INTRODUCTION
One way to describe a speciation process is the evolution of genetic differences between populations that are adapted to different conditions as a result of divergent selection (Rundle and Nosil 2005). The ecological species concept is founded on the importance of disruptive selection on the formation of host races while, on the other hand, the biological species concept emphasizes the evolution of pre and postzygotic isolation for species divergence (Dres and Mallet 2002). In the latter case, isolation is an indispensable factor in reducing encounters between populations, thus allowing the maintenance of races and species, a process that has been widely reported for insects (Coyne and Orr 2004, Drès and Mallet 2002). Both species concepts are applicable to pest species when reproductive barriers prevent gene flow. Examples of host race evolution include the well-known fall armyworm Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae) on corn (Zea mays L.) and rice (Oryza sativa L.) in all of the Western Hemisphere (Prowell et al. 2004). Studies on prezygotic and postzygotic isolation carried out in Colombia have shown partial assortative mating since corn strain females do not mate with rice strain males (Saldamando-Benjumea et al. 2014); and F1, F2 generations have reduced viability, fertility, and fitness, particularly in females, suggesting the Haldane Rule (Velásquez-Vélez et al. 2011). Another case is the moth species Ostrinia nubilalis (Hbn., 1796) (Lepidoptera, Crambidae), also known as the European corn borer. In France, Bourguet et al. (2000) found that populations of this insect in maize (Zea mays) are genetically different from sympatric populations found on sagebrush (Artemisia sp.). Genetic differences between these two races are due to differences in pheromone composition and attraction (Malausa et al. 2005).
Another moth recently studied in Colombia that has evolved into host races is the tomato fruit borer Neoleucinodes elegantalis (Guennée, 1854) (Lepidoptera: Crambidae). This insect is a quarantine pest that causes relevant economic damage to Solanaceous fruits, including the Solanum sp. and Capsicum sp. genera (USDA 2005, Díaz and Solis 2007, Díaz et al. 2011) and to vegetables across the Western Hemisphere. Actually, it is the main entomological limitation for Solanaceae fruit exportation in Colombia. N. elegantalis has adapted to different ecological zones and elevations (0-2600 m a.s.l.) including six Holdridge life zones Díaz-Montilla et al. (Díaz et al. 2011). Laboratory studies have shown that its life cycle is from 25.6 to 124.1 days, and the number of eggs laid depends on both temperature and host fruit (such as tomato, eggplant, or lulo) used for larvae development (Marcano 1991a, b, Serrano et al. 1992). Oviposition and pupation habits studied in laboratory and nature also show host plant variation (Marcano 1991a, b, Serrano et al. 1992). Natural enemies are diverse and depend on the host (Serrano et al. 1992, Viafara et al. 1999, Díaz-M and Brochero 2012).
This species meets the criteria described in Dres and Mallet (2002) for host race speciation through host plant association, partial sympatric coexistence of the species range of distribution and genetic differentiation (Díaz and Solis 2007, Díaz et al. 2011, Díaz-Montilla et al. 2013). Díaz-Montilla et al. (2015) described the evolution of four races in a study based on collections of larvae attacking cultivable and wild species of the Solanaceae family throughout Colombia. These races were divided into four groups according to variation in six genitalia sizes as follows: a) S. acerifolium (small genitalia), b) S. atroporpureum (small-medium genitalia), c) Capsicum annuum, S. hirtum, S. lycopersicum, and S. quitoense, (medium-sized genitalia), d) S. betaceum, S. crinitum, and S. melongena (large genitalia). In addition, Díaz-Montilla et al. (2013) sequenced the mitochondrial gene CO1 (cytochrome oxidase I) of 103 individuals from Colombia, finding four haplotypes that were genetically structured (Fst = 0.57, P < 0.0001) due to both host plant association and the Andean mountains acting as a barrier to gene flow. Finally, Obando (2011) applied a morphometric wing analysis to approximately 1500 males and females sampled on a variety of hosts in Holdridge life zones, demonstrating that wing size and wing shape varied significantly according to host. These three separate studies support the possibility of genetic divergence and host race formation facilitating speciation.
The purpose of this study was to determine the reproductive compatibility of N. elegantalis in Colombia using intrapopulation (homotypic) and interpopulation (heterotypic) crosses between adults collected on S. lycopersicum, S. quitoense and S. betaceum. We raised insects under laboratory conditions, characterized pupae and adults from Solanaceae hosts, measured postzygotic isolation parameters in parental and F1 generations and proposed that investigating the evolutionary biology of N. elegantalis is also important in determining the potential of host-specific associations for use as an additional control tactic.
