INTRODUCTION
The Dry Tropical Forest (DTF) is a biome that comprises a group of ecosystems similar in their physiognomy and vegetation (Hernández-Camacho 1992). The main characteristic of this biome is a marked seasonality of rain with several months of drought. Therefore, the ecological changes in the communities of this biome are affected by this seasonality (Pennington et al. 2006). Due to its climatic and soil type characteristics, the Caribbean region of Colombia has the largest extensions of DTF in Colombia (Pizano and García 2014).
Pseudoscorpiones is an order of arachnids currently including more than 3400 species that belong to 437 genera and 26 families (Harvey c2013). They are small animals that range between 0.3 and 12 mm in size. They exhibit a great variety of body shapes and trophic habits (Buddle 2010). Pseudoscorpions occupy a variety of habitats, including edaphic and cave environments and even canopy forests (Murienne et al. 2008, Battirola et al. 2017a, b). They display phoretic associations and are frequently found in nest of birds, mammals, and on other animals (Poinar Jr. et al. 1998, Mahnert and Adis 2002, Turienzo et al. 2010).
Recent studies such as Bedoya et al. (2014, 2015), Bedoya (2015), and Romero-Ortiz (2015, 2017) have contributed to the knowledge of pseudoscorpions in Colombia, others (i.e. Lacava et al. 2015, Bedoya et al. 2016, Garcia et al. 2016), have helped to explain the ecological role of pseudoscorpions in certain ecosystems. However, the ecological and taxonomic diversity of pseudoscorpions still remains underexplored, alongside their relationship with different habitats. Few studies on the pseudoscorpions of the Colombian DTF have been published, with only a few reports from the Magdalena department (Ceballos and Florez 2007).
Several studies have discovered different patterns of spatio-temporal variation in some communities of arthropod inhabitants of the DTF in the Caribbean region of Colombia (Barraza et al. 2010, Quijano and Martínez 2015, Román-Garrido et al. 2016). However, there is still a gap in knowledge related to arachnid communities. Understanding these patterns in organisms such as pseudoscorpions could help establish their role as indicators, especially since changes in environmental factors affect the abundance or permanency of a community (Yamamoto et al. 2001).
This study develops an inventory of species of pseudoscorpions in two DTF fragments and relate it with environmental data. In order to determine the spatiotemporal variation of the species and the relationship of this variation with the fluctuations in environmental variables such as environmental temperature, relative humidity, canopy cover, and rainfall.
MATERIALS AND METHODS
Study area
This study was conducted in two locations of the Caribbean region. First, the Reserva La Flecha (RLF) in the municipality of San Jacinto (Bolívar), (09°51'09,74" North, 75°10'32,32" West), with an altitude of 324 m and an extension of 149 ha. This is included within the climatic subunit D2, according to the classification proposed by Rangel-Ch and Carvajal-Cogollo (2012), with a precipitation average of 1972 mm and a temperature average of 25 °C per year (Castaño 1999). The rainfall regime is bimodal, with a period of intense drought between the months of December and March, and a less-marked drought period between June and July (Rangel-Ch and Carvajal-Cogollo 2012). The vegetation is hygrotrophophytic, however, on some areas the vegetation is subhigrophic, whereby the evergreen vegetation is present (Díaz et al. 1986).
Second, the Reserva Campesina La Montaña (RCM) located between the municipalities of Juan de Acosta and Usiacurí, Atlantico (10°46'02,06" North, 75°02'34" West), with an elevation ranging from 160 to 250 m and an extension of 47 ha. This is included in the climatic unit B1, with an annual precipitation average of 1161 mm. The rainy season is between May to November and the dry season from December to April (Rangel-Ch and Carvajal-Cogollo 2012). The average temperature of the place is 27.6 °C, and the average relative humidity is 62 % (García-Atencia and Martínez-Hernández 2015). The vegetation is hygrotrophophytic. Therefore, during the prolonged dry season, the forest completely loses the canopy layer. The forest fragment is surrounded by areas dedicated to livestock and crops. In the vicinity of the fragment, wood coal production, wood extraction for firewood, and construction lumber for houses, are frequent practices (García-Atencia and Martínez-Hernández 2015).
Fieldwork
During 2016, two sampling efforts per fragment were conducted taking into consideration rainfall seasonality (Dirzo et al. 2011) which strongly affects arthropod activity (Barraza et al. 2010).
