out between Feb 2011 and Jan 2012. Birds were captured in secondary vegetation, using 10 mist nets (12 x 2.5 m, 36-mm mesh). Nets were open to ensure a monthly effort of 150 net-h in three days, working from 6:00 -17:00 h but closing them by midday. Individuals of Blue-black Grassquit and Gray Seedeater were marked with a unique combination of colored bands, processed and released, following standard methodologies (NABC 2001). Birds were sexed by plumage coloration and presence of cloacal protuberance/brood patch, aged using plumage and molt criteria (see Moreno-Palacios et al. 2013, 2017) and adopting the age classes suggested by the WRP system (Wolfe et al. 2010, Johnson et al. 2011). Finally, birds were weighed using a digital scale (0.1 g precision).
Two methods were used to estimate molt duration of the primary feathers (data from recaptures were not included). All measures were performed only using data from the right wing. First, we estimated duration by using a linear regression between time and molt score of primary feathers, following Pimm (1976), using molt score as the independent variable. Molt score was obtained by following Newton (1966), with modifications suggested by Rohwer (2008). Old feathers received a score of zero (0), whereas the new ones were assigned a score of one (1). Each feather in growth was described as a fraction of its total length, by using decimal values from 0.1 to 0.9. Missing feathers were scored as 0.01, whereas entirely developed feathers, but with traces of the quill at the base, code 0.99 was used. The scores across all primaries were added, so that the total score described the molt status of each individual. As suggested by Dawson and Newton (2004) the molt score should be corrected by feather mass, because of a better correlation with time may be found. Thus, to adjust the raw molt score, the Dawson and Newton (2004) method was implemented by collecting six individuals of Blue-black Grassquit and eight of Gray Seedeater from which the nine primary feathers were removed from the right wing. Feathers were weighed with an analytic scale (Ohaus, 0.0001 g) after were being dried in a desiccator with silica for 30 min. An average mass was found for each of the nine primary feathers, and the contribution of the mass of each feather to the total mass of primary feathers was calculated by taking the average mass value of each feather and dividing it by the total average weight of all the primary feathers. The molt score obtained of each primary feather was transformed to a score corrected by the feather mass. This was done by taking the value of the feather’s molt score and dividing by 10 (the number of fractions available i.e. 0.1 to 1). This value was multiplied by the average mass of each feather. Finally, the scores of each primary feather were added to obtain the total molt score, corrected by feather mass for each individual of the sample. With these transformed data, linear regressions were conducted between the molt score and time.
Upon finding the linear equation, the value of x (molt) was replaced by the maximum and minimum molt score (0 and 1), thus obtaining the values of y (time) to find the molt duration estimate, with its respective confidence intervals in the population of both species. Likewise, molt initiation and end dates were calculated. Events of complete molt were detected during various periods of the year for each species, which can affect the duration estimate. Some molt events included individuals that showed a clear suspended flight feather molt; others may represent different cohorts. Outliers were removed from the distribution following Cook's distance. Individuals with suspended molts were also removed from the analysis. This process considerably diminished the sample (~30 %) without visibly affecting normality of the data.
The second method used to estimate molt duration of primary feathers was that proposed by Rohwer and Wang (2010), using data of molt intensity, length, and growth rate of primary feathers. First, molt intensity was evaluated counting the average number of primaries growing simultaneously in all molting birds. Second, using the primary feathers obtained from individuals collected per species, the length of each of these was measured and the average total length of the primary feathers was obtained. Third, the growth rate of primary feathers was obtained from the growth bars. For this, at least six growth bars were measured in each primary feather and then the average was obtained for each individual. The growth rate was then averaged among the number of individuals from the sample.
According to Rohwer and Wang (2010), assuming no interruption in the molt of primary feathers, the number of days it takes for and adult individual to replace all primary feathers is given by the following expression: d = l / (r y), where d is the number of days, l is the total length of the primary feathers, r is the rate of growth of primary feathers in mm/day, and y is the average number of primary feathers growing simultaneously. Additionally, confidence intervals were calculated for each of the variables.
Molt duration of primary feathers for each species was estimated for adults and immature individuals (birds undergoing a pre-formative molt) independently, however it was not possible to discriminate by sex due to a small sample size. The difference in flight feather molt intensity between Blue-black Grassquit and Gray Seedeater and between age classes, within and between species, was evaluated with Mann-Whitney U tests. The represented age classes, and their abbreviations follow the classification system based on WRP molt cycles (Wolfe et al. 2010, Johnson et al. 2011).
