INTRODUCTION
In June 2015 a soft scale insect was collected on sapodilla, Manilkara zapota (L.) P. Royen (Sapotaceae) in the grounds of the Colombian Corporation for Agricultural Research (Agrosavia), Palmira Research Station. The soft scale insect was identified as the Marques' soft scale, Alecanochiton marquesiHempel, 1921 (Hemiptera: Coccomorpha: Coccidae). Other samples collected on Melastomataceae from Costa Rica were also identified as A. marquesi. This is the first record of A. marquesi from Colombia and Costa Rica. Herein we give a brief description of the insect in life and a short redescription of the species based on slide-mounted specimens and published literature. The first-instar nymph of A. marquesi is described and illustrated based on specimens collected in Colombia.
The Marques' soft scale, A. marquesi, was originally described by Hempel (1921) based on specimens collected on coffee (Coffea sp.) in the municipality of Angatuba, in the state of Sao Paulo, Brazil. Alecanochiton marquesi is considered a pest of coffee in Brazil (Pickel 1927, Costa-L 1928). In the present study, we found high infestations of A. marquesi on M. zapota, and associated with symptoms of sooty mold. Furthermore, A. marquesi is for the first time recorded on Conostegia xalapensis (Bonpl.) D. Don ex DC., Miconia trinervia (Sw.) D. Don ex Loudon and on M. schlimii Triana (Melastomataceae) in Costa Rica. We also report five other species of scale insects collected on M. zapota in Colombia in the present study and discuss the affinities of Alecanochiton with other scale insect genera
MATERIALS AND METHODS
Scale insect specimens were collected from the twigs and peduncles of their host (see material studied). Samples were identified by OJDL, JMMR and TK. Specimens from Colombia were slide-mounted according to the protocol of the Systematic Entomology Laboratory, United States Department of Agriculture (USDA c2014); specimens from Costa Rica were slide-mounted following the method by Williams and Granara de Willink (1992), for the exception that xylene was used instead of clove oil prior to sealing the specimens in Canada Balsam. The soft scale insect was identified using the keys found in the identification manual to genera of Coccidae by Hodgson (1994). Photographs of live specimens were taken with a Canon IXY 640 digital camera and a Nikon digital sight DS-5M. The terminology for the first-instar nymph follows that of Kondo et al. (2005). The first-instar nymph was drawn with the aid of a drawing tube attached to an Olympus BX40 compound microscope and the Adobe program Photoshop CS2.
Specimens were deposited at the Colección Taxonómica Nacional de Insectos "Luis María Murillo", Corporación Colombiana de Investigación Corpoica, Mosquera, Cundinamarca, Colombia (CTNI).
RESULTS AND DISCUSSION
Herein we report Alecanochiton marquesi (Fig. 1) for the first time in Colombia based on specimens collected on Manilkara zapota (Sapotaceae) in Palmira, Department of Valle del Cauca and in Costa Rica on Conostegia xalapensis, Miconia trinervia andM. schlimii (Melastomataceae) in the Agua Buena District, Puntarenas Province. The hosts recorded from Costa Rica are all new host records for A. marquesi. The adult female is diagnosed based on published literature and the first-instar nymph is described for the first time based on specimens collected on M. zapota in Colombia. An updated list of hosts and geographical distribution of A. marquesi is provided.

Figure 1 Alecanochiton marquesi Hempel on sapodilla Manilkara zapota. a. On twig, notice first-and second-instar nymphs on lower right corner; b. Close-up, notice lighter color of younger adult female; c. Sooty mold on leaf of M. zapota; d. Ant cartons of Azteca sp. ants; e. Crypticerya abrahami (Newstead) on tree bark of M. zapota; f. Azteca sp. ant. Photos a, c and d by T. Kondo; b, e and f by O.J. Dix-Luna. Scale bar: b and f = 1mm, e = 5mm.
