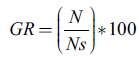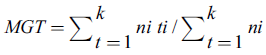INTRODUCCION
Páramo ecosystem is located in the humid zone over equatorial Andes region between latitudes 11° N and 8° S and altitude between 3000 and 5000 meters, mainly in Venezuela, Colombia, Ecuador, and Northern Peru, but it is also found in some areas in Costa Rica and Panama (Sklenář et al. 2005). This ecosystem possesses high biodiversity and endemism with its key biological relevance in the regulation of hydrological cycles, making it an important water reservoir for different regions in South America (Sklenář et al. 2005).
In Colombia, Páramos cover only 2.5 % of the territory. Nonetheless, 4700 species of plants have been recorded in this ecosystem, representing 17 % of the country (Marin and Parra 2015). Factors such as anthropogenic pressure, climate change, and reduced resilience after a disturbance are altogether threats of Páramo biodiversity and ecosystem services. For this reason, it is important to preserve their natural diversity through the implementation of in situ and ex situ conservation strategies. One of the long-term strategies of ex situ conservation of plant genetic resources are seed banks (de Viana et al. 2009). This strategy has been applied mainly on species of agricultural or commercial use, leading native species to represent less than 1 % of stored taxa in these banks (Rao et al. 2007). Furthermore, biological information such as longevity, storage conditions according to desiccation tolerance, germination requirements, and viability (Hong and Ellis 1995), crucial to establish successful ex situ conservation plans of native species, is scarce in tropical countries. Additionally, little is known on germination requirements of tropical high mountain species (Baskin and Baskin 2014).
Quality and desiccation tolerance information is important, not only for conservation purposes, but also for medium and long-term propagation strategies. Moreover, data about recruitment, natural seed bank dynamics, and reproductive phenology are the basis of restoration and reproductive ecology projects (Vieira and Silveira 2010).
In Colombia, 25 genera and 543 species of Bromeliaceae have been reported (Betancur c2015). Puya, represented by 37 species in Colombia from which 26 are endemic, it is also the fifth most diverse genus of the family in the country (Betancur c2015). This genus is mainly distributed in the high Andean forests and Páramos, between the 2500 and 4500 m of elevation, and 90 % of its species are under thread of extinction (Betancur c2015). Detailed information about reproductive cycles are a cornerstone in the implementation of conservation plans of endemic and threatened species (Schemske et al. 1994). Therefore, given the scarce knowledge of reproductive biology about these species, procuring such information is needed to advance in conservation plans of the genus Puya. The main goal of this study is to evaluate germination, viability, and desiccation tolerance of four Colombian species of Puya, and elucidate if there are significant physiological tendencies among this group of closely related species.
MATERIAL AND METHODS
Study location and species
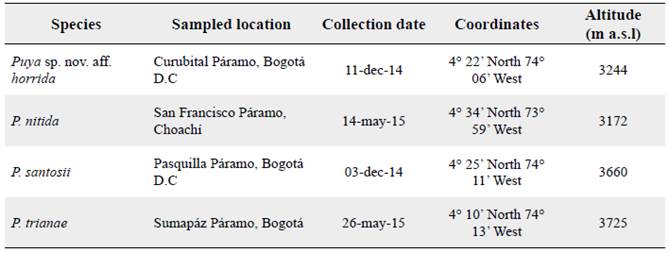
Seeds were collected in four Páramos in Cundinamarca, Colombia from 2014 to 2015 (Fig. 1). These localities have different levels of anthropogenic disturbance, although potato (Solanum tuberosum L.) cultivation and cattle breeding are the more important. Four Puya species were studied (Table 1): Puya sp. nov. aff. horrida, an undescribed species currently undergoing publication process (Betancur, pers. com.), Puya nitida Mez, Puya santosii Cuatrec, and Puya trianae Baker. According to the IUCN Red List criteria, P. nitida and P. santosii are Near threatened (NT), whereas P. trianae is Least concern (LC) (Betancur and García 2006). The conservation status for the new species has not been assessed. All the species are native and endemic to Colombia except Puya trianae, which is also distributed in NW Venezuela (Betancur c2015). The four species are monocarpic acaulescent rosettes, mostly found in frailejón-grassland plant communities proper of andean Páramos (Varadarajan 1990, Betancur 2000, Mora et al. 2007), common in poorly drained soils and peatlands (Vargas 2002, Pedraza-Peñaloza et al. 2004). The identity of target taxa was verified by vouchering specimens at the herbarium of the Bogotá Botanical Garden (JBB).
Figure 1 Location of seed sources for the species from Puya in Bogotá, Cundinamarca, Colombia, South America.
