INTRODUCTION
San Andres Island is part of the International Biosphere Reserve Seaflower, one of the largest marine reserves in the world (CORALINA-INVEMAR 2012); however, the marine flora of the island has historically received little attention (Albis-Salas and Gavio 2011). Recently, collections of macroalgae have been undertaken in the whole archipelago, revealing a flora much more diverse than previously appreciated (Albis-Salas and Gavio 2011, Ortiz and Gavio 2012, Reyes-Gómez et al. 2013, Gavio et al. 2013, Barrera et al. 2016, Reyes-Gómez and Gavio 2017).
In 2003, Díaz-Pulido and Díaz-Ruiz presented an updated list of macroalgae for the Caribbean coast of Colombia, including a total of 565 taxa, 201 of which were reported for the Archipelago of San Andres, Old Providence, and Santa Catalina. From 2011 to date, phycological studies have been resumed in the archipelago: Albis-Salas and Gavio (2011) included six new records of red epiphytic algae, growing over Thalassia testudinum K.D. Koenig. Ortíz and Gavio (2012) listed 80 macroalgae species, reporting 23 as new records, 17 for the archipelago and six for Colombia. Reyes-Gómez et al. (2013) recorded seven species of Cyanobacteria, of which five were new records for Colombia. Additionally, Gavio et al. (2013) described a new species for science, Crouania pumila B. Gavio, V.P. Reyes-Gómez & M.J. Wynne, which was found in samples taken at different coastal ecosystems of the archipelago. Rincón-Díaz et al. (2014) reported a new record of the red alga for Colombia, Ceramium bisporum D.L. Ballantine, exhibiting variation in reproductive structures from the original description.
Vega-Sequeda et al. (2015) explored the northernmost ocean reef complex of Colombian Caribbean: Bajo Alicia cay (Joint Regime Area between Colombia and Jamaica) and Serranilla cay, where they made rapid ecological evaluations to determine the composition and abundance of the most representative groups of marine biota. For the macroalgae, they recorded 16 species of brown algae, 22 of green algae, and 23 of red algae, and several cyanobacterial morphotypes. Their results included seven new records for Colombia and for the International Reserve Seaflower: Caulerpa lanuginosa J. Agardh, Caulerpa paspaloides var. laxa Weber-van Bosse, Jania cubensis Montagne ex Kützing, Gracilaria cornea J. Agardh, Chamaedoris peniculum (J. Ellis and Solander) Kuntze, Cladophora prolifera (Roth) Kützing, and Hydrolithon chamaedoris (Foslie and M. Howe) M.J. Wynne.
Gavio et al. (2015) published a list of species collected in the northern key of the archipelago, specifically at Quitasueño, also known as Queena Reef. A total of 75 taxa were listed: 14 were new records for Colombia and nine new records for the archipelago. Albis-Salas and Gavio (2015) reported nine species as new records for the Archipelago, all found growing as epiphytes on Thalassia testudinum.
Rincón-Díaz et al. (2016) found as a new record for the Western Atlantic the species Griffithsia capitata Børgesen, originally reported exclusively from Macaronesia. Barrera et al. (2016), exploring the intertidal rocky shore habitats associated with the mollusk Cittarium pica (Linneaus, 1758) on San Andrés Island, reported 25 new records, 16 for Colombia and nine for the Archipelago. Finally, Reyes-Gómez and Gavio (2017) reported two new records of brown algae for Old Providence Island.
To date, the list of species of seaweed registered for the archipelago includes a total of 303 taxa. Most of these species are small filaments or filiform in morphology and difficult to detect and to identify, although they appear abundant and are epiphytes of larger algae or seagrasses. These findings reveal the high diversity of benthic flora in the Archipelago and the need to continue exploring for them.
To complete the biodiversity study of the International Biosphere Reserve Seaflower, we discuss our recent findings on 10 species, four of which are new records for the Archipelago and the other six new for the Colombian Caribbean.
MATERIALS AND METHODS
San Andrés (12°28’ North; 81°40’ West) is an oceanic island of coral origin located in the southwestern Caribbean, Colombia (Fig. 1). The island has, on the eastern side, a barrier reef running parallel to the coast, which encloses a shallow lagoon. The western side is characterized by two submerged terraces, parallel to the coastline: one terrace is shallow (4-10 m depth), while the second is deeper (10-20 m depth) (Chaves-Fonnegra et al. 2007). All the specimens were collected randomly by SCUBA diving over the coral reef formations on the western side of the island, at the localities “Wild Life” (12°30’30” North; 81°43’45” West) and “Green Moon” (12°29’07” North; 81°44’01” West), at depths ranging from 9-17 m. This sector is characterized by a reef terrace with coral mat formations starting at a depth of 9 m, characterized by high species diversity of corals, sponges, octocorals, and algae (Díaz et al. 2000).
