INTRODUCTION
The species Setophaga petechia (Linnaeus, 1766) (Yellow Warbler) is considered a wetland habitat specialist on its breeding grounds (Humple and Burnett 2010). The migratory subspecies that breed in North America are obligate riparian specialist (Humple and Burnett 2010, Quinlan and Green 2012). For tropical resident subspecies, the habitat specificity corresponds to mangrove vegetation (Barrantes 1998, Salgado-Ortiz et al. 2008, Vincenty et al. 2009), with evidence of being more restricted to their primary breeding habitat, compared to migratory subspecies (Lowther et al. 1999) . A recent study with the subspecies S. p. gundlachi (Cuban Yellow Warbler) in Cuba, found that despite the reduction and disturbance to coastal mangrove areas in Havana city, the subspecies is still restricted to mangrove vegetation patches, and does not use the surrounding non-native secondary vegetation (Márquez 2013).
S. petechia is recognized as vulnerable and its populations are declining on a regional scale, because of its habitat specialization in the breeding season (Beissinger et al. 2000) . In California, it is considered as a species of special concern, because its populations have shown a local and regional decline (Humple and Burnett 2010). The breeding habitat loss is their main threat; thus many studies have been conducted on migratory subspecies to better understand habitat use (Tremblay and St. Clair 2011, Quinlan and Green 2012, Drake 2013). For tropical resident subspecies, habitat loss is likely to be the main threat, but there are no studies that report this impact on their populations. Only in Florida, S. p. gundlachi is reported as a species of special concern due to its limited range and threats to mangrove habitats (Prather and Cruz 1995). The mangrove is one of the most impacted ecosystems worldwide. In the period 1980-2001, between 19 % and 35 % of the total area occupied by this vegetation was lost in the world (Luther and Greenberg 2009). In Cuba, more than 30 % of mangrove ecosystems have been affected by both, natural phenomena and human activity (Menéndez 2013). Studies are needed to evaluate how S. petechia resident populations are affected by the modification and loss of these habitats.
Studies of habitat ecology support the identification and prioritization of habitats for conservation, based on their quality for the species of interest (Johnson 2007). Habitat quality is defined as the capacity of an environment to provide the appropriate conditions for the persistence of individuals and their populations (Hall et al. 1997). Johnson (2007) described two general ways to measure habitat quality for birds. The first, through direct measurements in which the habitat attributes that are critical resources are analyzed, such as food or nesting sites. The second way is through indirect measures, using individual or population variables of the birds; examples of demographic variables are density, survival, and reproductive success. Because the habitat quality affects the per capita contribution to population growth, demographic variables offer some of the best measures (Johnson 2007). However, there are many cases in which the population density is negatively related to survival or reproduction success, as habitat with human disturbance (Bock and Jones 2004). Currently, the use of density as indicator of habitat quality is questioned (Hall et al. 1997, Garshelis 2000). Therefore, it has been advised that density can be considered a habitat quality indicator, only if it is corroborated with other data (Garshelis 2000, Johnson 2007).
Márquez (2013) studied the two largest mangroves in Havana, Cuba: the mangroves of Laguna de Cobre-Itabo and Bajo de Santa Ana. Laguna de Cobre-Itabo mangrove is a management area within the Protected Natural Landscape Rincón de Guanabo, with important natural and socioeconomic values (Guzmán et al. 2006, Ferro et al. 2006). While Bajo de Santa Ana mangrove is under heavy pressure by urban development form the nearby Santa Fé village. In addition, their mangrove forest exhibits lower height, basal area and crown width due to constant deforestation (Guzmán et al. 2011). These effects may have direct consequences on S. p. gundlachi, as vegetation structure is a factor that influences their nesting site selection (Quinlan and Green 2012) and foraging behavior (Dobbs et al. 2009). However, recent studies report similar density in both areas (Márquez 2013). Considering the problems associated with population density as a measure of habitat quality (Garshelis 2000), it is necessary to consider other measures for evaluating how mangrove vegetation disturbance can affect populations of S. p. gundlachi.
Differences in food availability between habitats can reveal habitat quality (Lyons 2005), as this is an important variable during the reproductive season of birds, that exerts a strong influence on reproductive success (Murakami 2002, Lyons 2005). In addition, this is a more feasible measure of habitat quality in a complex ecosystem like mangrove forest. Because of this, the objective of this work is to compare the prey availability for S. p. gundlachi during the breeding season in two mangrove sites with different vegetation structure in Havana, Cuba.