Seed collection and measurements
Mature fruits from a minimum of five individuals per species and 500 seed per individuals were collected. The seeds were extracted manually in the Germination Laboratory at Bogotá Botanical Garden. Only mature seeds without visible damage were selected following the criteria by Ayala-Cordero et al. (2004).
Four features were measured in ten seeds per species: embryo length, endosperm length, body seed length, and overall seed length. The latter includes the dispersal appendix. The measurements were taken through the images obtained under a Motic® SMZ-168 stereoscope (Hong Kong, China) and were processed with the software Motic Images Plus 2.0 (Hong Kong, China).
Quality and desiccation tolerance tests
Moisture content (MC) was assessed on 3 ± 0.5 grams of seeds at 150°C during seven minutes, using a moisture analyzer OHAUS MB45 (Parsippany, Nueva Jersey). Germination tests were carried out in a Thermoline germination chamber (New South Wales, Australia) under controlled conditions with a 12 hours photoperiod, 20/10 ± 2.5 °C, and mean humidity of 75 ± 5 % following the criteria by Pérez-Martínez et al. (2014). Both variables were monitored with Data Logger EBCHQ 94150 (China). The germination of fresh seeds was evaluated at initial moisture content (MCi) and after desiccating the seed to 5.0 ± 2.0 % MC. Due to high MCi in fresh seeds, an additional experiment was carried out on P. nitida and P. trianae, with MC at 10 ± 2.0 %. Decrease in MC was reached through a silica-based desiccation chamber with a 2:1 ratio (silica: seeds) and the equation FSW = SSW×[(100 - MCi)/(100 - MCo)] where FSW is the Final Seed Weight, SSW is Starting Seed Weight, MCi initial moisture content, and MCo is object moisture content after desiccating (in this case 10 or 5 %) (Rao et al. 2007).
Four replicates per experiment were done, using 50 seeds each. Before setting the experiment, seeds were disinfected with 5 % sodium hypochlorite solution (Muñoz and Ackerman 2011), and then sowed into Petri dishes with double filter paper. Germination experiments were recorded every third day over two months until there was no more germination. A germination event was recorded as positive whenever the radicle emerges through the seed coat (≥ 2 mm) (Salisbury and Ross 1992).
Finally, viability was determined in seeds that did not germinate after completion of the previous experiment. For this purpose, a seed was considered viable if its embryo was white and rigid (Buhler et al. 2001, Sawma and Mohler 2002, Nurse and DiTommaso 2005). The tetrazolium test was not used in this study given that standardization was not possible with this technique.
Data analysis
Germination percentage (GR) and mean germination time (MGT) were calculated through the following equations (Tompsett and Pritchard 1998, Ranal and Santana 2006):
Where, N is number of germinated seeds, Ns total number of seeds, ni number of germinated seeds at the i-th data measurement; ti is time (in days) at the i-th measurement; and k is germination test extent (in days).
The normality test (Shapiro-Wilk, P < 0.05) was conducted on each data group. If the null hypothesis was not rejected, ANOVA and Tukey tests were applied, but if normality was rejected, a Kruskal-Wallis non-parametric analysis was applied, followed by a measure comparison analysis to evaluate differences. Comparisons were made both among and within species. Statistical analysis was performed using StatGraphics® Centurion XVI version 16.1.11.
RESULTS
Morphological measurements and moisture content
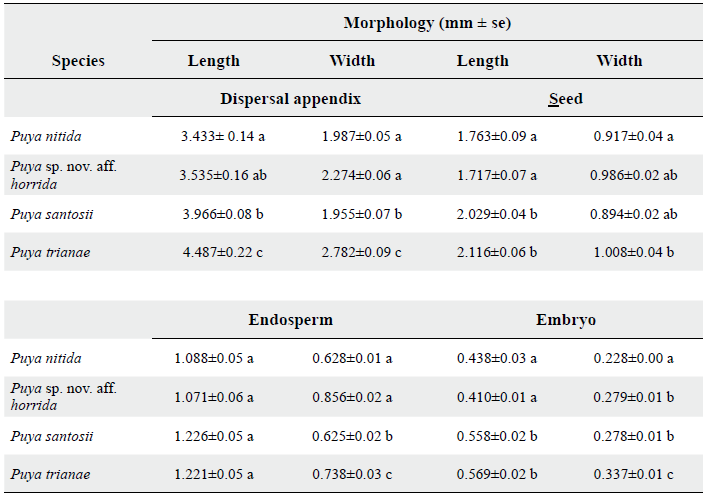
All four species have three-locule capsule fruits, with color ranging from olive-green to light brown at immature stage and to dark brown while ripening. Fruits contain numerous dark brown, ovate seeds with a rough surface and a beige winged edge surrounding the entire seed, which gives the dispersal unit a triangular shape. Inside, approximately 80 % of the seed is endosperm and 20 % is an ovate, smooth surfaced, white embryo (Fig. 2). Regarding the morphometric variables, there were significant differences between species (Table 2). Seeds of P. nitida and Puya sp. nov. aff. horrida are smaller than P. santosii and P. trianae, both in dispersal unit, seed and embryo. P. trianae presented the largest seed and dispersal unit size. Concerning MC, P. santosii, Puya sp. nov. aff. horrida, P. nitida registered a value below 15 %; 10.8, 11.8 and 14.3 % respectively, whereas P. trianae had 30.5 % MC.