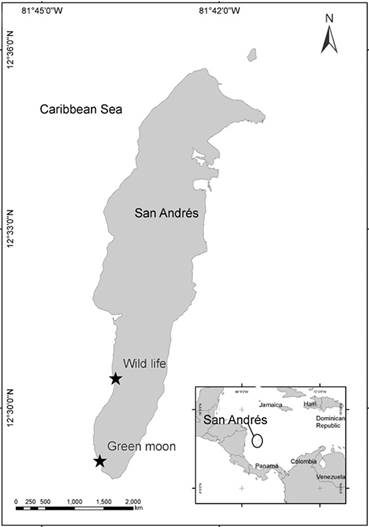
Figure 1 San Andrés Island with sampling sites. Wild Life (12°30’30” North; 81°43’45” West). Green Moon (12°29 07” North; 81°44′01” West).
Sampling was carried out during the rainy (from November 2012 to January 2013 and June 2013 to August 2013) and dry season (from February 2013 to May 2013). The algae were collected by hand and then preserved in a 4 % formalin/seawater solution. In the laboratory, algae were identified using an OLYMPUS BX51 optical microscope (Olympus, Tokyo, Japan) with specific literature for species identification such as Taylor (1960), Littler and Littler 2000, and Dawes and Mathieson 2008). Slide material was mounted in 50 % glycerin, with previous staining in aniline-blue solution. All specimens were deposited in COL, the Herbarium of the Universidad Nacional de Colombia. Information on type localities and nomenclature was obtained from Dr. Paul C. Silva’s Index Nominum Algarum (Silva c2017) and AlgaeBase (Guiry and Guiry c2017).
RESULTS AND DISCUSSION
We listed and commented on a total of 10 new records of red algae found attached to coral rubble or epiphytic on larger algae. Callithamniella tingitana, Frikkiella searlesii, Lejolisia exposita, Melanothamnus gorgoniae, Monosporus indicus, and Wrangelia gordoniae are new records for Colombia (**). Dohrniella antillarum, Halydictyon mirabile, Taenioma nanum, and Aglaothamnion cordatum are new records for the archipelago (*).
RHODOPHYTA
Order Ceramiales
Family Callithamniaceae
Aglaothamnion cordatum * (Børgesen) Feldmann-Mazoyer Figs 2a-b
Type locality: Virgin Islands, Caribbean Sea.
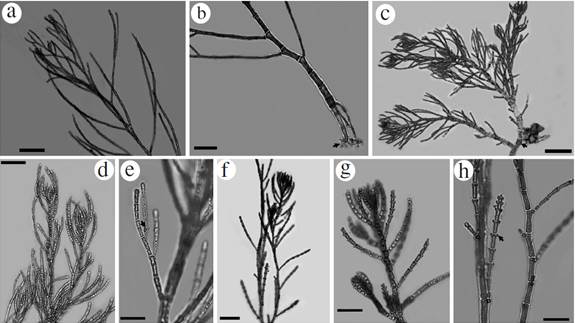
Figure 2 Habit of species reported from the International Biosphere Reserve Seaflower. Aglaothamnion cordatum: a. Principal branches, b. Detail of rhizoids with haptera (arrowhead). Callithamniella tingitana: c. Habit with detail of prostrate axis and multicellular rhizoids (arrowhead), d. Erect and alternate branches bearing glandular cells. e. Detail of glandular cells (arrowhead) located in the last dichotomy of the branches. Dohrniella antillarum : f. Habit and detail of erect axes, g. Main branch bear alternately decumbent branchlets with papilliform cells (arrowhead), h. Detail of basal cells of branches and papilliform cells. Scale bar: a, b, c and f = 200 µm; d, g and h= 100 µm; e= 50 µm.
Specimens observed: San Andrés Island: Wild Life: 02/IX/2012, depth 13 m; 22/I/2013, depth 17 m; 4/IV/2013, depth 13 m; 7/VI/2013, depth 10 m; 09/VIII/2013, depth 10 m. Green Moon: 6/II/2013, depth 12 m; 2/IV/2013, depth 9 m. All specimens were in a vegetative stage. Specimen collected at Green Moon, 6/II/2013, deposited at COL (COL 602242).