In each fragment, four plots of 100 m x 25 m were established, separated from each other by 200 m. Four points were marked, spaced 20 m apart where manual capture (MC) and sifting of leaf litter (SL) were performed. MC involved the intensive search of individuals in different places for 10 minutes/man/ day for a total of 50 min/plot. After each specimen was captured, descriptive data concerning its natural history were recorded. In the case of SL, a Winkler bag (Besuchet et al. 1987) was used. At each sampling place, 10 L of litter was collected, sifted, and transported in cloth bags. The extraction of pseudoscorpions was performed with Winkler bags (Battirola et al. 2017a), extraction time lapsed two days. Finally, the material was checked in white trays in order to capture the remaining specimens with the help of forceps and brushes.
Additionally, at each point environmental temperature (°C) and relative humidity (%) were measured with a thermo-hygrometer Extech RH101 and soil temperature with an Extech thermometer M0750. The canopy cover was measured for each sampling point following Strickler (1959). Furthermore, a rain gauge was installed in each fragment in order to determine the amount of rainfall in mm per month.
The collected specimens were stored in flasks labeled with ethanol 70 % and identified with the taxonomic keys of Muchmore (1990), Harvey (1992), Mahnert and Adis (2002), Buddle (2010), and Mahnert et al. (2011), using a Leica M125 stereomicroscope, only adults were included in the analyses. The identifications were confirmed by comparison with the collection of pseudoscorpions at the Instituto de Ciencias Naturales (ICN-APs) Bogotá. The specimens are registered in the Museum of Zoology of the Universidad del Atlántico and the Collection of Arachnology-ICN.
Data analyses
Richness and abundance were recorded by technique, location, sampling season and fragment. The community structure by sampling and fragment was determined by means of range-abundance curves (Magurran 1988). To explore spatial or temporal patterns in terms of species composition and abundance, a Bray-Curtis similarity matrix was made, previous transformation by Log (X + 1), which avoid positive biases, and therefore homogenize variances (Zar 1996). The matrix was used to perform other three analyses:
First, a cluster analysis by hierarchical classification, using the method of grouping pairs according to the unweighted arithmetic mean (UPGMA), was made with the software Primer v6.0 (Clarke and Gorley 2006). This technique represents, by means of a graph or dendrogram, the similarity between the communities of pseudoscorpions in the fragments.
Second, an analysis of similarities (ANOSIM) was performed using Primer v6.0 (Clarke and Gorley 2006). This test determines the statistical support of spatial or temporal patterns by comparing the variances within and between groups (Clarke 1993), thereby allowing for the statistical corroboration of whether the spatial variation or the temporal observation in the dendrogram is statistically significant.
Third, a canonical correspondence analysis (CCA) with the package Vegan (Oksanen et al. c2018) in R version 3.5.1 (R Core Team c2017) was conducted, to determine the extent to which abiotic variables such as ambient and soil temperature, environmental humidity and precipitation, and canopy cover may explain the variation of richness and abundance of pseudoscorpions by time and fragment.
RESULTS
A total of 260 individuals belonging to five families and eight species were collected with the two methods, of which the most abundant was Pachyolpium granulatum (Beier, 1954) Olpiidae, with 119 individuals, followed by Paratemnoides nidificator (Balzan, 1888) Atemnidae, with 73 individuals. The highest abundance was found during the rainy season with 147 individuals, while during the dry season it decreased to 113 individuals.
The RLF showed more richness and abundance of pseudoscorpions than the RCM. However, abundances differed per season, RLF had 98 individuals in the dry season and 61 in the rainy season, whilst the RCM had a higher abundance in the rainy season (83 individuals) and decreased in the dry season (thirteen individuals) (Table 1).
Table 1 Richness and abundance of variation of the pseudoscorpions collected in RCM and RLF for two seasons. Abbreviations: MC = manual capture, SL = sifting leaf litter, R = richness, N = abundance, text within parenthesis after species names refer to abbreviations used in figures. *New record for the department.
Richness in the RLF was similar between the two seasons with five species each. Concerning the composition, there were differences in Pachychernes sp. (Chernetidae), that was typical of dry season and Paratemnoides sp. that was only found in the rainy season. Despite differences in the abundance, the richness in the RCM was almost same between seasons with four species in dry and five in rainy seasons. Parachernes sp. (Chernetidae) was found only in rainy and Cacodemonius sp., the only species of the Withiidae, was typical of dry season, the remaining species were shared among seasons (Table 1).