Adult female
Unmounted specimens (Figs. 1a, b and d). Hempel (1921) described the live insects as follows: "Body of adult female oval and convex, whitish grey in young individuals, darker grey in older specimens. Margin thick and darker than rest of dorsum. Dorsal surface covered with small particles of a transparent material, which also forms a fringe around margin. Length 2.0 mm, width 1.5 mm and height 1.25 mm." The above description fits well with the external morphology of the specimens collected in the present study. Additionally, specimens with a yellowish appearance were also observed (Fig. 1a). The glassy waxy material that covers the insect body is brittle. Specimens of A. marquesi are found on twigs and peduncle of leaves and fruit. They are often associated with sooty molds that grow on leaves and twigs and wherever their excreted honeydew accumulates (Fig. 1c).
Diagnosis. Slide-mounted specimens (not illustrated). Adult female oval to elongate oval, with no distinct stigmatic clefts. Anal cleft rather short, about 1/8th of body length. Length 1.6-1.8 mm long, width 1.2-1.4 mm. Dorsal derm membranous. Dorsal setae minute, each only as long as wide, sparse. Dorsal pores round, with thick walls and small inner ductule; arranged along dorsal setae in a reticulate pattern on dorsum. Preopercular pores arranged in a small compact group of 14-19 pores present anterior of anal plates, with another small group of 11-19 pores dorsad to a point midway between antennal bases. Dorsal tubular ducts absent. Dorsal tubercles present submarginally, each convex and rather tall. Anal plates together quadrate, each plate with posterior margin longer than anterior margin, outer angle forming a right angle, with a large discal setae, which is either fimbriate or has a slightly bulbous distal apex, and three small apical setae. Anogenital fold with 6-8 setae present along anterior margin and three or four pairs laterally. Anal ring with about 6 setae. Marginal setae quite long, finely setose with pointed or fimbriate apex. Stigmatic clefts absent; stigmatic setae totaling 3 (rarely 2) per stigmatic area, median spine very long, spiniform and tapering, lateral spines usually spiniform, but frequently with one (usually the anterior) or both setiform and fimbriate. Eyespots present near margin. Ventral derm membranous. Pregenital pores each mainly with 10 loculi. Spiracular disc pores mainly with 5 loculi. Ventral microducts most abundant marginally when compared to mid areas. Ventral tubular ducts of about two types present: 1) a smallish duct with a filamentous inner ductule, rather sparse mediolaterally on abdomen and medially and mediolaterally on thorax; and 2) a similar duct but with inner ductule broader with a large terminal gland, frequent anterior to mouthparts. One or two long pairs of ventral setae present between antennae; also, with three pairs of long pregenital setae; small setae frequent laterally on abdomen and submarginally. Spiracles normal. Legs well developed, each with a small articulatory sclerosis; tarsal digitules slender; claw digitules similar, with broad apices. Antennae with 7 or 8 segments (adapted from Hodgson 1994).
Description of first-instar nymph
Unmounted material. Crawlers elongate oval, cover in a thin layer of glassy wax, greyish to yellowish in color (Fig. 1a).
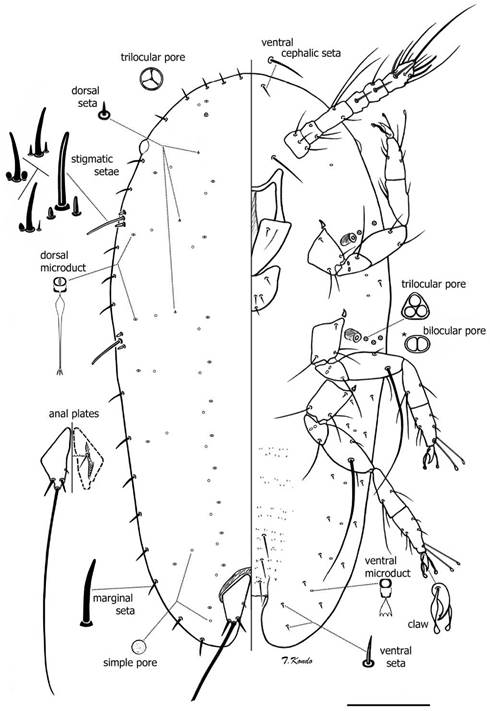
Mounted material: Body elongate oval, somewhat tapering posteriorly. Length 350-430 urn, width 180-220 um. Stigmatic clefts very shallow or absent. Anal cleft well developed (n = 3).