Finally, dynamics of flight feather molt intensity were described according to Rohwer (2008), detailing the number of growing flight feathers, related to the outermost growing primary feather. This allows observing the distribution of the number of flight feathers in simultaneous growth as the molt of primary feathers took place.
RESULTS
After a sampling effort of 1800 h-net, 94 individuals of the Blue-black Grassquit (5.2 ind/100 net-h) were captured, including to 52 males, 39 females, and three individuals for which sex was not determined. For Gray Seedeater, 85 individuals (4.7 ind/100 net-h) were captured, corresponding to 39 males and 46 females. A recapture rate of 11 % was obtained for Blue-black Grassquit (10 ind) and of 21 % for Gray Seedeater (18 ind).
Among Blue-black Grassquit individuals, 65 % (61 ind) registered molt. Of these, 52 % were birds undergoing the definitive/second pre-basic molt (DPB/SPB), 33 % were undergoing pre-formative molt (FPF), 10 % first/definitive pre-alternate molt (FPA/DPA), and 5 % first pre-basic molt (FPJ). For Gray Seedeater, 67 % (57 ind) of the individuals were in molt. Of these, 54 % were in the definitive pre-basic molt (DPB/SPB), 42 % in pre-formative molt (FPF), and 4 % in first/second pre-alternate molt (FPA/SPA).
Complete molts (FPF, DPB/SPB) were observed between Jan and May (Blue-black Grassquit, N = 53) and Dec and Feb (Gray Seedeater, N = 56). Although the age classes overlapped during the primary molt (Fig. 1), an independent analysis was performed by age.
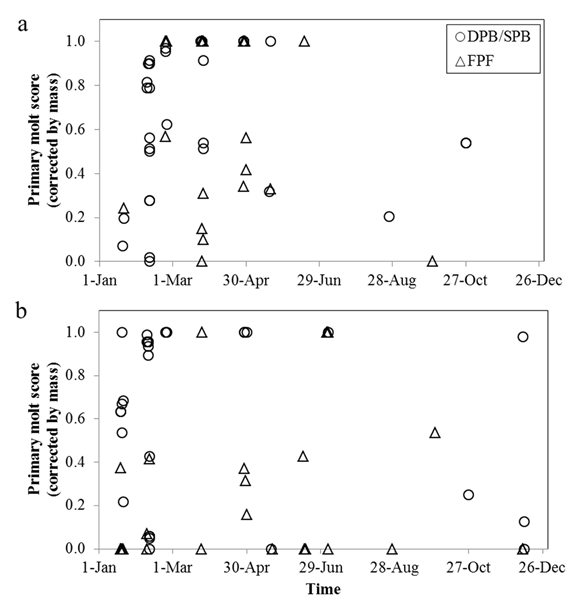
Figure 1 Temporal dispersion of the primary molt score for a. Blue-black Grassquit and b. Gray Seedeater individuals with replacement of body (scores 0-1) and/or primary remiges. FPF = Immature pre-formative individuals, DPB/SPB = adult definitive/second pre-basic individuals.
The estimated duration of molt of primaries in adults (DPB/SPB) under Pimm’s method was 80 days (CI 95 % = 64-96 days) for Blue-black Grassquit (N = 17, r 2 = 0.34, t = 2.7711, P = 0.01), and 100 days (CI 95 % = 75-124 days) (N = 16, r 2 = 0.62, t = 4.7488, p < 0.001) for Gray Seedeater (Fig. 2).