Thallus filamentous, erect, delicate (Fig. 2a), up to 5 cm high; uniseriate main axes ecorticate throughout, attached to the substrate by descending multicellular rhizoidal filaments (Fig. 2b). Main axis 50-110 µm in diameter, tapering distally; cells 180-250 µm long (Fig. 2a). Branchlets alternate and radial, composed of cells 15-20 µm in diameter and up to 125 µm long. Apical cells of secondary branchlets are longer and obtuse, 4 µm in diameter and 7.5 µm in length, and basal cell 50 µm diameter and 120 µm in length. Basal portion formed by multicellular rhizoids with haptera (Fig. 2b). No reproductive structures were observed. All specimens were found growing as epiphytes on Canistrocarpus cervicornis (Kützing) De Paula et De Clerck, Dictyota pulchella Hörnig et Schnetter, Lobophora variegata (J.V. Lamouroux) Womersley ex E.C. Oliveira, and Halimeda opuntia (Linnaeus) J.V. Lamouroux.
Remarks: To date, there are 28 species of Aglaothamnion taxonomically accepted (Guiry and Guiry c2017). Most species are differentiated by the presence or absence of rhizoidal cortication, the branchlet disposition and the shape of carposporophytes. In the tropical and subtropical Western Atlantic, there are 11 recognized species of Aglaothamnion (Wynne 2017). Aglaothamnion collinsii Aponte, Ballantine & Norris, A. felipponei Aponte, Ballantine & Norris, A. herveyi (Howe) Aponte, Ballantine & Norris and A. priceanum Maggs, Guiry & Rueness are easily distinguishable from our specimen because they are corticated. A. gallicum, A. halliae (Collins) Aponte, Ballantine & Norris, A. roseum (Roth) Maggs & L'Hardy-Halos and A. uruguayense (W. R. Taylor) N. E. Aponte, D. L. Ballantine & J. N. Norris have distichous branching, while our specimen has radially distributed branchlets. We can also discard A. boergesenii (Aponte & Ballantine) L'Hardy-Halos & Rueness because of its coarser habit: according to the original description (Aponte and Ballantine 1990, as Callithamnion boergesenii) the main axis is 160-220 um in diameter, while our specimens are in the range of 50-110 um in diameter.
The remaining species, A. cordatum and A. flexibile (Collins) Aponte, Ballantine & Norris share many characters. However, they are easily distinguishable by the shape of carposporophytes, and the overall shape of the apices, which are distinctly flat topped in A. flexibile and pyramidal in A. cordatum. In the absence of reproductive structures, the apex outline in our specimens fits the description of A. cordatum. The species has been previously reported for the continental coast of Colombia, for the National Natural Park Tayrona (near the town of Santa Marta) and for the Darien region, close to the border with Panama.
Family Ceramiaceae
Callithamniella tingitana ** (Schousboe ex Bornet) Feldmann-Mazoyer Figs 2c-e
Type locality: Tangier, Morocco.
Specimens observed: San Andrés Island: Green Moon: 12/VIII/2013, depth 12 m, vegetative specimen.
Very small algae (up to 1 mm), epiphytic on Halimeda tuna (J. Ellis & Solander) J.V Lamouroux. Branched thallus with prostrate and erect uniseriate axes (Fig. 2c). Prostrate axis consisting of cells that are 70-80 µm in diameter and 110-135 µm in length. From these cells originate either a rhizoid or an erect axis. Rhizoids are multicellular, with lobate haptera. The axial cells in the middle thallus are about 68 µm in diameter and 80 µm in length. Apical cells are 15-25 µm in diameter and 30-60 µm in length. Determinate erect axis with 1-2 orders of branches made up of cylindrical cells of 20 µm diameter at the base, and 50 µm long; these branches have dichotomous to pseudodichotomous divisions. Cells of the erect branches with 15 µm diameter at the apex and 25 µm long. Divisions of branches have small basal cells of 15 µm in diameter and 15 µm in length near the apex; these cells have a 6.5 µm diameter and a 7.5 µm in length (Fig. 2d). The specimens observed had few inconspicuous glandular cells that are usually located in the last dichotomy of the branches. These gland cells are 5-7 µm in diameter, are located at one end of the cells and are in contact with just one cell of the branch (Fig. 2e). No reproductive structures were observed.
