INTRODUCTION
Seasonally Dry Tropical Forests (DTF) are largely deciduous forests with a mean temperature above 25 °C, annual precipitation between 700 and 2000 mm, and three or more dry months with monthly precipitation below 100 mm (Sánchez-Azofeifa et al. 2005). Important neotropical DTF are found along the Pacific coast of Mexico and Central America, on the Yucatán peninsula, in the Greater Antilles, the Caribbean region of Colombia and Venezuela, the inter-andean valleys, the Pacific coast of Ecuador and Peru, southwest Brazil to Bolivia and Argentina, and the interior of northeast Brazil (Pennington et al. 2009, Portillo-Quintero and Sánchez-Azofeifa 2010, Linares-Palomino et al. 2011, Dryflor et al. 2016).
Neotropical DTF show a floristic differentiation into 12 groups in six major clusters (Dryflor et al. 2016): (1) Antilles, (2-4) central inter-andean valleys, central andean coast of Ecuador and northern Peru, and the Peruvian Apurimac-Mantaro region or southern inter-andean valleys; (5-7) Mexico, Central and northern South America, including northern inter-andean valleys in Colombia and Venezuela; (8) Tarapoto-Quillabamba cluster in Ecuador and Peru; (9-10) Piedmont and Misiones regions in northern Argentina, Bolivia, Paraguay, and parts of southern Brazil; and (11-12) Central Brazil and the northeast Brazilian Caatinga.
Besides showing distinctive phylogenetic patterns (Pennington and Lavin 2016, Rezende et al. 2017), these phytochoria correspond to phytogeographic provinces (Takhtajan 1986, McLaughlin 1994): (1) West Indies province, (2-4) Central Andes province, (5-7) Central American and Northern Andes provinces, (9-10) Amazon and Chaco provinces, and (11-12) Central Brazilian uplands and Caatinga provinces.
DTF are considered the most threatened tropical forest biomes (Janzen 1988, Trejo and Dirzo 2000, Miles et al. 2006, Derroire et al. 2016). Less than 10 % of their original extension remain in many countries (García et al. 2014, Dryflor et al. 2016), with as little as 5 % for Peru and as much as 55 % for Bolivia, and an average of 34 % across the entire Neotropics, although often without protected status (Portillo-Quintero and Sánchez-Azofeifa 2010). Apart from continuous threat through urbanization and land use change (Grau et al. 2008), DTF are considered vulnerable to global climate changes (Allen et al. 2017).
Colombia is situated in the center of the neotropical realm, although its dry forest flora is phytogeographically and phylogenetically closer to Central America, the Antilles, and the Central Andes, than to the dry forests of Bolivia, Brazil, Paraguay and Argentina (Dryflor et al. 2016). Colombian DTF belong to two groups in the same cluster (6-7): the Central and northern South American group (Central American province) and the northern inter-andean valleys group (Northern Andes province). In Colombia, both groups are well separated geographically and comparable in extension, with the first largely confined to the Colombian Caribbean and the second along the Andes (Dryflor et al. 2016). Based on remote sensing data, Portillo-Quintero and Sánchez-Azofeifa (2010) estimated that 33 % of DTF remain in Colombia, whereas another study reported an extension of 720 000 ha or just about 8 %, more than half corresponding to small fragments of less than 25 ha (García et al. 2014).
Colombian Caribbean DTF is mostly located in the departments of La Guajira, Cesar, Magdalena, Atlántico, Bolívar, Sucre, and Córdoba, exhibiting a high level of transformation (Arcila-Cardona et al. 2012, González et al. 2018). Atlántico contributes with only 3.7 % or 12 158 ha (García et al. 2014), largely due to a 60 % loss of coverage that occurred between 1990 and 2005 (IDEAM 2010). Atlántico has three protected areas, none of them categorized as national: the Distrito de Manejo Integrado Luriza (Corporación Autónoma Regional del Delta Magdalena and Corporación Autónoma Regional de la Cuenca Baja del Río Magdalena, Acuerdo 0003 del 2011); the Parque Natural Regional Los Rosales; and the Reserva Forestal Protectora El Palomar (Corporación Autónoma Regional del Atlántico, Acuerdo 00019 del 2013). Floristic studies in the DMI Luriza and the RFP El Palomar reported 141 and 246 species of plants, respectively (Rodríguez and Banda-R 2011, 2012).