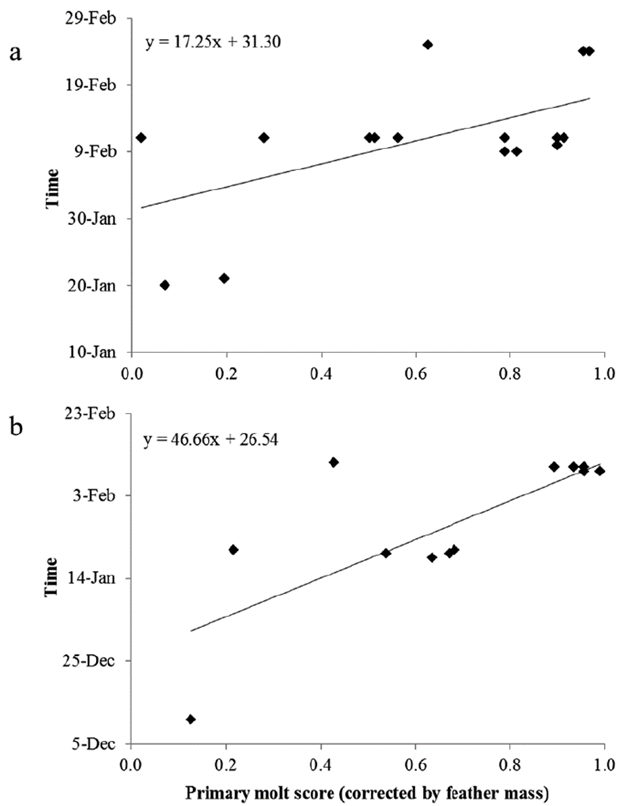
Figure 2 Pimm’s regression of primary molt scores adults over time. a. Blue-black Grassquit and b. Gray Seedeater.
Upon analysis of immature individuals (FPF), the linear regression showed a slope not significantly different from zero in both species (Blue-black Grassquit N = 9, r 2 = 0.040, t = 0.5463, P = 0.6; Gray Seedeater N = 7, r 2 = 0.037, t = 0.4392, P = 0.6); hence, no estimation of duration was possible. Individuals of Blue-black Grassquit were not recaptured during primary feather molt. Two individuals of Gray Seedeater were recaptured during two periods of molt; the duration of primary feather molt of one of the adult females (DPB) was estimated at 79 days, whereas that of the other was estimated at 359 days. However, this last female showed a clear suspension in the molt of primary feathers.
The estimated date for the start of primary feather molt in adults of Blue-black Grassquit was 20 Dec, and the end date was 10 Mar, while for Gray Seedeater it was 14 Nov and 22 Feb, respectively. It is likely that, because of the asynchrony observed in both species, these results represent an average of initiation and end dates for the population, with adult individuals of Blue-black Grassquit ending the pre-basic molt by mid- May, prior to the dry season, and with some adult individuals of Gray Seedeater undergoing pre-basic molt between Apr and Jun.
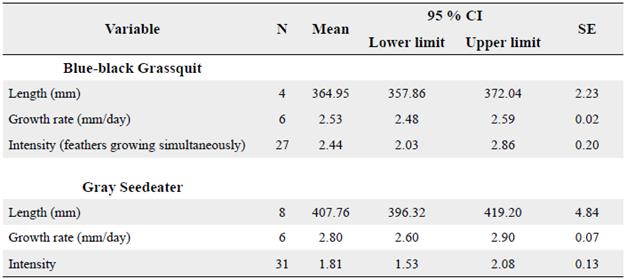
Following the Rohwer and Wang (2010) method, the estimated duration of the molt of primary feathers in adults was 59 days (CI 95 % = 48-74 days) for Blue-black Grassquit, and 80 days (CI 95 % = 66-105 days) for Gray Seedeater (Table 1). In both species, the estimated duration of primary feather molt using the Rohwer and Wang (2010) method was lower than those estimated through linear regression (21 days for Blue-black Grassquit and 20 days for Gray Seedeater). However, confidence intervals (95 %) between estimates overlapped by 10-30 days, which indicates similarity between both calculations.
Table 1 Variables used to estimate molt duration of primary feathers in Blue-black Grassquit and Gray Seedeater. SE = Standard error
No significant differences were found in flight feather molt intensity between immature (FPF) and adult (DPB/SPB) individuals of both species (Blue-black Grassquit Mann-Whitney U = 56.5, z = -1.82, P = 0.07; Gray Seedeater U = 71.5, z = -1.341, P = 0.2), so data were combined for further analysis.