Remarks: The genus Callithamniella was described by Feldmann-Mazoyer (1938), based on the species Callithamnion tingitana Schousboe ex Bornet (1892). Later, Feldmann-Mazoyer (1941) provided a more detailed description, depicting the creeping axes giving rise to erect axes, on which pedicellate tetrasporangia were produced. Accounts of this species have also been given by Schneider and Searles (1991) and by Dawes and Mathieson (2008) for material occurring on the coasts of North Carolina and Florida, respectively. More recently, Secilla (2012) has described material of this species from the coast of Spain. In the specimens collected, the branches are simple or with a pseudo-dichotomy in the second or third cell from the base, do not exceed 500 μm and are composed of up to 20 cells, while for Callithamniella flexilis Baardseth, the thallus has simple branches, up to 1000 μm and composed of up to 30 cells.
Dohrniella antillarum * (W.R. Taylor) Feldmann-Mazoyer Figs 2f-h
Type locality: Baie Anglais, Aquin, Haiti.
Specimens observed: San Andrés Island: Green Moon: 2/IV/2012, depth 9 m, vegetative specimen.
Thallus delicate (2.5-3 mm high) attached to the substrate by multicellular rhizoids (Fig. 2f). Main branch erect, uniseriate, with basal cells up to 65 µm diameter, 130 µm long, tapering towards the apex (Fig. 2g). Cells in the middle part of the axis 34 µm in diameter, 62 µm in length, and at the apex, 25-30 µm in diameter, 30 µm in length. The main axis bears alternately decumbent branchlets, which are 500 to 600 µm long and have 1-2 elliptical papilliform cells (12 x 18 µm) located at distal cells of each branch (Fig. 2h). No reproductive structures were observed. Specimens epiphytic on Dictyota spp.
Remarks: the genus Dohrniella includes only three species: D. neapolitana Funk, Dohrniella nana Mayhoub, which are restricted to the Mediterranean basin, and D. antillarum, described from Haiti, in the Caribbean Sea, and reported for the tropical Western Atlantic and the Atlantic coast of Africa (Guiry and Guiry c2017). These algae are characterized by their small size, and the presence of papilliform cells, which is considered a characteristic of the genus (Mayhoub 1975). Our specimen can be easily distinguished from D. nana by the absence of unicellular rhizoids and from D. neapolitana by the absence of secretory cells (Mayhoub 1975). Due to its small size, it is easily overlooked. For the continental coast of Colombia, it has been previously reported for National Natural Park Tayrona and the Darien region (Díaz-Pulido and Díaz-Ruiz 2003).
Family Dasyaceae
Halydictyon mirabile* Zanardini Figs. 3a-b.
Type locality: Adriatic Sea.
Specimens observed: San Andrés Island: Green Moon: 6/II/2013, depth 12 m. 12/VIII/2013, depth 12 m. These were vegetative specimens. Specimen collected at Green Moon, 12/VIII/2013, deposited at COL (COL 602241).
Thallus soft, forming turfs like a mesh. Light-pink to pale-red branches. Uniseriate axes formed of cylindrical cells, branched, with branches fusing to form a mesh. The branchlets are 170 µm in diameter, cells up to 700 µm in length (Fig. 3a). The main axis is indistinct. At the apex, cells are rounded to semi-acute (Fig. 3b). Growing on Canistrocarpus cervicornis and Halimeda tuna (J. Ellis et Solander) J.V. Lamouroux, and attaching by small rhizoids.
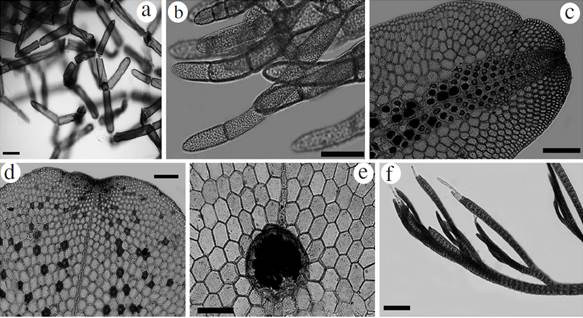
Figure 3 Habit of species reported from the International Biosphere Reserve Seaflower. Halydictyon mirabile: a. Filament branching in the center of thallus, b. Detail of filaments apex. Frikkiella searlesii: c. Blade with central tetrasporangial sori, d. Blade bearing spermatangial sori. e. Blade bearing a cystocarp. Taenioma nanum: f. Upper portion of blades with two monosiphonous “hairs” at apex. Scale bar: a, c, d, e and f = 200 µm; b = 100 µm.
Family Delesseriaceae
Frikkiella searlesii ** M.J. Wynne et C.W.
Schneider Figs. 3c-e.
Type locality: Mona Island, Playa Carabinaro, Puerto Rico, Greater Antilles.