MATERIALS AND METHODS
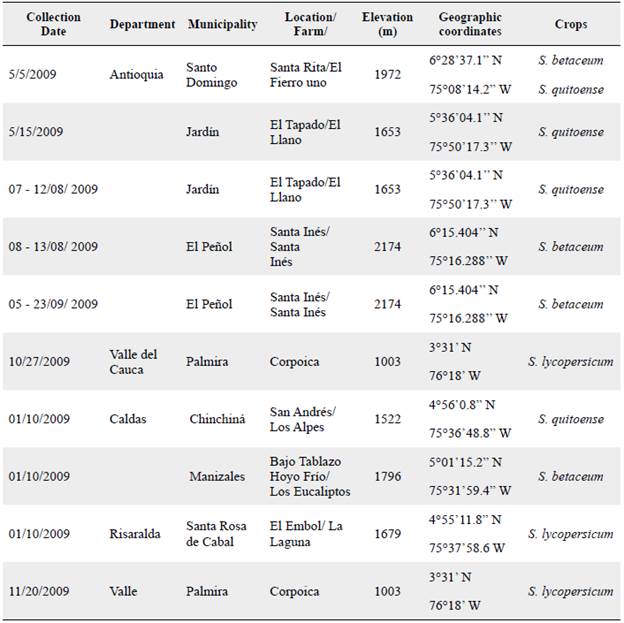
Two trials were carried out at two locations and seasons of the year to facilitate larvae collection. In both experiments, populations of N. elegantalis were collected from tree tomato (S. betaceum), tomato (S. lycopersicum) and lulo (S. quitoense) in the Departments of Antioquia, Caldas, Risaralda and Valle del Cauca (Colombia, South America) (Table 1). These populations were chosen because they differ in female genitalia size and genetics according to DNA sequencing of mitochondrial gene COI (cytochrome oxidase I). S. betaceum females have large genitalia and S. quitoense and S. lycopersicum females have medium-sized genitalia (Díaz-Montilla et al. 2015) and are genetically differentiated based on COI haplotype composition (Díaz-Montilla et al. 2017). The other host N. elegantalis populations were not included in this study as the most affected crops in Colombia are S. betaceum, S. lycopersicum, and S. quitoense.
Table 1 Collection sites and hosts studied for the analysis of reproductive compatibility and fitness differences in N. elegantalis host races in Colombia.
Insect collection and rearing/raising
Trial 1 collections of N. elegantalis larvae took place between May and November 2009 from S. betaceum, S. lycopersicum and S. quitoense infested fruits in the departments indicated in Table 1. All samples were taken to ICAs (Instituto Colombiano Agropecuario) Tulio Ospina Entomology Laboratory in Bello, Antioquia: at 1459 m, 6°19'12.8" North, 75°33'13.4" West. The average temperature at this location is 23.95 °C and RH 76 %. Trial 2 collections were made from January to June of 2014 in S. lycopersicum, S. quitoense and S. betaceum fields in the municipality of Anserma in the Department of Caldas (5°45'58.2" North, 75°45'07.8" West) located at 1846 m. This second trial was made five years later due to difficulties in sampling conditions. All fruits were transported to the Agrosavia La Selva Research Center in Rio Negro, Antioquia (6°7'57.9'' North, 75°25'8.7'' West) at 2120 m. There they were kept at room conditions described as follows: Photoperiod 12:12 day/ night, average temperature 14.25 ± 1.26 °C (CV 8.8 %), with a maximum average temperature 18.02 ± 4.76 °C (CV 26 %) during the day and a minimum average temperature 16.82 ± 4.24 °C (CV 25 %) at night, RH 82.11 ± 19.2 % (CV 23 %), sun radiation 202.68 W/m2 (CV 136 %). For both trials, sampled fruits were separated according to host and were kept in different cages with paper towels for pupation. To obtain virgin adults, all pupae were sexed (Serrano et al. 1992) and deposited separately in plastic containers (11.5 cm x 7.5 cm); they were left there until emerging as adults.