Lichens are not well studied in tropical regions, although research has taken up speed in the past two decades (Staiger 2002, Frisch et al. 2006, Aptroot et al. 2008, Lücking et al. 2008, 2009a, Rivas-Plata et al. 2010, Rincón-Espitia et al. 2011, Aptroot 2012, Sipman et al. 2012, Lücking 2014, Moncada et al. 2014, 2015, Cáceres et al. 2014, 2017, Aptroot and Lücking 2016, Lücking et al. 2017a). Few studies focused on tropical lichen communities (Rivas-Plata et al. 2008) and even fewer included DTF (Cáceres et al. 2008). The total number of species in Colombia has been estimated at 3,600 (Lücking et al. 2009b), with less than half (1,674) catalogued (Bernal et al. 2015). Most species were recorded in the Andes, whereas the Colombian Caribbean remains little studied. Between 1990 and 2015, 54 out of 470 works on the biological diversity of the Colombian Caribbean targeted the floristic composition of DTF, and among those, just four focused on lichens (Aldana-Domínguez et al. 2017). The only recent lichen inventory in the region (Rincón-Espitia et al. 2011) reported 215 species from five localities in the departments of La Guajira, Cesar, Atlántico, and Córdoba. For Atlántico, based on that study and the Catálogo de Plantas and Líquenes de Colombia, 27 species are known, in contrast to well over 700 for Cundinamarca. In an assessment of potentially threatened bryophytes and lichens in Colombia, Aguirre-C and Rangel-Ch (2007) listed five critically endangered species for the Caribbean, but only from the Sierra Nevada de Santa Marta, not considering dry forest lichens.
In order to close this gap, the objective of the present study was to conduct a first inventory of lichens in Átlantico, with the aim to obtain a broader picture of lichen diversity and community composition in Colombian Caribbean DTF and to compare biogeographic patterns between woody plants and lichens, with the future goal to use lichens to monitor the conservation status of dry forest fragments (Rivas-Plata et al. 2008).
MATERIAL AND METHODS
Study sites
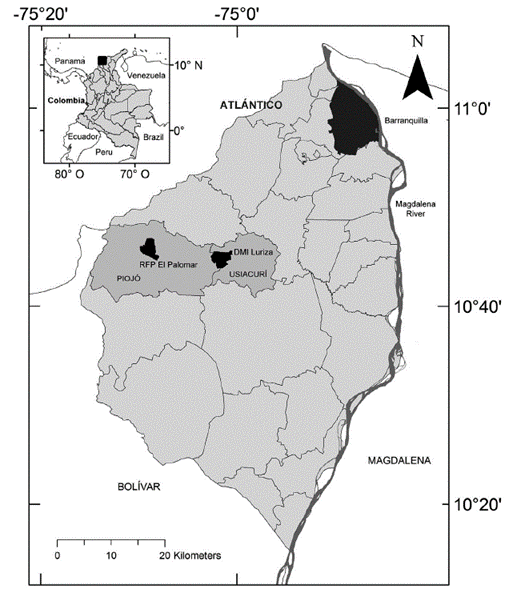
The study was conducted in two of the three protected areas in Atlántico: Distrito Regional de Manejo Integrado (DMI) Luriza and Reserva Forestal Protectora (RFP) El Palomar. The DMI Luriza (Fig. 1) has an area of 339 hectares and is located in the municipality of Usiacurí, at 10°45' North and 75°01' West, with an elevation of 100-200 m. The region corresponds to the upper basin of the Luriza river, part of the Canal del Dique sub-area in the Magdalena-Cauca hydrographic area (Alexander et al. 2009, Molina-Acosta 2013). The average temperature is 27.5 °C and annual rainfall is 980 mm. The reserve is composed of dry forest (19 %), sclerophyllous vegetation (54 %), pastures (25 %) and open woodland (2 %; Rodríguez and Banda-R 2011). The best preserved areas of DTF are located along the low terrains of the Luriza basin; other areas are covered with successional intermediate forest, with low to medium levels of intervention. Most of the study area is surrounded by subsistence crops. Some species of mammals are present, including the howler monkey, Alouatta seniculus (Linnaeus, 1758) (Rodríguez et al. 2012).
The RFP El Palomar (Fig. 1) has an area of 312 hectares and is located in the municipality of Piojó, at 10°46' North and 75°09' West, with an elevation of about 500 m. The average temperature is 26 °C and annual rainfall amounts to 980 mm. The reserve is part of the El Palomar-Sierra del Águila mountain range and is characterized by having a hilly topography, where the steepest slopes are the areas with the most conserved forest, harboring a population of the endemic and critically endangered Tití cabeciblanco, Saguinus oedipus (Linnaeus, 1758). El Palomar is located in the Litoral watershed and its waters reach the Caribbean Sea and the Totumo swamp. The vegetation is composed of secondary forest (35 %), sclerophyllous vegetation (48 %), pastures (6 %), and grassland mixed with crops (4 %) (Rodríguez and Banda-R 2012), with some transitional forest towards subxerophytic vegetation (Rodríguez et al. 2012).