Dorsum: Derm membranous throughout and smooth. Dorsal setae numbering three pairs: one seta on head region on area dorsad to each antennal base; one seta dorsad to area between clypeolabral shield and body margin on each side, and one seta dorsad to area between labium and body margin on each side; setae very short, hard to detect, each about 2 μm long. In one specimen, four pairs of dorsal setae were observed, the fourth pair present on area dorsad to mesothoracic coxae. One pair of trilocular pores present on head near margin, each about 2 μm at widest point. Dorsal microducts small, each 2 μm wide and appearing bilocular, found in two submedial rows and in a submarginal row. Simple pores small, each about 1.5 μm wide, one pore present near each dorsal microduct. Anal plates sclerotized, each 33-35 μm long, 15-16 μm m wide; antero-lateral margin each 23-26 μm long, postero-lateral margin each 19-20 μm long. Each plate with four dorsal setae: one inner margin seta and three apical setae (including long median apical seta), median apical setae, each 150-160 μm long, other setae about 4 μm long. Anal fold with one pair of anterior margin setae. Anal ring with a row of irregularly shaped pores and six anal ring setae.
Margin: Margins rugose. Stigmatic clefts shallow or absent. Marginal setae sharply spinose, straight or slightly bent, each 8-13 Um long. Total number of marginal setae 31-32: eight anteriorly between eyes, one or two between each eye and anterior spiracular setae, two between each group of anterior and posterior spiracular setae, and eight between posterior spiracular setae and body apex. Stigmatic setae totaling three, lateral setae shape variable, sharply or bluntly spinose, sometimes with rounded apices and wider bases, each seta 3 to 5 μm m long, median seta sharply or bluntly spinose, longest, 22-25 Um long. Eyespots present on margin about level of antennal scape.
Venter. Derm membranous, with microtrichia present on mid areas of abdominal segments. Spiracles small, round or oval in shape; peritremes each 4 to 5 μm wide. Spiracular pores each 2 μm wide with 3 loculi, occasionally with 2 loculi; two or three lateral to each anterior spiracle and three laterad to each posterior spiracle. Ventral submarginal setae slender, each 3-5 μm long; with seven pairs in two parallel longitudinal rows on each side of body between posterior spiracle and anal cleft, with outer row of submarginal setae slightly longer than setae on inner row; one seta on area between anterior and posterior stigmatic areas, and one pair of longer ventral cephalic setae, each 7-10 μm long. Three pairs of ventral submedian setae on posterior abdominal segments, each 13-31 μm long; and one pair of interantennal setae between antennal scapes, each 2430 μm long. Ventral microducts present submarginally, each about 1.5 μm wide, with one present near each antennal base, two between anterior and posterior stigmatic areas, and six between inner and outer submarginal setae in abdominal region on each side of body. Legs well developed, with separate tibia and tarsus, without tibio-tarsal scleroses; claw with a small denticle. Tarsal digitules slender, knobbed, subequal in length and longer than claw digitules; latter also knobbed, longer than claw and subequal in length. Dimensions (all legs): coxa 33-35 μm long (at widest point), trochanter + femur 53-55 μm long, with a very long femoral seta on each meso- and metathoracic legs, each very long femoral seta as long as or longer than length of tibia + tarsus + claw, tibia + tarsus, 59-60 μm long, claw 12-13 μm long. Antennae six-segmented, 91-102 mm long. Mouthparts normal, clypeolabral shield, 62-65 μm long, 54-56 μm wide; labium one segmented, 20-22 μm long, 31-32 μm wide; with four pairs of setae.
Geographic distribution. Neotropical region. Brazil (Hempel 1921, Vernalha 1953, Silva d'Araujo et al. 1968, Hodgson 1994); Colombia and Costa Rica (Present study); French Guiana (Hodgson 1994) and Puerto Rico (Jenkins et al. 2014).