Trial 1: Reproductive compatibility
Paired homotypic and heterotypic crosses consisted of introducing one male and one female in a cage (42 cm x 42 cm x 64 cm) with a S. lycopersicum Torrano hybrids plant with fruit from 1.5 to 4 cm long. Males and females were between 0-2 days old to ensure having virgin adults for mating behavior experiments. After mating, the number of copulated females was counted by examining the spermatophore transfer produced at the "bursa copulatrix" (Burns 1968). The "bursa copulatrix" was stained following Prophet et al. (1995). Dissections were kept in 2.5 ml tubes with glycerin.
Postzygotic isolation
Once mating occurred, adults were removed from each cage to evaluate female fertility, and the number of eggs produced per female per fruit on each tomato plant was counted in parental generations. To test for differences in oviposition behavior, orifices generated by females were identified with a pen mark, and then classified as basal, medial, and superior, according to position/location on the fruit. Paper towels were placed on the bottom of each cage fifteen days after hatching to facilitate pupation. Pupae were removed from each cage daily and placed in plastic containers (5 cm x 7.5 cm). All pupae were sexed and kept separated until adulthood. The following life history traits were recorded from the parental and F1 generations from the different sampling sites to test for postzygotic isolation: 1) number of pupae, 2) pupae longevity (days), 3) pupae weight (gr), 4) adult longevity (days) and 5) proportion of sexes in all hosts.
Trial 2. Reproductive compatibility
Virgin individuals were obtained from previously sexed pupae, as mentioned above. The number of homotypic and heterotypic crosses depended on the availability of both adults and plants. In this case, paired crosses consisted of placing five males and five females in an entomological cage (42 cm x 42 cm x 64 cm) with either a S. lycopersicum, a S. quitoense or a S. betaceum plant. Plants were grown in greenhouses to facilitate female oviposition and larvae development because this species does not feed on artificial laboratory diets and does not easily mate in captivity. On this trial, females were enclosed with their native host species for oviposition. The number of copulations that occurred per female was recorded by counting the number of spermatophores transferred to the "bursa copulatrix" (Burns 1968). Dissected genitalia were kept in glycerin in 2.5 ml tubes.
Postzygotic isolation
The following parameters were measured daily in parental and F1 generations: 1) number of laid eggs per female per cross on the basal, medium or apical parts of the fruit as well as on the sepals or peduncles; 2) number of larvae produced per female by counting the number of orifices on the fruit epidermis; 3) pupae longevity from oviposition time to pupae formation, this measurement is performed when larvae left the fruit to produce perforations for pupae formation; 4) the number of pupae per fruit; 5) pupae development time (days); 6) pupae weight (gr); 7) wingspan (cm); 8) adult longevity (days) and 9) proportion of sexes. In cases where fruit damage was recorded without identifying pupae, transversal cuts on the exocarpus or epicarpus were made to locate larvae and evaluate survival rate (Chiarini and Barboza 2007).
Data analysis
To test whether differences in the number of copulas among N. elegantalis paired crosses (i.e. homotypic vs heterotypic) were significant, a Chi-square test was employed in both trials. In Trial 1, a Yates correction was carried out due to the low number of copulas observed in this experiment (Sokal and Rohlf 1995). In addition, differences in oviposition behavior of N. elegantalis females (from intrapopulation and interpopulation crosses) were tested using another Chi-square analysis. To analyze fitness components in both trials, comparisons tests (ANOVA, Welch-F, Kruskall Wallis or Mann Whitney tests) were carried out on N. elegantalis populations obtained from S. lycopersicum, S. quitoense and S. betaceum. To test the assumptions of the variance analysis, Shapiro Wilk tests for normality and Levene's tests for variance homogeneity were conducted (Sokal and Rohlf 1995). Depending on the results of these tests, a parametric variance analysis test (ANOVA), followed by a Tukey test, a Welch F statistic test (followed by a Tukey test), or the non - parametric Kruskal Wallis or Mann Whitney tests were subsequently employed (Sokal and Rohlf 1995). All analyses were carried out using Past 1.34 (Hammer et al. 2001) software.