Specimens observed: San Andrés Island: Wild Life: Tetrasporic specimen, 4/IV/2013, depth 13 m; Spermatangial and tetrasporic specimens, 09/VIII/2013, depth 14 m; Cystocarpic specimens, 4/IV/2013, depth 13 m. Green Moon: Spermatangial specimen, 12/VIII/2013, depth 12 m. Specimens collected at Wild Life, 09/VIII/2013, deposited at COL (COL 602237 and COL 602240).
Blades primarily monostromatic, 2-3 mm long and 1.5-2 mm wide. Cells of mature blades have pentagonal or hexagonal shapes in surface view (Fig. 3c), the blade arising from a short rhizoidal holdfast, and also exhibiting marginal multicellular rhizoids in some portions of the blades. Third-order rows of cells produced only from outer cells of second-order rows. Narrow tetrasporangial sorus in the center and distal region of the blade (Fig. 3c). Tetrasporangial tetrahedrally divided and to 70 µm diameter. Spermatangial sori scattered over the surface of blades (Fig. 3d). Cystocarps on the mid-line of 370-400 µm in diameter and 380-400 µm in length, with a ostiole measuring up to 100 µm in diameter (Fig. 3e).
Remarks: The genus Frikkiella was described by Wynne and Schneider (1996) based upon specimens from Bermuda and the Caribbean Sea (Bahamas, Puerto Rico and Martinique). It comprises two species: Frikkiella searlesii and F. pseudoprostrata (D.L. Ballantine and M.J. Wynne) M.J. Wynne and C.W. Schneider. They are easily distinguishable from one another by the arrangement of the tetrasporangial sori. Our tetrasporangial specimen fits well the description of F. searlesii. In the Caribbean Sea, this is the southernmost record of the species.
Taenioma nanum* (Kützing) Papenfuss Fig. 3f
Type locality: Naples, Italy.
Specimens observed: San Andrés Island: Wild Life: 22/I/2013, depth 17 m; 4/IV/2013, depth 13 m; 07/VI/2013, depth 14 m. Green Moon: 6/II/2013, depth 12 m; 02/IV/2013, depth 9 m; 12/VIII/2013, depth 12 m. All the specimens were vegetative.
Thalli to 1 mm, creeping, observed growing over larger algae such as Amphiroa tribulus (J. Ellis et Solander) J.V. Lamouroux, Canistocarpus cervicornis, Lobophora variegata, Halimeda spp., Sphacelaria sp., and the cyanophyte Phormidium sp. Often found as part of turf mats. Polysiphonous prostrate axes, with four pericentral cells; axes 60-80 µm in width, with uniseriate rhizoids of 13-20 µm in width. The apices of erect thalli have one apical cell or two apical hairs (Fig. 3f). No reproductive structures were observed.
Remarks: To date, there are only two recognized species of Taenioma: Taenioma nanum and T. perpusillum (J. Agardh) J. Agardh. The first species was described from the Mediterranean Sea and has been reported in most oceans, thus with a cosmopolitan distribution. In the Caribbean Sea, Taylor (1960) reported it as T. macrourum, which was later put in synonymy with T. nanum. Taenioma perpusillum, the type species, whose type locality is the Pacific coast of Mexico, has a cosmopolitan distribution similar to the former taxon, including the Caribbean Sea. The main characters to differentiate the two species is the number of hairs at the apex of the erect axes, which are one or two in T. nanum and three or more in T. perpusillum, and the number of segments of erect determinate branches, which range from eight to 20 in T. nanum and from 20 to 30 in T. perpusillum. Despite that some authors in the past have considered T. nanum and T. perpusillum to be conspecific (Hollenberg 1967, cited in Womersley 2003), at present the two taxa are recognized as separate. Our specimen fits well the description of Taenioma nanum.
Family Rhodomelaceae
Melanothamnus gorgoniae ** (Harvey) Díaz-Tapia et Maggs Figs 4a-d
Type locality: Key West, Florida, USA.
Specimens observed: San Andrés Island: Wild Life: Cystocarpic, spermatangial, tetrasporic and vegetative specimens, 22/I/2013, depth 17 m; Cystocarpic specimens, 04/IV/2013, depth 13 m; Cystocarpic specimens, 7/VI/2013, depth 14 m; Cystocarpic specimens, 09/VIII/2013, depth 14 m. Green Moon: Tetrasporic specimens, 7/XII/2012, depth 10 m; Vegetative specimens and cystocarpic specimens, 6/II/2013, depth 12 m; Vegetative, tetrasporic and cystocarpic specimens, 2/IV/2013, depth 9 m; Cystocarpic and vegetative specimens, 05/VI/2013, depth 10 m; Vegetative specimens and cystocarpic specimens, 12/VIII/2013, depth 12 m. Cystocarpic specimen collected at Green Moon, 02/IV/2013, deposited at COL (COL 602236).