MATERIALS AND METHODS
Study sites: The study was conducted in two mangrove sites, Laguna de Cobre-Itabo and Bajo de Santa Ana, both in Havana Province, Cuba. Both sites had been highly transformed (Roig 2005), although they show different degrees of disturbance. The sites were selected considering that they have a large area, enough to maintain their functional ecosystems values (21.9 ha Bajo de Santa Ana and 41.0 ha Laguna de Cobre-Itabo).
Laguna de Cobre-Itabo mangrove (23°10' North, 82°10' West) is in the Ensenada de Sibarimar, northeast of the city of Havana. This site constitutes the most preserved and largest extension of coastal wetland of the northern coast of Havana (Guzmán et al. 2006). This is a monodominant forest of Laguncularia racemosa ((L) C.F.Gaertn) (White Mangrove), with only one canopy stratum up to 8 m in height. Rhizophora mangle (Linnaeus) (Red Mangrove) and Avicennia germinans (Linnaeus) (Black Mangrove) can also be found, forming in some areas, mixed forests with L. racemosa. Impacts to this site include fragmentation and high vegetation reduction (Suárez 2011, Menéndez 2013). Nonetheless, Guzmán et al. (2006) highlight that the mangrove forest presents a good structure.
The other mangrove was Bajo de Santa-Ana (23°03' North, 82°31' West), located east of Santa Fé town, west of Havana, there the vegetation receives strong pressure from urban development. The mangrove exhibits sections with monodominant vegetation, and others with mixed vegetation of L. racemosa, A. germinans and R. mangle. In addition, it presents variable heights, with forests that do not exceed 5 m, and others between 10 m and 12 m. According to Guzmán et al. (2011), among the main consequences of the pressures in this mangrove, are the fragmentation and reduction of forest, the replacement of mangrove areas for crops and house constructions, and the structure vegetation changes of the mangrove forest.
Structural characteristics of vegetation: To characterize differences of vegetation structure between mangrove sites, variables were collected in April 2017.
Sampling was carried out between two to three quadrants of 4 x 4 m taken randomly in 100 m transects. Transects were distributed considering accessibility and representativeness of the area. A total of 32 quadrants were sampled in Laguna Cobre-Itabo and 18 quadrants in Bajo de Santa Ana. The number of trees per mangrove species, and the diameter at breast height (DBH) (1.30 m) was calculated using the perimeter data with a measuring tape (error ± 1 cm) and considering only the branches with a perimeter greater than 5 cm. In addition, the individual height was recorded, using a stick with marks every 50 cm as reference. The vegetation density per square meter was estimated considering all the branches that separated from the base of the tree below the breast height, regardless of whether they were from the same individual.
Foraging behavior of S. p. gundlachi: To establish the sampling design for prey availability estimation, behavioral observations were made first, between May 26 and June 25, 2016, from 07:30 to 11:00 am. All behavior records were made by the same observer and were conducted in the same transects where vegetation variables were measured. To avoid autocorrelation, no more than one measurement was taken of an individual on the same day (Johnson 2000). For this, behavior records transects were separated at least 50 m, based on the species territoriality (Salgado-Ortiz et al. 2008), in each transect, only a female and a male could be recorded on the same day. Only observations with duration greater than 20 s, and those in which the individual performed a capture maneuver or looked for food, were considered. The record was finalized when the individual began to perform other behavior, such as territory defence or preening, or when it was lost from sight by the observer. A total of 48 observations (26 males and 20 females) of foraging behavior of S. p. gundlachi were registered at the two mangrove sites, 25 in Bajo de Santa Ana and 23 in Laguna de Cobre-Itabo. The total observation time was 84.4 min for the two areas, with an average observation time of 1.6 ± 1.0 min.
The foraging height and the tree species on which S. p. gundlachi foraged were recorded (A. germinans, R. mangle, L. racemosa, Conocarpus erectus (Linnaeus) (Button Mangrove) and others). The variables were obtained as percentage of use of each foliage height and of each tree species. The recorded foraging maneuvers were classified into two general categories. The category of "capture attempt on substrate", was recorded when a perched individual attacked the food on the substrate, and includes the categories previously described by Remsen and Robinson (1990) as: gleaning, reaching, and hanging. The category "capture attempt in flight", was recorded when individual took the food by aerial maneuver and included the behaviors of hawking and hovering. For each maneuver, the number of capture attempts per minute was calculated. This variable included both, successful and unsuccessful catches; because most catching were on small-sized preys, it was difficult to determine capture success.