RESULTS
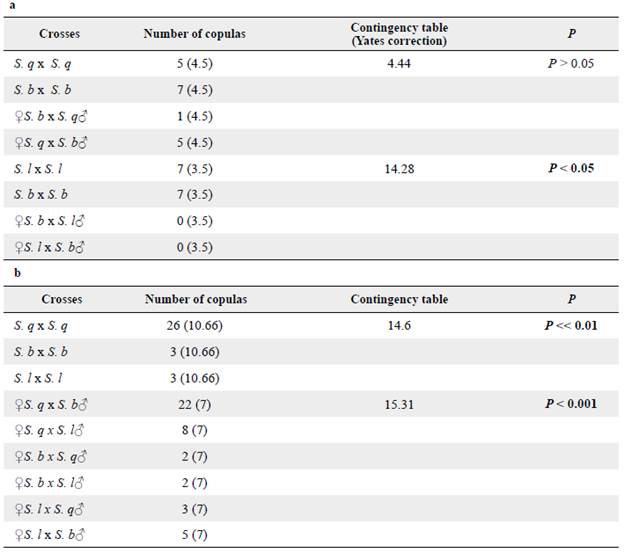
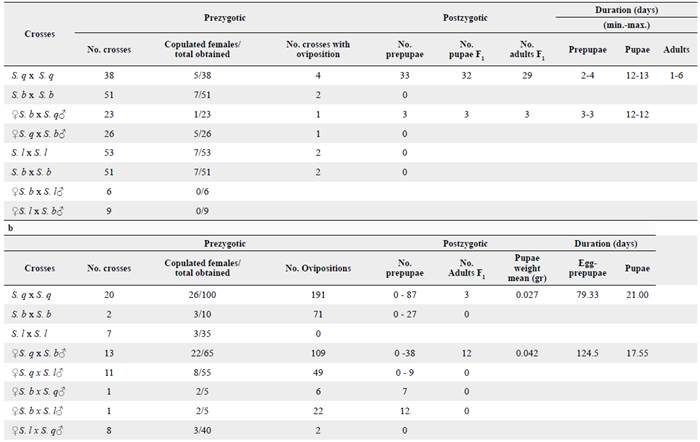
In Trial 1, 138 crosses were made between adults obtained from S. quitoense and S. betaceum. From these crosses, 119 matings occurred and a total of 257 females were obtained to analyze spermatophore transfer (Table 2a). In Trial 2, 72 crosses were made (29 intrapopulation and 43 interpopulation crosses) and 360 females were obtained from them and analyzed (Table 2b). In total, 617 females were tested in both trials and only 106 copulated. Overall, N. elegantalis females mated once with only one spermatophore transfer per bursae copulatrix, thus the moth is a monoandric species.
Table 2 Reproductive incompatibility obtained for N. elegantalis evaluated in populations collected on S. lycopersicum (S. 1), S. quitoense (S. q) and S. betaceum (S. b). a = Trial 1, b = Trial 2.
Mating compatibility in N. elegantalis (Prezygotic isolation)
These populations exhibit a higher number of homotypic than heterotypic crosses (Table 3). On the first trial, we found differences between the homotypic and heterotypic crosses between individuals from S. lycopersicum and S. betaceum (P < 0.05) (Table 3a). In fact, reproductive isolation was found between individuals from S. lycopersicum and S. betaceum where no crosses were obtained regarding sex. On the second trial, more crosses were obtained between individuals with similar genitalia size (n = 48), including the S. q x S. q, S. l x S. l and S. b x S. b homotypic crosses and the two heterotypic crosses, ♀ S. lycopersicum x ♂ S. quitoense, and their reciprocal progeny. Lesser successful crosses were obtained between individuals with medium size genitalia and individuals with large sized genitalia (n = 24), including ♀ S. betaceum x ♂ S. quitoense and its/their reciprocal progeny, and ♀ S. lycopersicum x ♂ S. betaceum and it's/their reciprocal progeny (Table 3b). These differences were also statistically significant, thereby supporting the results obtained in the first trial (Table 3a).
Postzygotic isolation in N. elegantalis
On Trial 1, according to the number of females that copulated (Table 2), N. elegantalis produced more successful crosses between homotypic populations than the heterotypic populations. However, only the homotypic cross from S. quitoense and the heterotypic cross (♀ S. b x ♂ S. q) produced eggs that later reached pupae and adulthood. On Trial 2, the most successful crosses were between S. q x S. q followed by ♀ S. q x ♂ S. b, and ♀ S. q x ♂ S. 1 as more copulated females were found on them. Ovipositions occurred on all crosses except for S. 1 x S. 1 and S. 1 x S. b, and pupae in all crosses except for S. l x S. q. However, only the S. quitoense homotypic cross produced eggs that reached adulthood and, of the heterotypic crosses, only the reciprocal progeny from Trial 1 (♀ S. q x ♂ S. b) (Tables 2b, 4). On fruits without observable pupae, the larvae apparently died in/during mesocarpus since no more damage was found. We did not find sex bias in this species; sex ratios were 1:1 in all crosses. Longevity or duration of pupae and adult longevity were shorter in Trial 1 than in Trial 2.