Epiphytic and erect plant up to 3 cm high, found growing on Hypnea spinella (C. Agardh) Kützing, Dictyota spp., Coelothrix irregularis (Harvey) Børgesen, Halimeda tuna and Riphocephalus phoenix f. longifolius A. Gepp et E. Gepp. Thalli with ecorticate segments and four pericentral cells. Main axes 135-150 µm in diameter comprised of segments 150-160 µm in length, fixed by a basal disc with unicellular rhizoids 450 µm length (Fig. 4a). Branchlets of 110 µm diameter and 120 µm length narrowed towards the apex. Scar cells occur one per segment. Apex with hair-like filaments dichotomously divided. Apical and spherical tetrasporangia spirally arranged up to 100 µm in diameter (Fig. 4b). Spermatangial branches up to 100 µm width and 300 µm long, with sterile cell at apex (Fig. 4c). Numerous dichotomically divided trichoblasts, up to 800 µm in length, of up to 10 cells in length, of 1-4 for each dichotomy. Cystocarps ovoid 250 µm in width, and 250 µm in length. Ostiole up to 130 µm long. Carpospores ovoid to 55-60 µm length and 25 µm with in the widest dimension (Fig. 4d).
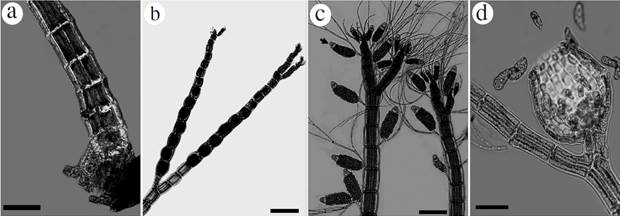
Figure 4 Habit of species reported from the International Biosphere Reserve Seaflower. Melanothamnus gorgoniae: a. Basal region of thallus on Dictyota sp., showing a disc of fixation with rhizoids, b. Tetrasporangial branches, c. Spermatangial branchlets having sterile cell at apex (arrowhead), and with many trichoblasts, d. Detail of cystocarp showing ostiole and carpospores. Scale bar: a, b and c = 200 µm; d = 100 µm.
Remarks: Melanothanmus gorgoniae was originally described as Polysiphonia gorgoniae (Harvey, 1853), with a type locality of Key West, Florida (USA). It was later transferred to Neosiphonia by Guimarães and Fujii (in Guimarães et al. 2004). Most recently, it was transferred to Melanothamnus by Díaz-Tapia and Maggs (in Díaz-Tapia et al. 2017), who provided both molecular and morphological evidence for their treatment.
This species is easily recognizable because it is the only entirely erect species of Polysiphonia-group in the area. Two other species of erect “Polysiphonia” are listed for the Caribbean Sea: Polysiphonia binneyi Harvey and Melanothamus harveyi Bailey Díaz-Tapia et Maggs. However, Polysiphonia binneyi differs from our species in that the branches arise in the axil of trichoblasts, while in M. gorgoniae they replace the trichoblasts (Schneider and Searles 1991); M. harveyi is corticated at the base, while our specimen is completely ecorticated. At this time, it is the only erect species in this “Polysiphonia complex” for the Archipiélago.
Family Wrangeliaceae
Lejolisia exposita ** C.W. Schneider et Searles Figs 5a, b

Figure 5 Habit of species reported from the International Biosphere Reserve Seaflower. Lejolisia exposita: a. Habit with ovoid tetrasporangia, tetrahedrally divided, borne laterally or terminally on erect axes, b. Detail of lateral tetrasporangia. Monosporus indicus: c. Detail of distal branchlets portion, d. Prostrate axis and unicellular rhizoid, e. Oval one-celled propagules attached by triangular pedicel (arrowhead). Scale bar: a and e = 100 µm; b = 50 µm; c and d = 200 µm.
Type locality: Onslow Bay, North Carolina, USA.
Specimens observed: San Andrés Island: Wild Life: Tetrasporic and vegetative specimens, 2/XI/2012, depth 13 m. Tetrasporic specimen collected at Wild Life, 02/XI/2012, deposited at COL (COL 602239).