Host plants. Ebenaceae: Diospyros kaki Thunb. (Silva d'Araujo et al. 1968). Malvaceae: cotton, Gossypium sp. (Costa-L 1936, Silva d'Araujo et al. 1968). Melastomataceae: Conostegia xalapensis (Present study), Miconia trinervia (Present study); Miconia schlimii (AJK, personal observation). Myrtaceae: Melaleuca sp. (Costa-L 1936, Silva d'Araujo et al. 1968). Oleaceae: Jasminum sp. (Silva d'Araujo et al. 1968). Rubiaceae: Coffea arabica L. (Fornazier et al. 2017), Coffea sp. (Hempel 1921, Pickel 1927, Moreira 1928, Costa-L 1928, 1936, Silva d'Araujo et al. 1968), Genipa americana L. (Costa-L 1936, Silva d'Araujo et al. 1968), Gardenia jasminoides J. Ellis (Silva d'Araujo et al. 1968), Ixora sp. (Jenkins c2013), Gonzalagunia spicata (Lam.) M. Gómez (Jenkins c2013). Sapotaceae: Chrysophyllum cainito L. (Costa-L 1936, Silva d'Araujo et al. 1968); Manilkara zapota (Costa-L 1936, Silva d'Araujo et al. 1968); Pouteria guianensis Aubl. (as P. caimito) (Costa-L 1936, Silva d'Araujo et al. 1968, Hodgson 1994).
Additionally, Silva d'Araujo et al. (1968) listed a species of conifer, citing a personal observation, however, this record needs confirmation.
Biology. Probably with multiple generations per year. Females and males of various stages usually found together. In Costa Rica, A. marquesi was found almost exclusively in restored forests and mixed-use agricultural areas but was nearly absent from primary forest. This is likely due to the early-successional characteristics of their host plants, which are rarely present in old-growth forest. On Melastomataceae, A. marquesi predominantly occupies nodes, internodes, and meristems of seedlings and saplings, with females occasionally found on leaves and rarely observed on adult trees. Infestations of ~20 or more scales were often tended by Wasmannia auropunctata (Roger, 1863) (Hymenoptera: Formicidae: Myrmicinae), a native tramp ant, and occasionally by other unidentified ants (AJK, personal observation). In Colombia, on M. zapota, A. marquesi was found mainly on adult M. zapota trees, on nodes, internodes, meristems, leaf petioles and fruit peduncles. First-instar nymphs were found settled alongside the midrib of leaves. Specimens of A. marquesi on M. zapota were often found under ant cartons and tended by Azteca sp. (Hymenoptera: Formicidae: Dolichoderinae) (Fig. 1d, f) (OJDL, personal observation).
Material studied. COLOMBIA. Valle del Cauca: 5 slides (5 first-instar nymphs). Palmira, Corporación Colombiana de Investigación Agropecuaria (Agrosavia), Centro de Investigación Palmira, 03°30' North, 76°19' West, 1005 m, 13 feb. 2017, coll. O. J. Dix Luna, ex. Manilkara zapota (Sapotaceae), CTNI (235, 235-1, 235-2, 235-3, 235-4). 5 slides (5 adult females): Same data except, 26 nov. 2015, CTNI (233, 233-1, 233-2, 234, 234-1). COSTA RICA. Puntarenas: 3 slides (2 adult females + 1 nymph), Coto Brus, Agua Buena, 19 Jul. 2016, coll. A. Kulikowski, ex Conostegia xalapensis (Melastomataceae), CTNI (241-1, 241-2, 241-3). Puntarenas: 3 slides (3 adult females + 1 nymph), Coto Brus, Agua Buena, San Gabriel, 20 jul. 2016, coll. A. Kulikowski, ex Miconia sclimii (Melastomataceae), CTNI (241-4, 241-5, 241-6).
Other scale insects collected on Manilkara zapota in the present study
During the present study, other scale insects were collected on M. zapota, these are namely Hemiberlesia lataniae (Signoret, 1869), Howardia biclavis (Comstock, 1883) (Diaspididae), Coccus viridis (Green, 1889), Saissetia coffeae (Walker, 1852) (Coccidae) and Crypticerya abrahami (Newstead, 1917) (Monophlebidae) (Fig. 1e). Crypticerya abrahami was found tended by Azteca sp. ants (Formicidae: Dolichoderinae) (Fig. 1d, f). This is the first record of C. abrahami found on M. zapota. According to the scale insect database ScaleNet (García-M et al. c2016) there are 42 species of scale insects recorded on M. zapota worldwide; with the addition of C. abrahami, the list of scale insects on M. zapota is increased to 43 species.