Lichen sampling and identification work
At both localities we performed opportunistic sampling following Sipman (1996) during several visits in 2015 and 2016. Specimens were air-dried and subsequently identified in the laboratories at the Universidad Distrital Francisco José de Caldas, Bogotá (UDBC) and the Botanic Garden and Botanical Museum, Freie Universität Berlin (BGBM), using standard techniques of light microscopy and thin-layer chromatography (TLC), with the following equipment: OLYMPUS SZX12, LEICA MS5, LEICA S6D (UDBC) and LEICA Zoom 2000 (BGBM) dissecting microscopes and OLYMPUS BH-2, ZEISS Axiostar Plus (UDBC), and ZEISS Axioskop (BGBM) compound microscopes; TLC performed in solvent C (Orange et al. 2010).
For initial identification to genera, we used Sipman (c2005a), Nash et al. (2002, 2004, 2007), and Cáceres (2007), and for species identifications, apart from the keys available in Nash et al. (2002, 2004, 2007) and Cáceres (2007) we used specialized keys (Arthoniales: Egea and Torrente 1993a, b, Tehler 1996, Kalb 2001, Aptroot et al. 2014a, Caliciaceae: Awasthi 1975, Aptroot 1987, Kalb 1987, Marbach 2000, Sipman c2011, Benatti and Jungbluth 2014, Aptroot et al. 2014b, Graphidaceae: Lücking et al. 2009a, Rivas-Plata et al. 2010, Lecanoraceae: Lumbsch et al. 1995, Malmideaceae: Breuss and Lücking 2015, Megalosporaceae: Sipman 1983, Parmeliaceae: Sipman c2005b, Physciaceae: Aptroot 1987, Moberg 1987, 1990, Sipman c2013, Benatti and Jungbluth 2014, Porinaceae: McCarthy 1995, Pyrenulaceae: Aptroot 2012, Trypetheliaceae: Aptroot and Lücking 2016). We also performed comparison with type material of candidate names, using our own data on tropical lichen types and JSTOR Global Plants (https://plants.jstor.org). Classification follows Lücking et al. (2017b) and nomenclature is based on the cited works and on Index Fungorum [http://www.indexfungorum.org]. Voucher specimens are being deposited in the herbario of the Fundación Universidad del Norte (UNO), with some duplicates at the Botanic Garden and Botanical Museum in Berlin (B).
