INTRODUCTION
Egg size and number are critical life-history traits in insects because the former influences offspring fitness by determining initial resources level (Fox 1994, Fox and Czesak 2000) and the latter influences overall progeny production (Fox and Czesak 2000, Kato et al. 2000). In many insects, the size and number of eggs produced by a female are known to correlate with female size and weight (Berrigan 1991, Honek 1993). Larger females tend to have a higher oviposition rate and/or larger eggs or larvae with higher larval survival rates (Honek 1993, Jikumaru et al. 1994, Fox and Czesak 2000, Yanagi and Tuda 2012). Several studies have suggested that egg size and/or the number of eggs produced vary depending on maternal conditions such as body size, nutritional status, age or weight (Fox and Czesak 2000, Kojima 2015). The size of the female's body is related to the number of eggs that a female can carry or oviposit (Juliano 1985, Lawrence 1990, Ito et al. 1992, Togashi et al. 1997, Kato et al. 2000, Kojima 2015), and this could be a function of the available space in the female's body (Honek 1993). Egg size is probably determined by the width of the ovipositor (Yanagi and Tuda 2012) or by the mother's nutritional state and age. Older or food-stressed females tend to produce smaller eggs or a lower number of eggs than young or well-fed females (Fox 1993, Kojima 2015). On the other hand, a negative correlation between the number and size of offspring (trade-off) is frequently reported (Berrigan 1991, Ernsting and Isaaks 2000, Fox and Czesak 2000). In general, it is argued that this trade-off is a consequence of a fixed level of resource availability that could affect the production of offspring (Smith and Fretwell 1974).
Arhopalus (Cerambycidae) is a Northern Hemisphere genus whose larvae develop in coniferous trees (Hosking and Bain 1977), mainly Pinus and Picea (Bense 1995). This genus is present in all major biogeographic regions of the world. Arhopalus has increased its population in the last years to levels at which it can be considered a pest (Ciesla 2011). The main damage is caused by the larvae that make holes and tunnels in the wood of the trees increasing levels of degradation of forest products (Cerezke 1977). Even, cerambycids cause the death of the plant, and occasionally also damage structural woods (Kolk and Starzyk 1996, Rassati et al. 2012).
Recently, some species of Arhopalus have colonized South America. In Córdoba Province (Argentina), Arhopalus rusticus (Linnaeus, 1758) and Arhopalus syriacus (Reitter, 1895) were first recorded in 2006 (López et al. 2008), probably introduced through wood trade from Buenos Aires Province where they were first observed in 2001 (Di Iorio 2004). Arhopalus rusticus, which is found in Western Europe, is the most important borer affecting pine forests in Córdoba Province (Pedemonte 2014). Arhopalus syriacus is an east Mediterranean species that has been observed in dead standing trees of Pinus elliottii Engelm. in the southern region of Córdoba Province (López et al. 2008). Currently, both species are well established in Córdoba and distributed throughout the province, although A. rusticus is more abundant and more widely distributed in the Calamuchita Valley than A. syriacus (Pedemonte 2014). Adults of both species emerge from the trees in summer and generally show high copulation activity and dispersal at dusk (Brockerhoff and Hosking 2001, Suckling et al. 2001). Arhopalus rusticus has a life cycle of two to three years (Bense 1995, Kolk and Starzyk 1996), while A. syriacus completes its life cycle in one or two years (Webb and Eldridge 1997). However, there are no studies about how many larval instars these species could have. In Argentina, previous studies on these Arhopalus species have concentrated on their taxonomy (Di Iorio 2004, López et al. 2008), life history characteristics (Fachinetti et al. 2015), and abundance (Pedemonte 2014).
This study aimed to analyze how female body size affects potential fecundity, size and number of mature eggs in ovaries, in both invasive species. Additionally, we compared egg morphometric characteristics such as length, width, elongation index and volume, and larval measurements such as length and exuvia head capsule width, development, and survival between these two species.