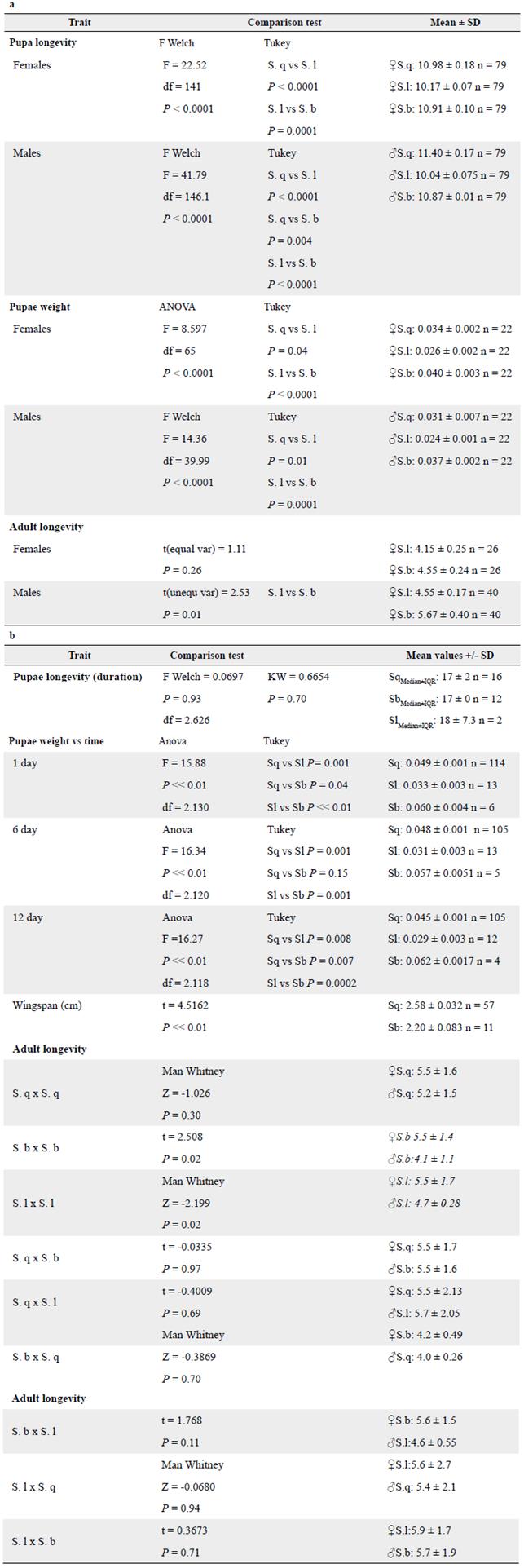
Fitness components in parental generations
In both trials, N. elegantalis pupae longevity or duration were correlated with environmental conditions. The higher the temperature and the stronger the solar radiation, the lower the pupae duration (Table 4). This result was observed in this work as two different locations were considered for rearing N. elegantalis and thus they were crucial to determine the importance of temperature and radiation in pupae longevity in this pest. In Trial 1, we found that pupae with the longest average duration came from S. quitoense fruit, followed by S. betaceum and S. lycopersicum (P < 0.05, Table 4). On both trials, the heaviest pupae were found in S. betaceum, followed by S. quitoense, and S. lycopersicum (Table 4). On Trial 1, differences were found between pupae from S. lycopersicum and the other hosts. On Trial 2, pupae weight differed significantly among hosts (Table 4). We found differences in the longevity of males by the host on Trial 1 (Table 4). On Trial 2, we only observed longevity differences between S. betaceum and S. lycopersicum males and females.