Molt intensity in flight feathers in both species increased as the molt of the most internal primary feathers advanced (P1-5), and then decreased as primary feather molt reached P9 (Table 2, Fig. 3). In Blue-black Grassquit, the most internal secondary feathers (S7-9) began to grow with P3. When the molt proceeded toward P4, the rectrices also started being replaced, along with external secondary feathers S1-6 in Blue-black Grassquit and S1-5 in Gray Seedeater. It is not clear what happend during the molt of P7-8 in Blue-black Grassquit; however, upon detailing the increase in averages, it may be suggested that during the molt of P7-8, feathers from various molt series would be growing simultaneously (i.e. secondaries and external rectrices), perhaps showing the point of highest molt intensity. The replacement process of remiges and rectrices reached its maximum intensity during molt of P7 in Gray Seedeater, with a total of 13 feathers in growth. Then, while molt is proceeding to P9, some series may have ended replacement of feathers; thus, the number of feathers in molt drops rapidly in both species, showing an average of four in molt when S9 is the most external primary feather in growth.
Table 2 Average number of flight feathers in growth for each molt series in Blue-black Grassquit and Gray Seedeater, organized according the most external primary feather in growth. A dash indicates no data available. In the Gray Seedeater the internal secondaries include S6 (see Moreno-Palacios 2013)
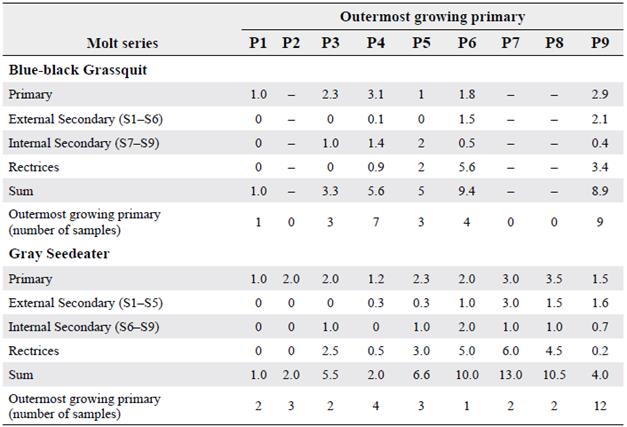
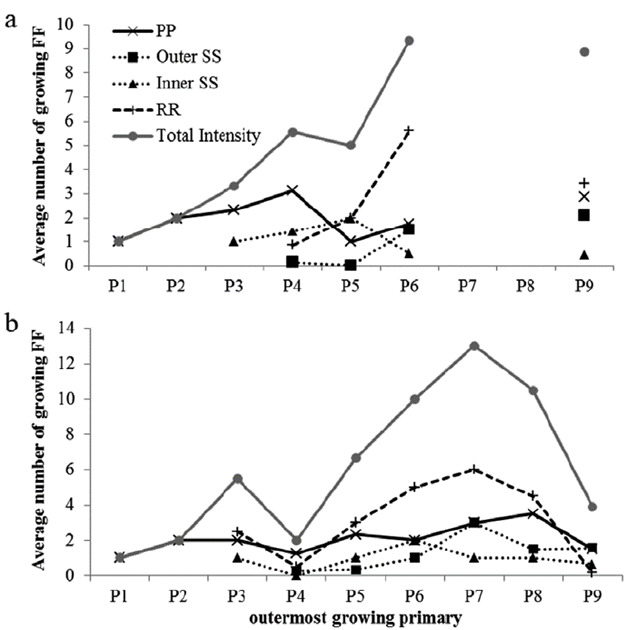
Figure 3 Average intensity of flight feather molt related to the most external primary in growth a. Blue-black Grassquit and b. Gray Seedeater. PP = Primaries, SS = Secondaries, RR = Rectrices, FF = Flight feathers
The measurements of Gray Seedeater molt suggest that internal secondary feathers delay their molt until P5 is in growth, which is why it was not possible to observe the increased molt intensity in P4, giving the impression of having diminished. However, an increase in the number of feathers in growth is evident when P5 starts to grow, and the entire molt series is active.
No significant differences were found in flight feather molt intensity between the two species (Mann-Whitney U = 476.5, z = -0.6749, P = 0.5), among adult individuals (Mann-Whitney U = 252, z = -0.728, P = 0.5), or among immature individuals (Mann-Whitney U = 35, z = -0.0493, P = 0.9) of these species.
DISCUSSION
The estimates obtained for molt duration of primary feathers in adults from both species were consistent between both methods, in spite of their methodological differences, in which the estimated duration of the molt of primary feathers was lower in Blue-black Grassquit than in Gray Seedeater. Blue-black Grassquit showed lower body mass (average of 9.3 g ± 1.3, N = 89) than Gray Seedeater (mean 10.8 g ± 1.65, N = 78), and although the mass difference is only 1.5 g on average, our results are consistent with the hypothesis that the molt duration increases with relation to body mass (Rohwer 2008, Silveira and Marini 2012), without it necessarily compromising a linear relationship (Rohwer et al. 2009).