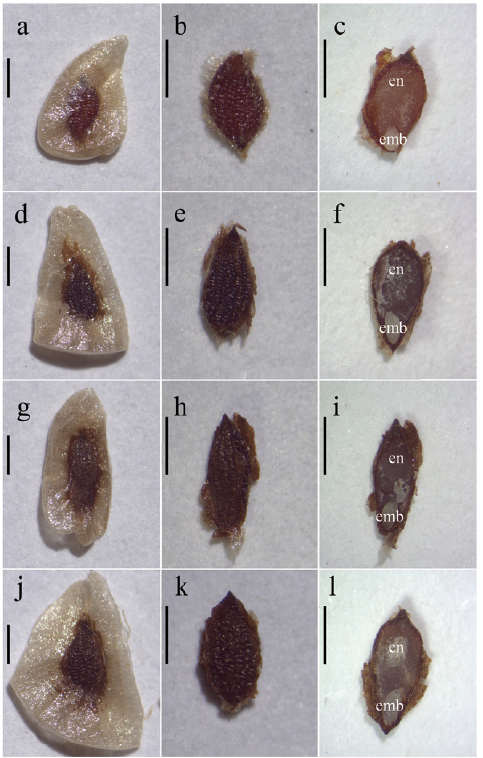
Figure 2 Seed of the Puya species, details of: dispersal unit, seed and longitudinal cut of the seed. a., b., c. P. nitida. d., e., f. Puya sp. nov. aff. horrida. g., h., i. P. santosii. j., k., l. P. trianae. en = endosperm, emb = embryo. Scale bars = 1 mm.
Germination and desiccation tolerance
The four species had high germination rates under all MC evaluated. However, there were significant differences of GR at 5 % of MC (P = 0.04). Puya nitida was the only species with a GR below 80 % while the rest of species showed a GR near 100 %. There were not differences between other MCs (P = 0.61).
Regarding species, there were differences in GR at different MC in P. nitida (P = 0.03) and Puya sp. nov. aff. horrida (P = 0.02). In the case of P. santosii (P = 0.14) and P. trianae (P = 0.41), there were not significant differences (Fig. 3). However, in either species there was no decreasing on GR as MC decreased.
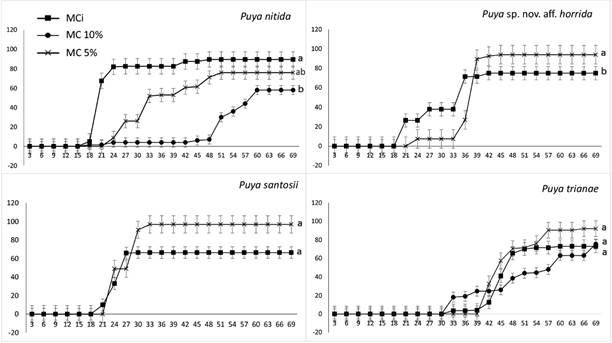
Figure 3 Germination curves at different moisture contents (MCi, MC 10 %, MC 5 %) for four species of Puya. The curves with the same letter are not significantly different according to the comparison test (P < 0.05). Perpendicular lines denote standard error (n = 4).
In the case of MGT at MCi there were significant differences among the studied species. P. trianae presented the highest MGT (48 days), while the remaining species registered an average of 26 days. At 5 % of MC there were also significant differences (P < 0.05); P. trianae showed the highest MGT with 47 days while P. santosii showed the lowest with 26 days. For P. nitida and Puya sp. nov. aff. horrida the mean MGT was of 36 days. Within species there were significant differences in MGT at each MC for P. nitida (P = 0.01), Puya sp. nov. aff. horrida (P = 0.03) and P. santosii (P = 0.01) which increased its MGT as the MC decreased (Fig. 4). Seed viability for the four species was high: P. santosii, 97 %; Puya sp. nov. aff. horrida, 94 %; P. trianae, 92% and P. nitida, 76 %.