MATERIALS AND METHODS
Female body size, number of eggs in ovaries, and egg mor-phometric characteristics
Adults of A. rusticus and A. syriacus were collected in the central area of Córdoba Province (32°33' South, 64°43' West) in Argentina. In this region, A. rusticus and A. syriacus were found in a vast pine forest dominated by P. elliottii and P. taeda L. (López et al. 2008, Pedemonte 2014). Collections were carried out using cross-vane panel traps made of two crosswise black plastic sheets (50 cm tall by 30 cm wide), mounted over a plastic funnel at 1.5 m above the ground. The cross-vane design was chosen because it is more effective than other trap designs for capturing borers (De Groot and Nott 2001, Pedemonte 2014). The traps were baited with 100 ml of turpentine, composed mainly of a-pinene, β-pinene, and other terpenes and 100 ml of 70 % ethanol (Pedemonte 2014). The turpentine and the ethanol were placed separately in two different bottles, and a collection cup with water with sodium chloride as a preserving agent was placed at the bottom of the funnel. Traps were placed in the field from November 2010 to March 2011. All the individuals collected were kept in 70 % ethanol.
A total of 20 females of each species were employed for the measurements. The length of the elytron from the scutel-lum to the tip was chosen as an indicator of adult body size (Berrigan 1991, Ciach and Michalcewicz 2013). Measurement was made with an ocular micrometer. Then, females were dissected and the number of mature eggs (length >1.2 mm) in the ovaries was recorded, which was considered as a conservative indicator of potential reproductive output (Juliano 1985). The eggs were carefully cleaned and their surface was washed (Ciach and Michalcewicz 2009), and four egg measurements were obtained from 200 eggs (ten per female): length (L), width (W), elongation index (E) and volume (V). The elongation index was calculated as E = L/W, and volume was calculated as V = 0.51 χ L χ W2, assuming the prolate spheroid shape (Ciach and Michalcewicz 2009).
The difference in egg number between the two species and the effect of body size, measured as elytron size, on the number of eggs were tested using Analysis of Covariance (ANCOVA). The relationships between the body size and egg morphometric characteristics (L, W, E, and V) in each species were tested using linear regression analysis. Female body size and egg morphometric characteristics were compared between species using t-tests. Analyses were conducted using InfoStat software (Di Rienzo et al. 2017).
Larval measurements, development, and survival
Larval development and survival were examined at 25 °C under laboratory conditions. The laboratory colony was started using Arhopalus adults of both species, collected from November 2013 to March 2014 from pine logs of weakened trees located in the same area as above. Pairs of adults, one male, and one female, were placed in 6 cm height and 4 cm diameter plastic tubes, with a piece of pine bark that served as a support to oviposition. Then, adults were placed in incubator chambers at 25 °C, with a photoperiod of 14:10 h (L:D) and high relative humidity (≥ 80 %). The pieces of bark were observed daily to detect the presence of eggs. Eggs were left in the bark until larvae were observed at the bottom of the plastic tubes. Newly hatched larvae (≤ 12 hours old) were handled using a fine hair brush and individually placed in Petri dishes (6 cm diameter) filled with 4 g of artificial diet and put back into the incubator chambers until the first pupa was observed (Fachinetti et al. 2015).
The artificial diet, modified from Iglesias et al. (1989), consisted of (for 1.5 kg) 600 ml of distilled water, 56 g agar, 8 g nipagin, 4 g benzoic acid, 250 g pine sawdust, 44 g yeast, 88 g corn flour, 176 g wheat germ, 30 g glucose, 48 g casein, 30 g sucrose, 4 g ascorbic acid, and two tablets of Supradyn® mix vitamins. Larvae food replacement and Petri dish cleaning were made every ten days. The artificial diet was removed and the Petri dishes were washed with ethanol 20 % while the larvae remained in another Petri dish. Food replacement and hygiene were monitored until the end of the experiment using the same Petri dish for each individual and no additional food was supplied during rearing.
The Generalized Linear Model with a Gamma error distribution was applied for statistical comparison of larval length and head capsule width between species in each instar. Survival of larvae was recorded by counting living individuals at each larval instar of development until the first pupa was observed. Differences in survival from the first instar to the first pupae in both species were analyzed by the Kaplan-Meier log-rank method (Altman 1991), using the Infostat software version 2015 (Di Rienzo et al. c2017).