Rohwer (2008) states that, on average, a bird with a mass of 10 g needs 179 days to replace all primary feathers one by one. However, birds that increase molt intensity may considerably diminish the time required. For example, Blue-black Grassquit had an average intensity of 2.4 primary feathers and a duration of 59-80 days, whereas in Gray Seedeater the average intensity value was 1.8 primary feathers and a duration of 80-100 days. If the upper and lower limits are taken for molt duration in both species, these intensities are sufficient to reduce the molt duration of primary feathers between 45 % and 68 % of the theoretical time required; similar to that found in the Northern Rough-winged Swallow (Stelgidopteryx serripennis Audubon, 1838) (Yuri and Rohwer 1997, Rohwer et al. 2009). When comparing the molt duration of Blue-black Grassquit and Gray Seedeater with small Passerine birds reported by Ginn and Melville (1983), the time required in European species is 65 days (CI 95 % = 61-68 days), which is close to the lower limit found for both species in this study. This suggests that these Neotropical species take more time growing the primary remiges than the typical species from the temperate region. Although Guallar et al. (2009) studied in detail the molt of Blue-black Grassquit in western Mexico, they did not estimate the molt duration of primary feathers. However, they documented a three-month pre-basic/pre-formative molt season, which agrees with the duration of primary feather molt calculated for the species in this study. Silveira and Marini (2012) found similar results in molt duration estimates of several Brazilian species, and argue that this longer time supports the idea of tropical birds having slower life cycles and rhythms than birds that breed in temperate and boreal zones (Wikelski et al. 2003, de la Hera et al. 2011).
We found low synchrony in molt timing among individuals from populations of Blue-black Grassquit and Gray Seedeater in this one location (Fig. 1), which could affect the estimates made through linear regression. Thus, considerations and decisions to filter the data could have significant consequences on the results, adding subjectivity to the process because it may depend on the researcher’s ability to detect suspended molts or different cohorts, consequently obtaining variable estimations. Individuals molting primary remiges outside the most regular molt period were possibly birds that have suspended the molt and have reactivated the process after the mid-year dry season (Jul to Sep), overlapping with the partial pre-alternate molt. Because the low synchrony observed in the molt of Blue-black Grassquit and Gray Seedeater, it is likely that the method by Rohwer and Wang (2010) would yield more accurate results on primary feather molt duration, due to the direct measurement of the variables required in the estimation, and because of the reduced variability that they show between individuals (Grubb 2006).
The method initially proposed by Pimm (1976) has been evaluated by other authors. Underhill (1985) underlined that this method is an alternative to eliminate de problem of heteroscedasticity inherent to molt data by treating molt score as the independent variable and time as the dependent variable. However, the lack of variance homogeneity is not apparently solved, but instead an epistemological inconsistency arise, there is no sense in which time depends on molt (Underhill 1985, Newton 2009). Underhill and Zucchini (1988) proposed a mathematical model specifically designed for molt data, but as they clearly stated, this model does not resolve cases of arrested molt, suspended molt, partial molt and Staffelmauser pattern. However, methods like those suggested by Underhill and Zucchini (1988) are complex, leading researchers to continue working on the classical proposal (e.g., Voelker 2000, Silveira and Marini 2012). Additionally, although estimation through linear regression may be more robust in statistical terms, it may lead to overestimation when dealing with low synchrony of molt between individuals or when the birds suspend the molting process. The Rohwer and Wang (2010) method could be an important alternative to work out some of these inconveniences, involving independent variables of the phenomena mentioned and for which an adequate sample can be gathered with little field effort.
It is important to highlight that in this study, we made the duration calculations for both species combining data from immature (FPF) and adult birds (DPB/SPB). However, in spite of these age classes undergo the complete molt at the same time, this not necessarily means that both ages are under same pressures. That is because the two molts have probably evolved for different reasons (Howell et al. 2003). Thus, it is possible that different pressures or drives may affect each age separately, so it is reasonable to think that pre-formative birds might take a different time lapse to replace the primaries than adult birds. A better sample by age is required to test this, for which long term studies will greatly be needed.