RESULTS AND DISCUSSION
We identified 61 taxa at the two localities (Table 1, Fig. 2). The inventory revealed four species new to science, all in Graphidaceae, described below: Fissurina linoana, Graphis lurizana, G. mokanarum, and Phaeographis galeanoae. Four other species, two in the Arthoniaceae and one each in the genera Bacidia and Pyrenula might be new to science (Table 1), but require further studies, including extensive revision of available type material from across the tropics; two further species in the genera Astrothelium and Sprucidea could not be identified with certainly to species level. Arthonia erupta and Coenogonium saepincola are reported as new to South America, whereas 16 species are new to Colombia (Table 1).
Table 1 List of 61 lichenized taxa recorded at DMI Luriza and RFP El Palomar , arranged alphabetically by family , genus, and species. Species new to science are marked as “new”, species new to the Neotropics with three asterisks, those new to Colombia with two, and those new to Atlántico with one asterisk; those known previously from Atlántico are indicated with “pre”. Voucher numbers and herbarium acronyms are indicated.
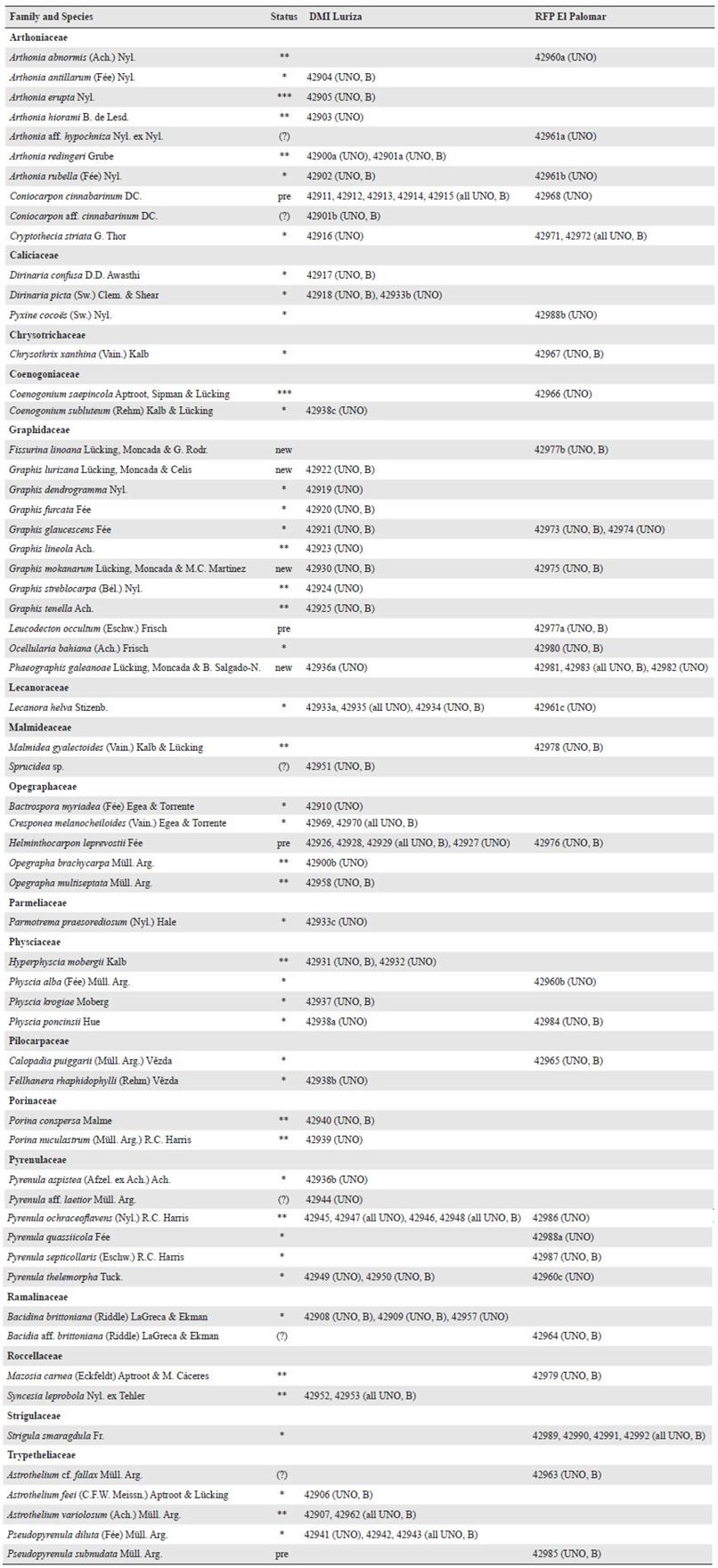
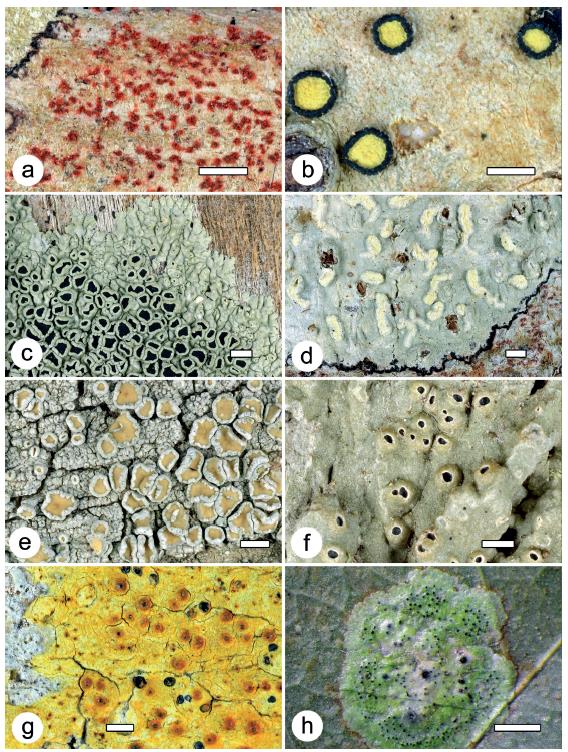
Figure 2 Examples of lichens found at the two localities and characteristic of DTF. a. Arthonia redingeri (42900a); b. Cresponea melanocheiloides (42970); c. Dirinaria confusa (42917); d. Helminthocarpon leprevostii (42976); e. Lecanora helva (42933a). f. Ocellularia bahiana (42980); g. Pyrenula ochraceoflavens (42945); h. Strigula smaragdula (42990). Thalli with ascomata, in h also with pycnidia. Scale = 1 mm.