RESULTS
Female body size, number of eggs in ovaries, and egg morphometric characteristics
Eggs of both species were symmetrically elongated, approximately three times longer than wide, and slightly curved along the longitudinal axis. The tips were rounded and there was a size difference between the two poles, with one wider. Eggs of both species were covered with a mucilaginous transparent layer.
Larval rearing started with 131 individuals of A. rusticus and 100 of A. syriacus. Each was observed daily until the instar in which the first pupa was observed, to register the development time in days. The first larval instar or larval instar I was considered as the period of time in days from egg hatching to the first molt, the second larval instar or larval instar II was considered as the period of time in days from the appearance of the larva instar I until the next molt, and so on for each instar. Larval length (cm) and exuvia head capsule width (mm) were recorded when each molted from one larval instar to the next one. Exuvia head capsule widths, as well as larval length, were measured using a caliper (with 0.1 mm accuracy). If the head capsule exuviae could not be found, but the larva had molted, as evidenced by an incompletely sclerotized head, only the larval body length was measured.
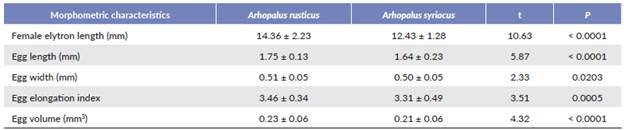
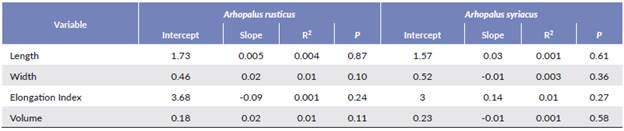
In both species, we found a significant positive relationship between female size and the number of eggs carried (Table 1, Fig. 1). Interaction between species and the female elytron length was not significant (Table 1). Female size had no significant effect on egg morphometric characteristics (L, W, E or V) in either species (Table 2). Females were significantly larger in A. rusticus than in A. syriacus (Table 3). We also found statistically significant differences in egg morphometric characteristics, with the eggs of A. rusticus being larger than those of A. syriacus (Table 3), but there was no significant difference between the species in the number of eggs in ovaries (Table 1).
Table 1 Results of ANCOVA using species and female elytron length as covariates and the number of eggs in the ovaries as dependent variable.
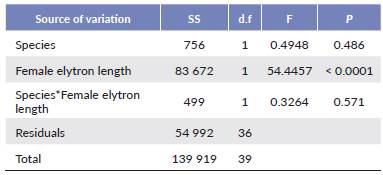
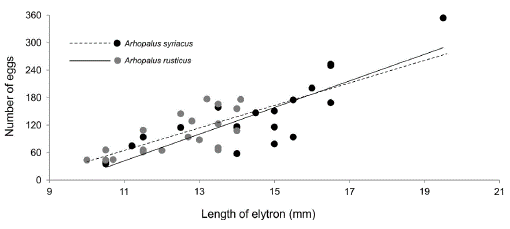
Figure 1 Relationship between the number of mature eggs in ovaries and female elytron length in Arhopalus rusticus (y = 29.036x - 277.05) and Arhopalus syriacus (y = 24.527x - 204.75).
Table 2 Regressions between female body size of Arhopalus rusticus and Arhopalus syriacus and morphometric characteristics of the eggs. None were significant.
Larval measurements, development, and survival
Larval body length and exuvia head capsule width were compared between the two species from instar one to instar six because this was the last instar before pupation in A. syriacus (Fig. 2). Larval body length increased to the fifth instar and there that remained almost stable in both species (Fig. 2a). Arhopalus rusticus was significantly larger than A. syriacus in almost all larval instars, while the head capsule width was significantly more prominent in A. rusticus from the fourth larval instar (Fig. 2, Table 4).