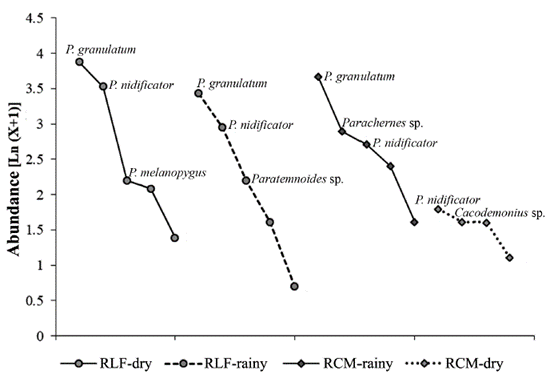
The range-abundance curves showed similar shape in the RLF-Dry, RLF-Rainy and RCM-Dry, with Pachy. granulatum as the dominant species, contributing with almost half of the total abundance in each one. Parat. nidificator exhibited great abundances in the fragments too, exceeded only by P. granulatum in RLF (Fig. 1). The RCM in dry season was completely different in shape of latter, this has a little number of individuals, therefore there is a major equitability in this season, Parat. nidificator was the most abundant species followed by Cacodemonius sp.
Manual capture (MC) was the most effective method with the collection of 56 individuals, eleven in dry season and 45 during the rains in RCM. In addition, this method achieved the greatest richness during the sampling, with four species. Sifting of leaf litter (SL) method captured 42 individuals, four during the dry season and 38 during the rains. Pachy. granulatum, was the only species found in both seasons with this technique (Table 1). In the case of RLF, the greatest number of individuals was recorded with the SL technique for the two seasons (88), although the abundance was distributed between two species: P. granulatum with 77 individuals and Ideobisium sp. (Syarinidae) with eleven individuals (Table 1).
Spatio-temporal variation of pseudoscorpions
The similarity in richness and composition was greater between seasons of the same places (temporal) than between collecting sites. The similarity dendrogram showed two groups, one corresponds to the dry and rainy seasons in RCM and the other to the RLF (Fig. 2). However, temporal variation in RCM must be highlighted, as we observed a greater variation which was represented by the lowest percentage of similarity (63 %) compared to that obtained for RLF (72 %).
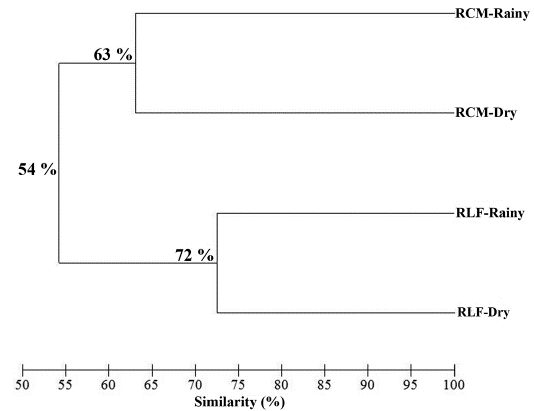
Figure 2 Similarity dendrogram for fragments and seasons applying UPGMA grouping and Bray-Curtis distance.
Temporal differences were statistically significant between the RLF and RCM (ANOSIM, R = 0.42, P = 0.03). However, the spatial differences, related with the specific species of each fragment (Table 1) and the variation in the structure of the community between the sites (Fig. 1) were not significant (R = 0.04, P = 0.36).
The environmental variables exhibited notable changes throughout the sampling periods, with RCM presenting the largest variation during the dry season. During this period, no individuals were found in SL, this corresponding to the maximum values of soil temperature (38 °C) and minimum canopy cover (0.16 %) (Table 2). In the rainy season, the variables remained uniform over time. Canopy cover values were higher, and soil temperature was lower (Table 2). RLF displayed a similar behavior between sampling times, with high values of canopy cover which prevented the soil temperature from being high and directly affected the conditions in the litter.
Table 2 Richness and abundance of pseudoscorpions in the study area and the environmental parameter values (min-max, mean, and standard deviation).