Rincón-Espitia (2011) and Rincón-Espitia et al. (2011) previously reported 19 species from Atlántico, all from La Batatilla in the municipality of Juan de Acosta. The Catálogo de Plantas and Líquenes de Colombia (Bernal et al. 2015) listed eight additional species for the department, for a total of 27. Of these 27, only Coniocarpon cinnabarinum, Helminthocarpon leprevostii, Leucodecton occultum, and Pseudopyrenula subnudata were found in the present inventory. The remaining 23, not found in our study, are Arthonia explanata Nyl. (Arthoniaceae), Phyllobaeis erythrella (Mont.) Kalb (Baeomycetaceae), Amandinea extenuata (Müll. Arg.) Marbach (Caliciaceae), Fissurina hyalinella Müll. Arg., Graphis chlorotica A. Massal., G. scripta (L.) Ach., and Melanotrema platystomum (Mont.) Frisch (Graphidaceae), Zwackhia viridis (Ach.) Poetsch & Schied. [as Opegrapha viridis (Ach.) Behlen & Desberger; Lecanoraceae], Malmidea granifera (Ach.) Kalb & Lücking and M. psychotrioides (Kalb & Lücking) Kalb, Rivas Plata & Lumbsch (Malmideaceae), Megalospora tuberculosa (Fée) Sipman (Megalosporaceae), Opegrapha dekeselii Ertz and O. longissima Müll. Arg. (Opegraphaceae), Porina imitatrix Müll. Arg. and P. simulans Müll. Arg. (Porinaceae), Pyrenula cubana (Müll. Arg.) R. C. Harris., P. nitidula (Bres.) R. C. Harris, P. ochraceoflava (Nyl.) R.C. Harris, P. santensis (Nyl.) Müll. Arg., and P. tenuisepta R. C. Harris (Pyrenulaceae), Lopezaria isidiza (Makhija & Nagarkar) Aptroot & Sipman and Ramalina usnea (L.) R.H. Howe (Ramalinaceae), and Astrothelium cinnamomeum (Eschw.) Müll. Arg. (Trypetheliaceae). Thus, of the 61 taxa found, 57 are new records for Atlántico (including the new species and those new to the Neotropics and Colombia), raising the total of lichenized species known from the department from 27 to 84, a more than twofold increase.
A higher number of species was found at Luriza (42) compared to El Palomar (31; Table 1). overlap in species composition was remarkably low, with only 12 species (20 %) shared between both sites (including two of the species new to science) and 30 species exclusively found at Luriza and 19 at El Palomar (Table 1). Observed species richness was comparable with that reported from three Brazilian Caatinga sites, with 23, 24, and 50 species (Cáceres et al. 2008). However, there are remarkable differences in species composition. Characteristic indicator species for the Caatinga were Baculifera pseudomicromera Marbach and Dirinaria leopoldii (Stein) D.D. Awasthi (Caliciaceae), Haematomma persoonii (Fée) A. Massal., and Lecanora hypocrocina Nyl. (Lecanoraceae), Maronina multifera (Nyl.) Hafellner & R.W. Rogers (Parmeliaceae), Pertusaria flavens Nyl., and P quassiae (Fée) Nyl. (Pertusariaceae), and Ramboldia haematites (Fée) Kalb (Ramboldiaceae). None of these was found in our study, and few of the species reported here are shared with the Caatinga sites (Cáceres et al. 2008). In contrast, species such as Graphis dendrogramma, Leucodecton occultum, and Helminthocarpon leprevostii, appear to be characteristic elements of dry forests in Central America and the Antilles (Lücking et al. 2008, Sipman et al. 2012), supporting the hypothesis that DTF lichens may behave similarly to woody plants in showing strong biogeographic differentiation between Central and northern South America and Central and northeast Brazil (Dryflor et al. 2016).
The remarkably low overlap in species composition between Luriza and El Palomar, as well as the low overlap between previously reported taxa and those found in the present inventory, suggests a high level of spatial heterogeneity, which could be genuine or more likely the result of sampling bias. Quantitative sampling may increase compositional overlap between different dry forest sites and also increase the overall number of species. On the other hand, fragmentation and degradation of dry forest remnants may also lead to stochastic 'relict' communities that represent a fraction of the original community, resulting in a high degree of heterogeneity. This was observed for lichens in Atlantic rain forest fragments in Brazil (Pereira et al. 2018). Vascular plant species overlap between Luriza and El Palomar amounts to 44 % (Rodríguez et al. 2012), also showing a rather high level of heterogeneity.
The four novel taxa discovered here underline the notion that small forest remnants may harbor undescribed species new to science (Pereira et al. 2018). This is of course not restricted to lichens or to DTF; in the past 25 years, an average of 744 new species have been described each year for the Americas (Ulloa-Ulloa et al. 2017), many of these from small remnants of intact vegetation. Since the Catálogo de Plantas and Líquenes de Colombia (Bernal et al. 2015), 152 new species of plants and 55 new species of lichen fungi were described from the country (Programa de Informática de la Biodiversidad del Instituto de Ciencias Naturales, pers. comm., Moncada et al. 2015, Aptroot et al. 2016, Lücking et al. 2017a, Soto-Medina et al. 2017). A striking case is the genus Cora, with dozens of new species discovered in threatened paramo fragments (Lücking et al. 2014, 2017a). It is estimated that between 50 000 and 100 000 plant species are yet to be discovered globally, and about 6 % of these are predicted to occur in Colombia (Pimm and Joppa 2015). Therefore, thorough studies of remaining fragments of intact vegetation in Colombia, in particular DTF, are urgently needed in order to complete our knowledge on Colombian biodiversity and to assess the importance of these undiscovered species in terms of ecosystem services, conservation, environmental monitoring, biochemistry, and food supply.