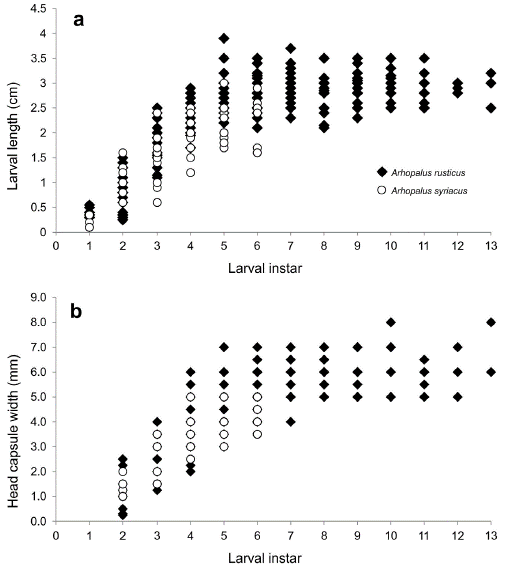
Figure 2 Changes in a body length, and b head capsule width during larvaldevelopment from arval instar 1 to the first instar in which a pupa was registered in Arhopalus rusticus and Arhopalus syriacus. Each point represents a single individual.
Table 4 Results of GLM for comparison of larval body length and head capsule width between Arhopalus rusticus and Arhopalus syriacus at each instar. ND: Data were not obtained.
Of 131 larvae of A. rusticus, 57 survived until the end of the experiment and of 100 of A. syriacus only 22 survived. Larval instar development time was variable between the species and different between instars. Arhopalus syriacus registered the slowest time of development in larval instars (Table 5). The first instar in which A. rusticus and A. syriacus pupated was instar thirteen and six, respectively (Table 5). The first A. syriacus pupa was obtained 172 days after the egg hatched, while the first A. rusticus pupa was observed after 278 days. Mean developmental time from the first instar to the first pupa for A. rusticus was 311.82 ± 56.75 (mean ± SD) days, while for A. syriacus it was 208.86 ± 56.38 days.
Table 5 Survival probability estimates (1.00 on day 0) for larvae of Arhopalus rusticus (from 131 individuals) and Arhopalus syriacus (from 100 individuals) reared on artificial diet at 25 °C. Survival indicates the proportion of surviving individuals. Mean and SD of larval instar development time of the two species are expressed in days.
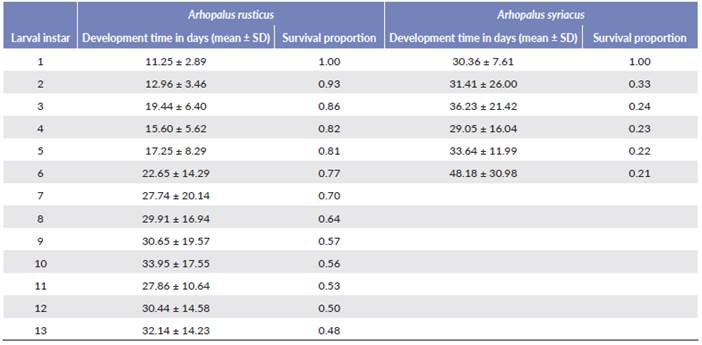
Survival was statistically higher in A. rusticus than in A. syriacus (Logrank χ2 = 44.794, P < 0.001) because the survival of A. syriacus showed a sharp fall in the early stages of larval development i. e. larval instars one and two (Table 5).
DISCUSSION
We observed that A. rusticus and A. syriacus show similar egg morphologies. Similar to eggs of some other species of Cerambycidae, eggs of both species are long, slightly curved along the longitudinal axis, with poles of different sizes, and covered with a mucilaginous transparent layer (Hernández 1990, Ciach and Michalcewicz 2009).
Some studies in cerambycids have found a relationship between female size and the egg characteristics (i.e. larger females tended to deposit larger eggs) (Togashi et al. 1997, Kato et al. 2000), but we observed no such relation. On the other hand, we found a positive correlation between female size and the number of eggs carried in the ovaries in both species. Kato et al. (2000) also reported this trend in the cerambycid beetle Semanotus japonicus Lacordaire, 1869, with the number of eggs deposited as well as egg size and weight of the first-instar larvae increasing slightly with the increase of female size. In Monochamus alternatus (Hope, 1843), Monochamus saltuarius (Gebler, 1830), and Paraglenea fortunei (Saunders, 1853), there were also positive relationships between female body size and egg productivity per ovariole (Jikumaru et al. 1994, Togashi et al. 1997, Togashi 2007). In Tetraopes tetraophthalmus (Forster, 1771), the number of larvae produced correlated positively with female body size (Lawrence 1990).