Epiphytic plant, pinkish-red, spreading, with prostrate axes attached to the substrate by short unicellular rhizoids, with digitate ends, 20 µm long (Fig. 5a) placed distally in the cells of prostrate exes. Prostrate axes 20 µm in width, formed of rectangular cells in surface view, to 100 µm long. Erect axes scattered unbranched, to 1.5 mm high, placed in the middle of prostrate cells, 15-20 µm in width, cells to 55 µm long in the basal portion of the axis. Erect axes slightly tapering, apical cell 12.5 µm in diameter. Tetrasporangia ovoid, 40-45 µm in diameter, tetrahedrally divided, borne laterally on erect axes, or short lateral branches, 1-5 cells long (Fig. 5b). Gametangia not observed.
Remarks: Díaz-Pulido and Bula-Meyer (1997) reported Lejolisia mediterranea Bornet for the Archipelago of San Andres based on vegetative material. Although we lack cystocarpic material, the tetrasporangial specimens fit the description of L. exposita better than L. mediterranea. In his description, Bornet (1859) reported that tetrasporangia are formed only on the basal cell of the erect filaments for L. mediterranea and are borne on a one-cell pedicel (Bornet 1859, plate 1), while in L. exposita they are located in the basal and middle part of the erect filament (Searles and Schneider 1989), borne terminally on 1-4 cell branches (Searles and Schneider 1989, Figs 5a, b). Our specimen has terminal tetrasporangia on 1-5 celled branches, which may grow in the basal through middle part of the erect filaments (Fig. 5b). Considering that a previous report of L. mediterranea was based on vegetative material (Díaz-Pulido and Díaz-Ruíz 2003), we contend that the record of this species for Colombia is doubtful and propose to eliminate it from the Colombian marine flora.
Monosporus indicus ** Børgesen Figs 5c-e
Type locality: Bombay, India.
Specimens observed: San Andrés Island: Wild Life: Vegetative specimen; 4/IV/2013, depth 13 m; Vegetative specimen, 7/VI/2013, depth 14 m; Tetrasporic and vegetative specimen, 9/VIII/2013, depth 14 m. Green Moon: Vegetative specimen, 05/VI/2013, depth 10 m; Vegetative specimen, 12/VIII/2013, depth 12 m. Specimen collected at Wild Life, 09/VIII/2013, deposited at COL (COL 602238).
Thalli filamentous to 1 mm high, with prostrate and erect axes. Principal axes to 55-60 µm in diameter, and 400-450 µm in length, secondary axes 120-150 µm in diameter, and 550-600 µm in length (Fig. 5c). Unicellular rhizoids, up to 650 µm long, one per node, and with clawlike ends (Fig. 5d). Branched dorsally bearing erect axes with subdichotomously division at every node, up to 800-900 µm high, cells 50-150 µm in diameter, to 400 µm in length. Apical cells broadly rounded (Fig. 5c). Oval one-celled propagules to 100 µm in length and 30-60 µm in breadth attached by a triangular pedicel (Fig. 5e). No gametophytes observed.
Remarks: Monosporus is a genus of red algae that produces one-celled propagules, and to date it accommodates five species (Guiry and Guiry c2017): Monosporus belangeri (Montagne) De Toni, M. herpesticus Vickers, M. indicus, M. inkyui G.H. Kim et D.-S. Choi, and M. pedicellatus (Smith) Solier. Three of these species are currently reported for the Caribbean Sea (Wynne 2017): M. belangeri, M. herpesticus and M. indicus. While the first two taxa have their type locality in the Caribbean basin (Martinique and Barbados, respectively), the latter was originally described by Børgesen (1931) from the Indian Ocean and was later reported from Australia (Huisman and Kraft 1982), the Caribbean (Bucher and Norris 1995, Ballantine and Aponte 1997, Delnatte and Wynne 2016) and Florida (Ballantine 1996). It is a widespread species reported for the Indian Ocean, the Western and Central Pacific Ocean (Abbott 1999, Lee and Kang 2001). It is also now known from the Mediterranean Sea, where it has been recently introduced (Hoffman and Wynne 2016). Our specimen fits well the description of the species. Similar to the situation for other species recently observed from Colombia (e.g. Griffithsia capitata, Rincon-Díaz et al. 2016), due to its small size, it is difficult to state if this taxon has always been part of the benthic flora of the Archipelago or if it is a recent introduction, either from other regions of the Caribbean Sea or elsewhere.