THE NEW SPECIES
Fissurina linoana Lücking, Moncada & G. Rodr. sp. nov. Mycobank MB 828114 (Fig. 3a).
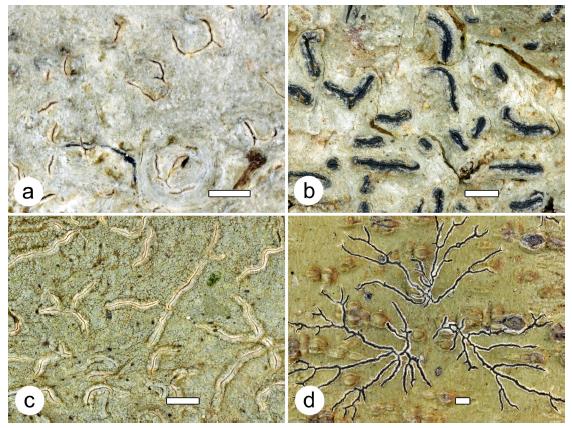
Figure 3 Species of lichens new to science. a. Fissurina linoana (42977b, holotype); b. Graphis lurizana (42922, holotype); c. Graphis mokanarum (42930, holotype); d. Phaeographis galeanoae (42981, holotype). Thalli with ascomata. Scale = 1 mm.
Diagnosis. Differing from Fissurina alligatorensis in the I-negative ascospores with thin walls and septa. Type. COLOMBIA. Atlántico: Piojó, Reserva Forestal Protectora (RFP) El Palomar; 10°45'47" N, 75°08'56" W, 200-600 m; 18 March 2016, R. Lücking, B. Moncada et al. 42977b (UNO, holotype).
Description. Thallus corticolous, up to 2 cm diam., continuous, white-grey with a silvery shine, minutely uneven-verrucose; prothallus not observed. Thallus partially immersed in the periderm, in section 50-80 μπι thick, ecorticate, dominated by the irregular photobiont layer, with large clusters of calcium oxalate crystals. Ascomata lirellate, more or less fissurine, unbranched to sparsely branched, immersed-erumpent, 0.5-1.5 mm long, 0.1-0.2 mm broad, 0.12-0.15 mm high; disc concealed; labia thin, entire, lighter than the thallus, along the slit with a thin, dark brown line, laterally covered by whitish thallus. Excipulum prosoplectenchymatous, 10-15 μm broad, yellowish, laterally covered by thallus including large clusters of crystals; periphysoids absent; hypothecium 10-15 μm high, light olive. Hymenium 90-100 μm high, clear; paraphyses unbranched, apically smooth. Asci 90-100 χ 15-20 μm oblong. Ascospores 8 per ascus, muriform, 15-20 χ 9-12 μm, ellipsoid-oval, with thin septa and rectangular lumina, constricted at the median septum, hyaline, I-. Secondary chemistry: no sustances detected by TLC.
Etymology. This new species is dedicated to Lino Olivares, one of the most knowledgeable and passionate empirical experts of the flora of the Colombian Caribbean dry forests and collaborator in the EcoSecos initiative and other conservation efforts in the region for 18 years (Castro-Vásquez et al. 2010, Rodríguez et al. 2012, Castellanos-Castro and Newton 2015).
Remarks. Currently, there are only two species known in Fissurina with a whitish, endoperidermal thallus, lacking substances, and muriform ascospores, namely F. alligatorensisLendemer & R.C. Harris and F. ilicicola Lendemer & R.C. Harris (Lendemer and Harris 2014). The first agrees with the new species in thallus and ascoma morphology, as well as ascospore size, but has distoseptate, I+ strongly amyloid ascospores. The second one differs in the more distinct (hemithecioid) labia and the larger (24-32 χ 12-14 μm), also distoseptate and I+ amyloid ascospores.
Graphis lurizana Lücking, Moncada & Celis sp. nov. Mycobank MB 828115 (Fig. 3b).
Diagnosis. Differing from Graphis illinata in the apically exposed, black labia and the smaller ascospores. Type. COLOMBIA. Atlántico: Usiacurí, Distrito Regional de Manejo Integrado (DMI) Luriza; 10°44'22" N, 75°01'24" W, 100-200 m; 1 December 2015; R. Lücking, B. Moncada et al. 42922 (UNO, holotype; B, isotype).