Our results also show that, although A. rusticus females were on average larger than A. syriacus, there was no statistical difference between them in the number of eggs in ovaries. If the female body size, i.e. the abdominal capacity, is not different between the two species, producing larger eggs should be compensated by a smaller number of eggs according to a trade-off between the size and number of eggs, which is commonly seen in insects (Smith and Fretwell 1974, Berrigan 1991). In A. syriacus, though smaller, this compensation would consist of producing smaller eggs.
The size of larvae and their survival were higher in A. rusticus, which produced larger eggs. Our results match with previous observations in other species of Coleoptera, such as Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae) and S. japonicus (Coleoptera: Cerambycidae), in which egg size influences the initial larval size and their survival (Fox 1994, Kato et al. 2000). In habitats where competition is severe, larvae with higher initial size may have advantages over smaller larvae (Togashi 1997), e. g. a higher size influences resistance against resin of host trees (Ito et al. 1992, Kato et al. 2000).
Well-fed organisms are typically larger, with high larval survival proportion and the time development is shortest than those feed with a poor quality diet (Chapman 1998, Keena 2005). Arhopalus rusticus and A. syriacus were reared using different artificial diets contained no host material (Notario and Baragaño 1978, Iglesias et al. 1989) and contained dried pulverized host material as the diet used in this study, with similar proportions of ingredients (De Viedma et al. 1985, Iglesias et al. 1989, Rogers et al. 2002 for A. rusticus, and Carle 1969, De Viedma et al. 1985, Iglesias et al. 1989 for A. syriacus). In this study, the most commonly rearing methods and environmental conditions were used; however, Arhopalus syriacus' first instar larvae low survival could be due to the diet moisture and environmental conditions (Keena 2017). Adverse effects on establishment and development were not observed related to moisture, neither the presence of mites, fungi or another type of contamination in the stored diets throughout the breeding. Moisture between 50-65 % has been commonly used (Galford 1969). In our case, 80 % for humidity could be considered excessive. Future assessments of larval resistance to desiccation may improve our knowledge about larval survival and development.
The present study registered for the first time that A. syriacus pupates after six larval instars and A. rusticus after thirteen larval instars at 25 °C. Although A. syriacus larvae hatch from smaller eggs, the time required to reach the pupae stage was shorter. These results do not match those found by Fox (1994) in C. maculatus, whose larvae hatching from larger eggs developed faster, which he considered a fitness advantage in interspecific competition allowing earlier access to mating and oviposition sites.
Although the two invasive species studied coexist in the study region, they have a different distribution within the province: A. rusticus is found throughout the area where pines are present, while A. syriacus is mainly restricted to the southern regions of the study area (Pedemonte 2014). Considering that A. syriacus can carry the same number of eggs as A. rusticus, it is possible that the smaller size of the eggs of the former is a disadvantage when colonizing new pine patches given the importance of initial larval size for these species.
However, to date there are no studies about competition between these two species in areas where both are found. Moreover, there are no data available about reproductive strategies such as courtship, the moment in which oviposition occurs, etc., for these species. In this context, specific studies on competition, behavior, mating and oviposition, distribution, and abundance of both species in Córdoba province are needed.
The successful invasion of exotic species, like those studied in this paper, can be separated into three processes: first, the arrival, in which human activity has an important role (Liebhold et al. 1992); second, establishment, in which a "minimum viable population" may survive in the proper environment, and finally, the spread. As soon as a population is large enough, it will expand into adjoining areas of suitable habitat, limited by its dispersal potential (Lander et al. 2014). This seems to be the situation with the studied species. Both species are recently established and from now on the spread phase will begin. It is clear that exotic species invasions have among many other consequences an effect on local biodiversity (Mack et al. 2000). In our case, a good understanding of the relationship of these two species and local forests is important in order to develop strategies for their management in order to minimize the environmental damage they can cause.