Other material studied. Hemiberlesia lataniae. COLOMBIA. Valle del Cauca: 2 slides (2 adult females). Palmira, Corporación Colombiana de Investigación Agropecuaria (Agrosavia), Centro de Investigación Palmira, 03°30' North, 76°19' West, ca. 1005 m, 13 feb. 2017, coll. O. J. Dix Luna, ex. on twig of Manilkara zapota (Sapotaceae), CTNI (236, 236-1); Howardia biclavis: Same data, on twig, 2 slides (2 adult females) CTNI (237, 237-1); Coccus viridis: Same data, on leaves and small twigs, 1 slide (1 adult female), CTNI (238); Saissetia coffeae: Same data, on twig, 1 slide (1 adult female), CTNI (239); Crypticerya abrahami: Same data, found on tree branch inside ant carton of Azteca sp. ants, 1 slide (1 adult female), CTNI (240).
Affinities of Alecanochiton with other soft scale insect genera
For a good description of the adult female see Hodgson (1994). Adult females of the genus AlecanochitonHempel, 1921 have sclerotized dorsal pores in a reticulated pattern, a characteristic shared with some other soft scale insect genera, for example, the mostly New Zealand genera Aphenochiton, Crystallotesta, Epelidochiton, Kalasiris, Plumichiton, Poropeza, and Umbonichiton (Hodgson and Henderson 2000). The above seven genera described by Hodgson and Henderson (2000) differ from Alecanochiton by the absence of dorsal tubercles and tibiotarsal scleroses, which are present in Alecanochiton. Sclerotized dorsal pores in a reticulated pattern are also seen in the closely related genus Avricus De Lotto, 1975, from the Afrotropical region and the genus Ctenochiton Maskell, 1879, which has been recorded from the Neotropical (Mexico), Oriental and Australasian regions. Hodgson (1994) separates Ctenochiton from Alecanochiton and Avricus by its smaller leg size, the different structure of the dorsal pores and its asymmetric mouthparts that are positioned close to one of the prothoracic legs. Another genus, Stictolecanium Cockerell, 1902, which members are distributed in southern South America (Argentina, Brazil and Chile) also produces a reticulate pattern on dorsum with preopercular pores and/or small and round cribriform plates (Hodgson 1994, Granara de Willink 2006, Kondo and Gullan 2010). Alecanochiton differs from Stictolecanium by the presence of dorsal tubercles and a discal seta on each anal plate, which are lacking in Stictolecanium.
In the first-instar nymph of A. marquesi, there is a long femoral seta on each meso-and metathoracic legs. Williams and Hodges (1997) reported the presence of long femoral setae in other species of Coccidae, i.e., Hemilecanium petasus (Hodgson, 1991) (as Etiennea petasus Hodgson, 1991), Kilifia americana Ben-Dov, 1979, Milviscutulus mangiferae (Green, 1889), Protopulvinaria pyriformis Cockerell, 1894 and Pseudokermes nitens (Cockerell, 1895), but did not specified on which of the three pairs of legs these very long setae are found. Ben-Dov et al. (1975) described the first-instar nymph of M. mangiferae (as Protopulvinaria mangiferae (Green, 1889)), as having a long femoral seta on each meso- and metathoracic legs, similar to A. marquesi. In the genus Hemilecanium, Hodgson (1993) listed four species (as Etiennea), i.e., H. ferox (Newstead, 1917), H. montrichardiae (Newstead, 1920), H. multituberculum (Hodgson, 1991), H. petasus and H. sinetuberculum (Hodgson, 1991), as having a long femoral seta on all three pairs of legs. Another species, Hallicoccus lomagundiae (Hall, 1935) also has long femoral setae on all three pairs of legs, and additionally has long trochanteral setae on all legs (Kondo 2007).
Hodgson (1994) did not place Alecanochiton in any of his subfamilies and considered the African genus Avricus to be a junior synonym of Alecanochiton. Further studies using molecular markers and studies on the morphology of the adult male may help to elucidate the relationship of A. marquesi within the Coccidae.