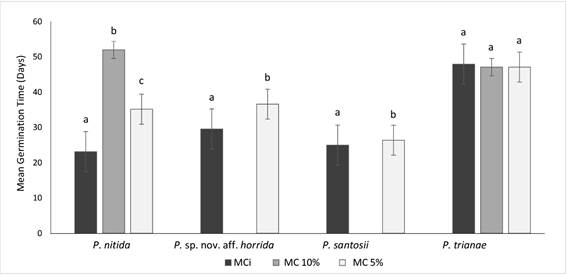
Figure 4 Mean germination time (Days) of four species from Puya at different moisture content (MCi, black bar; MC 10 %, grey bar; MC 5 %, white bar). Same letters indicate that these were not significantly different between the species according to Tukey test (P < 0.05). Perpendicular lines indicate the standard error (n = 4).
DISCUSSION
The high germination rates reported here for the four species of Puya agrees with previous experiments under light laboratory conditions for P. raimondii (Vadillo et al. 2004), P. nitida (Franco 2014), P. trianae (Pérez-Martínez et al. 2014); and are slightly greater than those registered for P. santosii (Pérez-Martínez et al. 2014). It is worth noting that the germination rates values for other Puya species such as P. cryptantha, P. trianae, P. santosii, and P. bicolor are lower under field or greenhouse conditions (28 % - 50 %, according to Mora et al. (2007) and Pico (2014 data not publ.)), which is related to non-controlled environmental factors. Despite of the lower germination rate registered for P. nitida, in general the values presented here are considerably higher than those previously reported for other páramo species (Pérez-Martínez et al. 2014). One of the biggest limitations in restoration projects are the availability of native plant material (Murcia and Guariguata 2014); these results evidenced the efficiency of propagation in controlled laboratory conditions for all four species studied which can be linked with propagation protocols for restoration projects.
This is the first known study of seed desiccation tolerance for high Andean tropical species. Here is demonstrated that germination was not affected by decreasing seed moisture content in the studied species of Puya; on the contrary, GR increased when seeds were at 5% of MC. This leads to conclude that seeds have an orthodox behavior and are tolerant to low moisture contents (Kermode and Finch-Savage 2002, Nkang et al. 2003).
The increase in the mean germination time while moisture content decreases agrees with expectations, since decreasing on MC of seeds also diminishes metabolic activity, taking more time to re-absorb the water needed to start germination (Tweddle et al. 2003). Nonetheless, increase on average MGT was six days, which do not imply a significant deceleration on the germination curve.
Although Hong and Ellis (1988) demonstrated that in Meliaceae all desiccation intolerant species have a high moisture content (20.3 - 52.5 %); in this study initial moisture content was not related with the result of desiccation tolerance. This agrees with Daws et al. (2006), who found that 104 orthodox species presented MCi range from 9.1 to 61.6 %.
Regarding seed morphology and morphometry, bigger seeds of P. trianae agree with its higher MGT linked to higher volume to imbibe. Heavy seeds with thin coats have been widely reported as poor tolerant to desiccation (Tweddle et al. 2003, Pritchard et al. 2004, Daws et al. 2005, 2006); these relations must be evaluated in contrasting ecosystems such as Páramo, where several morphological traits such as small size seeds, are usually constant. In turn, despite of the humid nature of Páramo, it is worth noting that seeds were collected during dry season a fact that is linked to anemochory of Puya and has been referenced in other desiccation tolerant species (Tweddle et al. 2003, Pritchard et al. 2004, Daws et al. 2005). Gathering information from different species and families from Páramo ecosystem will allow researchers to find tendencies within the ecosystem, as well as the development of probabilistic tools to improve our understanding of desiccation tolerance (Daws et al. 2006).
In conclusion, the four species evaluated have the potential to be conserved in germplasm banks, and is probable that we can extend the desiccation toleration type to the whole genus, contributing to diversity conservation of native species of Páramo, an endangered ecosystem. Germination protocols can be implemented due to both high viability and germination rate in controlled conditions, a process that will contribute to species propagation and reintroduction for restoration programs. Besides, in order to define seed desiccation tolerance, it would be useful to evaluate variables to foreseen seed physiological and evolutionary responses; variables that may vary at species, genus, family or ecosystem level. For this reason, it is recommended to continue evaluating desiccation tolerance of different species from various Páramo environments to find probabilistic tendencies and models that allow us to rapidly determine desiccation tolerance and to become a tool for decision making process for ex-situ conservation.