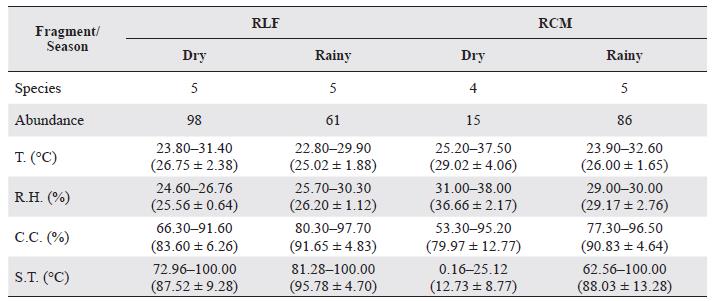
R.H. = relative humidity, C.C. = canopy cover, S.T. = soil temperature, T = environmental temperature
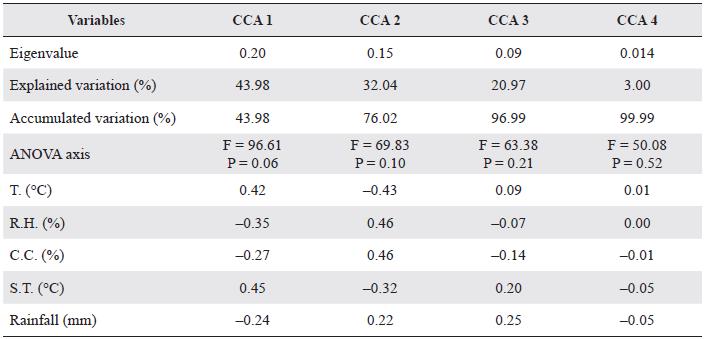
The species richness and abundance showed weak relations with the environmental variables measured and none of the variables or the axis of the canonical correspondence analysis (CCA) were statistically significant according to the ANOVA (P > 0.05) (Table 3). The first two axes of the CCA (Fig. 3) explained 76 % of the variation of the data (Table 3).
Table 3 Percentage of explained variation and variance analysis (ANOVA) for each axis in the canonical correspondence analysis (CCA).
R.H. = relative humidity, C.C. = canopy cover, S.T. = soil temperature, T. = environmental temperature
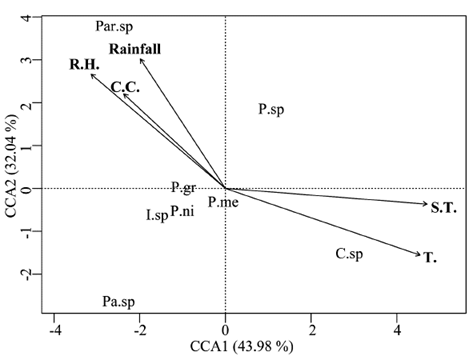
Figure 3 Canonical correspondence analysis (CCA) for the pseudoscorpions diversity and environmental parameters measured in field (abbreviations in Table 1).
The relative abundance of species such as Ideobisium sp. and Paratemnoides sp. was associated with negative values on axis 1, indicating that the abundance of these species is favored by high values of precipitation, relative humidity, and canopy cover, while their abundance declines in conditions with high soil and environmental temperature.
Cacodemonius sp. showed a direct relationship with the high environmental and soil temperatures, which indicates greater abundance of this species in high temperature conditions. Conversely, a decline in conditions with high precipitation, high relative humidity, and greater canopy cover. P. granulatum, Parac. melanopygus, Pachychernes sp., Parachernes sp., and Parat. nidificator showed weak relationships (Fig. 3).
DISCUSSION
This research found eight species, included in six genera and five families. These faunistic data are the first records of pseudoscorpions for the departments of Atlántico and Bolívar. Despite the fragmentation of the DTF in these departments, the selected fragments harbor a similar diversity to that currently reported for the DTF and the surrounding ecosystems such as the mangrove forest in the department of Córdoba where seven and eight species are known respectively (Bedoya et al. 2014, 2016). P. granulatum was the most abundant species in both fragments. This species is reported in DTF in the Sucre department and was registered with the highest abundance by Bedoya-Roqueme et al. (2017). This could indicate that P. granulatum can physiologically take advantage of the conditions of the litter to proliferate, as no other species was found in this microhabitat in RCM and only Ideobisium sp. was found in RLF, albeit in very low proportions.