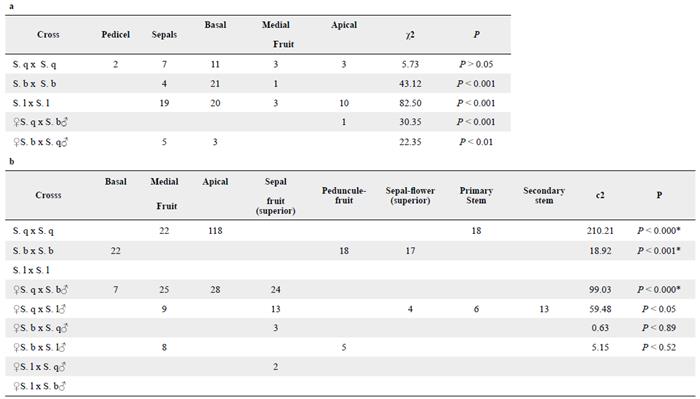
Oviposition sites
Contingency tables show that N. elegantalis female oviposition sites differ significantly according to host (Table 5). In general, on Trial 1, females of the S. q x S. q cross laid eggs on all parts of the fruit including sepal and peduncle areas, but they mostly oviposited on the apical part of the fruit. Females from the S. b x S. b cross preferred to lay eggs on the apical and basal fruit parts including the stem, and females from the S. l x S. l cross mostly laid eggs at the base of the sepals. In this last cross, females laid a larger number of eggs compared to other females. In generation Trial 2, females obtained from S. q, mostly laid eggs on the apical fruit parts, females from S. b laid eggs on the basal part of the fruit and on sepals, and females from S. l laid eggs on the sepals.
DISCUSSION
Prezygotic isolation
N. elegantalis host races mated assortatively. This was demonstrated by the number of females from both trials with spermatophore transfer counting both homotypic and heterotypic crosses, suggesting the possibility of chemical and/or mechanical reproductive isolation for this species. In the case of mechanical isolation, Díaz-Montilla et al. (2015) showed that N. elegantalis females with large genitalia originated from large fruits and females with small genitalia from small fruits. Therefore, if female genitalia size presents a correlation with fruit size, males of this species might also show this type of correlation demonstrating the possibility of a lock-key hypothesis in the four races of this species. Evolution of male genitalia is one of the most generalized evolutionary trends in animals with internal fertilization, where shapes of male genital traits often provide the only trustworthy characteristic for species identification (Arnqvist 1998). However, the evolutionary processes responsible for this pattern remain unknown. In a study comparing pairs of related clades of insects that differ in mating system, Arnqvist (1998) found that genital evolution in polyandric females is more than twice more divergent than in monadric females. Given that we only found one spermatophore transferred in N. elegantalis, our results do not support Arnqvist (1998)'s proposition. Similar observations were made of other Lepidoptera species. For example, Pashley and Martin (1987)'s mating study showed no spermatophore transfer from males to females on Spodoptera frugiperda corn and rice strains. Likewise, in Colombia, Saldamando et al. (2014) and Velásquez-Velez et al. (2011) observed no spermatophore transfer on S. frugiperda corn and rice females from a laboratory colony. In both studies, they suggested assortative species mating. In France Ostrinia nubilalis (Hbn., 1796), females from maize-Z and mugwort (Artemisia vulgaris L.) races mated with homospecific males in 95 % of the cases, indicating a lack of hybridization between them (Malausa et al. 2005).
Another possibility of prezygotic isolation in N. elegantalis is the evolution of chemical isolation through female pheromone differentiation among host races (Díaz-Montilla et al. 2017). Previous studies have shown attraction of N. elegantalis males to female pheromones or to synthetic pheromones (Jaffé et al. 2007, Díaz-Montilla et al. 2017). Jaffé et al. (2007) found that N. elegantalis males from tomato fruits were more attracted to pheromone blends produced by larger females, and even more attracted to a synthetic sex pheromone blend. Díaz-Montilla et al. (2017) tested two sex pheromones synthesized from females collected on S. lycopersicum from Venezuela in crops of S. lycopersicum, S. quitoense, and S. betaceum in Colombia. They obtained higher captures for males on S. lycopersicum versus the other crops, suggesting that N. elegantalis males are attracted to homotypic females. This attraction pattern could generate a reduction of matings between heterotypic individuals and, thus, a limited spermatophore transfer from one host race to another.