The average number of feathers in simultaneous growth increased during the molt of primary feathers, and the maximum intensity was reached when P6-7 were growing. These results are similar to those found in the Western Kingbird (Tyrannus verticalis Say, 1823) (Rohwer 2008) and the Northern Rough-winged Swallow (Yuri and Rohwer 1997), which achieved the maximum intensity when P7 was the outermost primary feather in development. This suggests the existence of a molt pattern probably associated with aerodynamic causes. In this sense, Hedenström and Sunada (1999) showed that molt gaps located inwards on the wing have the largest effect on aerodynamic efficiency compared with those found close to the wing tip. Thus, having more flight feathers molting simultaneously (i.e. having more molt gaps) at P6-7 could reflect an optimal position to increase molt intensity and reduce significant impacts on the ability to escape from predators. Although the molt gaps do not necessarily involve an additional energetic cost for birds (Slagsvold and Dale 1996, Hedenström and Sunada 1999), individuals with reduced wing areas may experience increased predation levels, as the case described by Slagsvold and Dale (1996) in which females of Pied Flycatchers (Ficedula hypoleuca Pallas, 1764) with induced molt gaps showed a significantly higher disappearance percentage (caused by predation) than control birds.
The Gray Seedeater reached a higher number of flight feathers in simultaneous growth, compared to Blue-black Grassquit (13 and 9.3 feathers, respectively), and it is more similar to Western Kingbird (12 flight feathers, Rohwer 2008). This contrasts with other Passerines such as: Northern Rough-winged Swallow (6.8 flight feathers, Yuri and Rohwer 1997), Wedge-tailed Grass-Finch (Emberizoides herbicola Vieillot, 1817) (7.0), Lesser Elaenia (Elaenia chiriquensis Lawrence, 1865) (6.0), Plain-crested Elaenia (E. cristata Pelzeln, 1868) (5.9), White-banded Tanager (Neothraupis fasciata Lichtenstein, 1823) (5.5), Narrow-billed Woodcreeper (Lepidocolaptes angustirostris Vieillot, 1818) (4.0), Rufous-fronted Thornbird (Phacellodomus rufifrons Wied, 1821) (4.0) and Grassland Sparrow (Ammodramus humeralis Bosc, 1792) (3.0) from central Brazil (Silveira 2011). Thus, flight feather molt intensity seems to be highly variable among Passerines (Rohwer 2008), which likely represents an adaptive response to important ecological and life cycle pressures, such as migration (de la Hera et al. 2011), or reproduction, in the case of resident Neotropical species (Svensson and Hedenström 1999).
Some of the birds studied by Silveira (2011) correspond to species from open areas, scrub, and natural grasslands, which could have similar conditions to the vegetation from the tropical dry forest in the upper valley of the Magdalena River. Their molt intensities were lower by 2.3-10 flight feathers from Blue-black Grassquit and Gray Seedeater in our study. This suggests that although these are species with related habits, different pressures exist that alter the intensity of molt. For example, the study area described by Silveira (2011) presents a marked rainy and dry period, while in the upper valley of the Magdalena River there is a bimodal rain pattern. It is likely that birds may adjust the molt duration through changing intensity, to carry out total replacement during a single period, even suspending the molt during the peak of the drought, to be reactivated during the following rainy season, as noted in some Blue-black Grassquit and Gray Seedeater individuals in this study. Although both species are taxonomically related and are similar in habitat and diet, it seems they also face similar pressures in the environment, which have led to their presenting high intensity in flight feather molt. Overall, molt duration of Gray Seedeater was higher than that of Blue-black Grassquit, although the seedeater also presented higher intensity; variations that could be associated with body mass. In general, a phylogenetic study that incorporates other life history traits (i.e. habitat, reproductive season duration, migration status, etc.) is necessary to better understand what drives this variation in molt intensity across species.
Both methods for evaluating molt duration showed similar estimates. The method by Rohwer and Wang (2010) presents methodological advantages that would permit evaluating these parameters in more species, so that they can be incorporated in comparative ecological and evolutionary studies. Increasing our understanding of how this key event in the life cycle of non-migratory species is regulated and develops will enable us to use a comparative approach to understand the proximate and ultimate factors regulating populations under current habitat transformations.