Wrangelia gordoniae** K.E. Bucher, D.L. Ballantine, C. Lozada-Troche and J.N. Norris Figs 6a-c
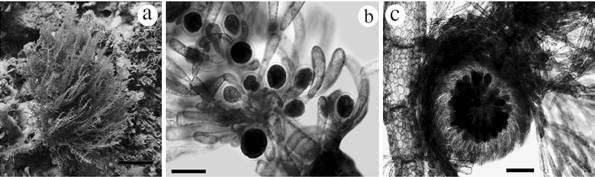
Figures 6 Habit of species reported from the International Biosphere Reserve Seaflower. Wrangelia gordoniae: a. Habit in situ, b. Whorl branchlets bearing clusters of tetraedric tetrasporangia, c. Mature cystocarp. Scale bar: a = 4 cm; b = 100 µm; c = 200 µm.
Type locality: on North Pinnacle, of a "seamount" off N end of San Salvador Is., Bahamas.
Specimens observed: San Andrés Island: Wild Life: Vegetative, female and tetrasporic specimens, 7/XI/2013, depth 14 m. Specimen collected at Wild Life, 07/XI/2013, deposited at COL (COL 602243).
Large erect alga, approximately 10-15 cm high (Fig. 6a). In its natural habitat it is red to pink and was found growing mainly on coral rubble. The main axis is completely corticated, 800 µm to 1 mm in diameter, and segments are 1.6 mm in length. Main axis bearing alternately corticated branches with 450-500 µm diameter, becoming smaller towards the apex. Each axial segment has alternate branches bearing whorled branchlets from the holdfast; these branchlets are not corticated and have dichotomous divisions, with rounded apical cells. Branchlets approximately 1-1.5 mm long; at the base, cells are 100 µm in length, and 20-25 µm in diameter; the apex is 170 µm long and 20 µm in diameter. Principal axis has 4-5 periaxial cells at the nodes. Tetrahedrally divided tetrasporangia occur in some of the branchlets, up to 50 µm diameter including the cell wall; these sporangia were found usually surrounded by involucral cells (Fig. 6b). Apex of the alternate branches have cells with partial and external cortication, different as observed in the principal axis. Cortical cells of mature portions have varied and irregular forms with a diameter of 30-70 µm, and superimposed over and around cells of inner cortical layer, these cells with 150-170 µm diameter.
Some specimens were found with cystocarps (650-800 µm in diameter, Fig. 6c) terminating the branchlets. Carpospores are 80-100 µm in length, and 60-70 µm in diameter. Spermatangia were not observed.
Remarks: Our specimens of Wrangelia gordoniae fit well the description and can be assigned to the species. It is a large alga, especially when compared to other species of Wrangelia, which normally are smaller and turf-like. It is also distinguishable from other species of the genus for its thick cortication, which is several cells in thickness. This taxon has been described relatively recently, from material of Puerto Rico (Bucher et al. 2014). The authors mentioned that it is a widely distributed species in the Caribbean and that it may be a possible introduction to the Caribbean Sea, due to the fact that herbarium specimens belonging to the species date from 1977 or later. We agree with the observation of the authors because it is a relatively large alga, at least for the Archipelago of San Andres, and it is very unlikely the species has been overlooked. Before the present work, we never observed the alga, but now it is widespread at several locations, and there are unpublished observations of it from the continental coast of Colombia (in Cartagena Bay, Rincón-Díaz, pers. obs.). We speculate that the alga might have been introduced from a region poorly explored from a phycological point of view and therefore never reported. Bucher et al. (2014) indicated that the species was confused with Wrangelia penicillata (C. Agardh) C. Agardh in the past. In the Archipelago W. penicillata is present, but it never reaches the size of W. gordoniae and is much less corticated. It would be worthwhile to determine if this alga is an introduction or not because in the past few years a number of species have been newly reported to the Caribbean Sea (e.g., Rincón-Díaz et al. 2016), but their status (autochtonous or allochtonous to the Caribbean basin) is yet to be determined.
CONCLUSIONS
Most of the species reported in the present work are small and epiphytic. Considering that the area has been poorly studied in the past, it is difficult to determine whether these algae have always been part of the marine flora or have been recently introduced. Wrangelia gordoniae is the only conspicuous species that we found in this study, and native fishermen and divers have said that they do not remember seeing this alga before (Jorge Sanchez, pers. comm.). Therefore, its presence in the Archipelago before this study is doubtful. All the other species, which are largely distributed in the Caribbean basin, were possibly overlooked. Turf algae are increasing in abundance on coral reefs worldwide (Swierts and Vermeij 2016), and turf algal assemblages, with a relatively high richness, are more resilient to competition with corals (Cetz-Navarro et al. 2015). Monitoring the composition, distribution and cover of turf assemblages at these sites should be improved, to determine their interactions with coral.