Description. Thallus corticolous, epiperidermal, up to 7 cm diam., continuous, white-grey, uneven, opaque; prothallus not observed. Thallus in section 100-150 μm thick, with thin, prosoplectenchymatous cortex and irregular photobiont layer, strongly encrusted with large clusters of calcium oxalate crystals. Ascomata lirellate, unbranched to sparsely branched, prominent, 1-3 mm long, 0.4-0.5 mm broad, 0.2-0.3 mm high; disc concealed; labia entire, apically exposed, black, with irregular, lateral thalline margin. Excipulum completely carbonized (thin at the base), 70-100 μm broad; hypothecium 20-30 μm high, hyaline. Hymenium 130-160 μm high, clear; paraphyses unbranched, apically smooth. Asci 120-130 χ 25-25 μm, oblong. Ascospores 1 per ascus, richly muriform, 80-110 χ 25-30 μm, oblong, with slightly thickened septa and more or less rounded lumina, hyaline, I+ violet-blue. Secondary chemistry: no substances detected by TLC.
Etymology. Graphis lurizana is dedicated to the Luriza community, who manages the Distrito Regional de Manejo Integrado (DMI) Luriza, the first protected area to be declared in the Atlántico Department (Molina-Acosta 2013).
Remarks. Graphis lurizana keys out in group 9 in the Graphis world key of Lücking et al. (2009a). There are four species in this group with large ascospores (> 80 μm), lacking secondary substances, and with elongate, prominent lirellae, namely G. acharii Fée, G. cleistomma Nyl., G. illinata Eschw., and G. subvernicosa Lücking. Graphis acharii and G. subvernicosa have (2-)4-6(-8) ascospores per ascus; in addition, in G. acharii the labia rather regularly become striate and the ascospores are longer (up to 170 μm), whereas in G. subvernicosa, the lirellae have only a basal thalline margin and the ascospores are narrower (15-20 μm broad). Graphis cleistomma and G. illinata agree with the new species in the single-spored asci; however, the first has shorter lirellae with an apically thin complete thalline margin, and larger ascospores (up to 180 χ 40 μm), whereas the second has lirellae with an apically thick complete margin and slightly longer ascospores (up to 150 μm).
Graphis mokanarum Lücking, Moncada & M.C. Martínez sp. nov. Mycobank MB 828116 (Fig. 3c).
Diagnosis. Differing from Graphis microsperma in the larger ascospores and the stictic acid chemistry. Type. COLOMBIA. Atlántico: Usiacurí, Distrito Regional de Manejo Integrado (DMI) Luriza; 10°44'22" N, 75°01'24" W, 100-200 m; 1 December 2015; R. Lücking, B. Moncada et al. 42930 (UNO, holotype; B, isotype).
Description. Thallus corticolous, epiperidermal, up to 10 cm diam., continuous, light greenish grey, uneven to minutely verruculose, opaque; prothallus not observed. Thallus in section 40-50 μm thick, with distinct. prosoplectenchymatous cortex and distinct photobiont layer, blurry through incrustation with numerous small crystals. Ascomata lirellate, irregularly branched, erumpent, 1-5 mm long, 0.3-0.4 mm broad, 0.15-0.2 mm high; disc concealed; labia entire, apically exposed, whitish, with thick, lateral to almost complete, uneven to verruculose thalline margin. Excipulum uncarbonized, 30-50 μm broad, orange-brown; hypothecium 10-15 μm high, hyaline to yellowish. Hymenium 80-100 μm high, clear; paraphyses unbranched, apically smooth. Asci 80-90 χ 20-25 μm oblong. Ascospores 8 per ascus, muriform, with (5-) 7 transverse septa and 1-3 longitudinal septa per segment, 25-30 χ 10-12 μm oblong-ellipsoid, with thickened septa and rounded lumina, hyaline, I+ violet-blue. Secondary chemistry: stictic acid, thallus in section with K+ persistently yellow efflux.
Etymology. This new species is dedicated to the Mokaná, the only indigenous community in Atlántico (Borda 2009, Montoya and Siegler 2010, 2011). The name is said to mean "those without feathers" in the Mokaná language, but this interpretation is disputed (Montoya and Siegler 2010). The Mokaná language has become extinct, but the community, which was considered near-extinct about three decades ago, is recovering its cultural traditions in a process of re-indigenization (Borda 2009).
Remarks. Because of its uncarbonized excipulum, this species corresponds to the concept of the genus Hemithecium, which has now been subsumed under the genera Graphis and Allographa (Lücking and Kalb 2018). Among these two genera there are only two species with uncarbonized excipulum, small, muriform ascospores, and secondary substances: G. microsperma (Chitale, Makhija & B. O. Sharma) Lücking & Kalb from India differs in the smaller ascospores (15-20 χ 8-14 μm) and the formation of norstictic instead of stictic acid, whereas Allographa dispersa (Redinger) Lücking & Kalb agrees in ascospore size, but also differs in the norstictic acid chemistry and the very short, prominent, white lirellae contrasting with the brownish thallus. The new species is morphologically similar to the widespread Graphis implicata Fée, which differs in the transversely septate ascospores and lack of secondary substances.
Additional specimen examined. COLOMBIA. Atlántico: Piojó, Reserva Forestal Protectora (RFP) El Palomar; 10°45'47" N, 75°08'56" W, 200-600 m; 18 March 2016, R. Lücking, B. Moncada et al. 42975 (B, UNO, paratypes).