Postzygotic isolation
Several fitness components tested on the F1 of both trials varied among most of the host races: number of eggs, number of developed larvae, pupae and adults (Tables 2-3). Moreover, on trials, oviposition behavior, pupae weight, pupae longevity, adult weight, adult longevity, and wingspan also varied in parental populations (Tables 4-5). Most data in Trial 2 demonstrated that parental populations had better fitness success compared to other crosses. Despite differences in laboratory conditions, individuals from S. quitoense were the most successful in reaching adulthood during both trials. In fact, the only heterotypic crosses that reached pupae and/or adulthood also involved a parental S. q male in Trial 1 and a female in Trial 2 (Table 2), suggesting that S. q host race populations are better adapted to environmental conditions compared to other N. elegantalis races.
In both trials, the ICA-Tulio Ospina Laboratory of Entomology seemed to provide better survival conditions for the N. elegantalis colonies tested. This may be attributable to warmer temperatures, lower relative humidity, and lower sun radiation compared to the Agrosavia-La Selva Laboratory. N. elegantalis pupae longevity was significantly different in both males and females in the three tested hosts and this longevity was influenced by differences in environmental conditions between these two trials. In fact, pupae duration was two times longer in individuals maintained at Agrosavia-La Selva compared to ICA-Tulio Ospina laboratories, suggesting that this pest is better adapted to warmer temperatures and lower altitude conditions. In addition, even though pupae weight was significantly different among hosts in both trials; the heaviest pupae originated from S. betaceum fruits followed by S. quitoense, and S. lycopersicum. Pupae from S. lycopersicum were the lightest in all trials.
Adult longevity in males was greater in S. betaceum than in S. quitoense in both trials. In Trial 2, adult longevity was also greater in S. betaceum than in S. quitoense. Differences in fitness components according to host have been previously found in other Lepidoptera species including Anticarsia gemmatalis Hübner, 1818 (Lepidoptera: Noctuidae) (Panizzi et al. 2004), Grapholita (Cydia) molesta (Lepidoptera: Tortricidae) (Belluti 2011) and Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Yponomeutidae) (Golizadeh et al. 2009), suggesting differences in fitness components due to host plant association.
On Trial 1, N. elegantalis females mostly laid eggs on S. lycopersicum. This result suggests that semiochemicals produced by this host plant might facilitate N. elegantalis oviposition behavior. Also, N. elegantalis females used different oviposition sites according to host. Previous studies of S. lycopersicum indicated that most N. elegantalis egg lying occurs on the peduncle, the sepals and the superior part of the fruit; however, this species never laid eggs on flowers (Marcano 1991b, Salas et al. 1991, Blackmer et al. 2001, Rodrigues-Fihlo et al. 2003). Our results coincide with the field pattern of oviposition explained by Marcano (1991b) and Blackmer et al. (2001). Differences in oviposition sites might be explained as a mechanism used by N. elegantalis females to prevent egg parasitoids such as Trichogramma sp. that has been widely used to control this pest in tomato (Blackmer et al. 2001). Salas et al. (1991) demonstrated that N. elegantalis females lay eggs in places that are not easily exposed to parasitoids such as hidden places under sepals and fruit receptacles.
The results of postzygotic isolation tests demonstrate the importance of verifying the physiological potential for N. elegantalis host race hybrid generation because traits related to this type of isolation are relevant for the improvement of the integrated pest management of this species in nature. One way to reduce population densities of this pest in Colombia could be by planting S. quitoense and S. betaceum in sympatry because the hybrids obtained between them will have a lower survival rates compared to parents or S. lycopersicum x S. betaceum and S. lycopersicum x S. quitoense where, according to our results, no adults were obtained.
In conclusion, N. elegantalis mate assortatively as more crosses between homotypic individuals than heterotypic individuals took place in the laboratory. Spermatophore transfer occurred only once, suggesting species monoandry. In addition, postzygotic isolation shows that hybrids between individuals with medium sized genitalia vs large genitalia show a reduction in viability. Our results suggest future research into the potential use for N. elegantalis hybrids (between races) in pest management in Colombia. More studies on postzygotic isolation, pheromone differentiation among races, and biological control (using Trichograma sp. to parasite N. elegantalis eggs) in this species are necessary. The results obtained here accentuate the importance of studies on the evolutionary biology of the pest in order to improve the management of this species in nature.
AUTHOR'S CONTRIBUTION
AEDM concept design of the project. NBB data collecting for Trial 1 as part of her undergraduate research project at the Universidad del Valle. AEDM, JML, and NBB analysis of Trial 1 and writing the text; AEDM data collecting for Trial 2; AEDM and CISB analysis for Trial 2. AEDM and CISB statistical analysis and writing the text.