Olpiids are known for their tolerance to desiccation and environmental changes (Weygoldt 1969), which explain their high abundance in various ecosystems such as the DTF. The genus Pachyolpium show high frequency in Colombia, being the most representative taxon in the collection of pseudoscorpions in the Instituto de Ciencias Naturales (Romero-Ortiz 2017).
The seasonal abundance patterns of pseudoscorpions in the RCM are similar to reports presented for other groups of arthropods from the DTF including those of the same locality, such as coprophagous beetles (Scarabaeinae) (Barraza et al. 2010, Rangel-Acosta et al. 2016, Rangel-Acosta and Martínez-Hernández 2017), bugs (Reduviidae) (Román-Garrido et al. 2016), and spiders (Quijano and Martínez 2015), which have low abundance during the dry season and increase during the rains. However, the ANOSIM results showed that there is no significative temporal variation because the species composition is similar (Table 1).
Additionally, many of the species found are of cortical habits and therefore, they are not seriously affected by changes related to external variables such as soil warming. On the other hand, pseudoscorpions have showed adaptations to life in environments to be subjected to drastic changes in their structural conditions (Mahnert and Adis 1985, Adis et al. 1986, Battirola et al. 2017b).
In RCM the structural change in the vegetation is evident because canopy cover measurements during the dry season are zero in many cases. It causes the desiccation of litter and high soil temperatures, and affects the pseudoscorpions associated with this microhabitat. Contrarily, in RLF no significant variation in composition or structure was found during the sampling periods. This is directly related with the canopy cover that is not completely lost in this fragment during the dry season. So, the temperature of the soil has fewer fluctuations and the variables remain relatively constant. The above statements reinforce the proposal of Pinheiro et al. (2013), who suggested that arthropod populations should be temporarily less variable in places where climatic conditions do not undergo sudden changes, although this will be directly related to the availability of resources during the seasons.
Different researches on the seasonality of pseudoscorpions in Amazonian forests in Brazil where there is a marked seasonality of rainfall, have shown that the abundance of pseudoscorpions tends to increase during the dry season (Morais 1985, Adis and Mahnert 1990, 1993, Aguiar et al. 2006), just like in the RLF in this study. It is because the soil fauna moves slowly to places or refuges in more specific microhabitats during times of low water availability, which are often sampled easier (Eijsackers 2001, Aguiar et al. 2006).
In the study, two litter and five cortical species were found, so sampling techniques are complementary since most of the species occupy a certain microhabitat and were not found to be shared. Even though a few individuals were obtained with MC, it was the sampling method that showed more richness. Considering that the cryptic habit of the representatives of this order often makes this type of sampling difficult, it is inferred that with greater efforts in MC, the observed richness can be increased.
Although some environmental variables were positively or negatively related to the diversity of some pseudoscorpions species, this relationship was not statistically significant. The various responses may arise from specific biological or ecological characteristics or requirements of each of the species, as each one may respond differently to changes in environmental variables (Adis and Mahnert 1993, Aguiar et al. 2006) due to the specificity of the microhabitat that each species occupies in the ecosystem.
The non-significant relationships with environmental variables are similar to these found by Bedoya et al. (2016) in mangrove ecosystems of the department of Córdoba, that did not find any significant relationship of these arachnids with the humidity of the litter and soil, soil salinity and salinity of the flood zones. In the Brazilian Amazon region soil moisture has been found to be a key factor in the abundance of pseudoscorpions because many species tend to dry out easily (Weygoldt 1969, Adis and Mahnert 1990, 1993, Battirola et al. 2017a). However, this factor was not considered in the present study.
Relationships between pseudoscorpions and environmental variables are frequently not clear, as they often show different results even in the same place if sampling techniques are changed (Aguiar et al. 2006). Therefore, although the significance of abiotic variables should not be overlooked, it is necessary explore the relationship of pseudoscorpions with the dynamics of prey and explore specific microhabitats such as bird nests, and colonies of bee, ants, and termites, as these can be home to many endemic and still unknown species for science, as well as the phoretic behavior that some species have displayed (Mahnert and Adis 2002).
Finally, although pseudoscorpions constitute a diverse group (Harvey 2002), a low number of species was observed. Future studies with greater sampling efforts in the DTF and considering the diverse ecosystems that occur in the Caribbean region will increase the knowledge. Yet, the scarcity of ecological works with pseudoscorpions makes it difficult to establish standards for comparison.