Phaeographis galeanoae Lücking, Moncada & B. Salgado-N. sp. nov. Mycobank MB 828117 (Fig. 3d).
Diagnosis. Differing from Phaeographis bicolor in the very long, narrow, dendroid lirellae and the smaller ascospores. Type. COLOMBIA. Atlántico: Piojó, Reserva Forestal Protectora (RFP) El Palomar; 10°45'47" N, 75°08'56" W, 200-600 m; 18 March 2016, R. Lücking, B. Moncada et al. 42981 (UNO, holotype; B, isotype).
Description. Thallus corticolous, epiperidermal, up to 5 cm diam., continuous, light olive-green, uneven-rugose, opaque; prothallus not observed. Thallus in section 80-150 μm thick, with prosoplectenchymatous cortex and irregular photobiont layer, strongly encrusted with clusters of calcium oxalate crystals. Ascomata lirellate, radiately branched (dendroid), erumpent, 5-20 mm long, 0.2-0.3 mm broad, 0.1-0.12 mm high; disc exposed but narrow, dark brown; labia inconspicuous, visible as thin, somewhat irregular, greyish-brown line between the disc and the thalline margin; thalline margin basal to lateral, white. Excipulum prosoplectenchymatous, 20-30 μm broad, dark brown to carbonized; hypothecium 15-30 μm high, hyaline. Hymenium 70-80 μm high, strongly inspersed, inspersion partially dissolving in K; paraphyses unbranched, apically smooth. Asci 70-80 χ 15-20 μm, oblong. Ascospores 8 per ascus, 7-9(-11)-septate, 25-35 χ 6-8(-10) μm, oblong, with thickened septa and lens-shaped lumina, grey-brown, I+ vine-red. Secondary chemistry: stictic acid, thallus in section with K+ persistently yellow efflux.
Etymology. We dedicate this beautiful new species to the memory of the late Gloria Amparo Galeano Garcés (1958-2016), one of the foremost Colombian botanists and agronomists and internationally renowned specialist in the taxonomy of Arecaceae (palms), an important element in tropical forests including DTF (García 2016, Olivares and Balslev 2016). One of her last published papers was on the use of the bitter palm, Sabal mauritiiformis, for roofing in Piojó, Atlántico (Andrade-Erazo and Galeano 2016), the municipality of the type locality of Phaeographis galeanoae.
Remarks. The genus Phaeographis is currently not well-studied, but we were able to examine most of the type material of names belonging here. We found only three species with transversely septate ascospores, inspersed hymenium, dark brown to carbonized excipulum, and stictic acid chemistry. Among these, the eastern paleotropical P. circumscripta (Kremp.) Vain. [syn.: Graphis pasaniae Vain. nom. inval.] differs in the distinct, apically strongly carbonized labia, fitting the concept of Platygramme (Staiger 2002) and the smaller (15-25 χ 5-6 μm), consistently 5-septate ascospores. Phaeographis bicolor Müll. Arg., also an eastern paleotropical species, forms much shorter and broader, irregularly branched lirellae, and the ascospores are larger (35-45 χ 9-11 μm). Finally, a third species also known from the eastern Paleotropics, P. concava Müll. Arg., differs in the consistently 5-septate ascospores and the shorter, irregularly branched lirellae with sunken disc and prominent margins covered by a thallus layer. The new species is quite conspicuous due to the dendroid lirellae and strong color contrast between the olive-green thallus and the dark brown disc and white margin of the lirellae.
Additional specimens examined. COLOMBIA. Atlántico: Piojó, Reserva Forestal Protectora (RFP) El Palomar; 10°45'47" N, 75°08'56" W, 200-600 m; 18 March 2016, R. Lücking, B. Moncada et al. 42982 (UNO, paratype), 42983 (B, UNO, paratypes). Usiacurí, Distrito Regional de Manejo Integrado (DMI) Luriza; 10°44'22" N, 75°01'24" W, 100-200 m; tropical dry forest, closed forest and disturbed forest patches and secondary vegetation along main access trail; 1 December 2015; R. Lücking, B. Moncada et al. 42936 (B, UNO, paratypes).
AUTHOR PARTICIPATION
TB, GB, RL, BM and MCMH conceived the study. All authors performed the field work; RL and BM identified the lichen samples and drafted the initial descriptions of the novel taxa; MCMH, BESN, MC, and GMRM provided context data for the etymology of each new species. RL wrote the first manuscript draft and all other authors contributed to subsequent installments, including the final version; MCMH, BESN, MC, ORZ, and GMRM thereby contributed context data on the study sites and their geography and aspects of the biodiversity and conservation of DTF in Colombia and Atlántico; ORZ designed the map and RL and GMRM provided field images and lichen macrophotographs.