Prey availability for S. p. gundlachi: In this study we used Wolda (1990) definition for arthropod availability, such as the abundance of potential prey within microhabitats that is used by insectivorous birds. To determine potential prey, we considered the orders and the maximum sizes of arthropods used by S. petechia, following available literature (Frydendall 1967, Busby and Sealy 1976, Greenberg, and Salgado-Ortiz 1994) and observations during feeding behavior records.
The samples of prey were taken during the S. p. gundlachi reproductive season of (April to July) (Prather and Cruz 1995), and just after the behavioral records. The samples were obtained between June 26 and July 1rst 2016, from 08:30 am to 15:00 pm. The stratified sampling design was based on the frequency of mangrove species and height range use in foraging by S. p. gundlachi (Table 1). Only mangrove species and height ranges that were most used on average by the bird, were sampled.
Table 1 Number of samples of prey availability for Setophaga petechia gundlachi, during the reproductive season, by location, mangrove species anc height range.
The sampling technique for prey availability was branch clipping method described by Johnson (2000). The advantage of this method is that it allows for sampling the most used microhabitats in the foliage for insectivorous passerine species (Johnson and Sherry 2001). The technique used a black plastic bag (65 cm wide and 90 cm long) to quickly enclose the branch and securing the bag by placing a string around the opening. Once the branch was cut, its wet weight was measured with a Pesola scale of 1 g of precision. Then, the bag was shaken vigorously to ensure that the arthropods were detached from the branch, and the branch was carefully removed from the bag and inspected thoroughly. Subsequently, the arthropods found in each sample were preserved with 90 % alcohol.
In the laboratory, preys were identified up to order level using dichotomous keys and the help of specialists. Due to the complexity in the larva taxonomy, these were grouped as Insect larvae. To measure arthropod maximum length (mm), individuals were photographed using a stereoscopic microscope with a coupled camera and a millimetre grid plate. The measurements were made with the programs ScopeImage 9.0 (X5) and tpsDig, version 2.12. The maximum size of the potential prey was limited to 20 mm for insect larvae (Greenberg and Salgado-Ortiz 1994) and 15 mm for adult arthropods, because individuals using prey larger than one centimetre were recorded in our behavioral observations. The prey biomass was obtained using the length-weight regression described by Johnson and Strong (2000), for the arthropods of Jamaica. The variable was expressed as the total arthropod biomass per 100 g of branch clipped.
To measure the differences of vegetation structure between mangrove sites, the effect sizes (ES) of the differences in height, DBH and mangrove density were calculated. The confidence intervals at 95 % (CI) of the effect sizes were calculated by resampling all the possible differences between mangroves, with 1000 iterations. This analysis allows us to see if there are statistically significant differences when the confidence interval is superimposed with zero.
In the analysis of foraging behavior, the 95 % confidence intervals were calculated in each locality for the capture attempt rate per type of attack, with 1000 iterations. To describe the prey availability for S. p. gundlachi, the distribution of arthropod biomass between mangroves sites and mangrove species was analysed using the 95 % confidence intervals obtained using the Monte Carlo method with 1000 iterations (Dunn and Shultis 2011). Additionally, the differences of averages for capture attempt rate on substrate, prey biomass by mangrove sites, and prey biomass by mangrove species, were calculated. Then, the probability of finding the observed difference within a null distribution obtained by resampling (1000 iterations), was estimated. All analyses were done using the Excel complement program, Poptools V.3.2.3 (Hood c2010.).
RESULTS
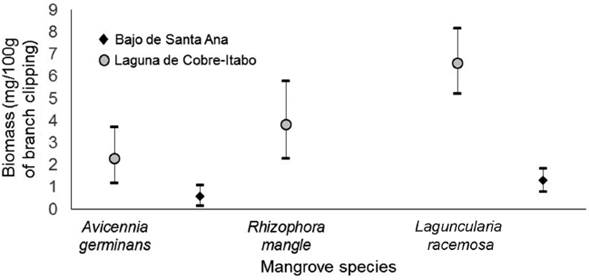
Structural characteristics of vegetation: The vegetation in Laguna de Cobre-Itabo mangrove, has a greater height and DBH, with effect sizes of 1.5 m (CI: 1.4-1.7) and 6.0 cm (CI: 5.8-6.3), respectively. While vegetation in Bajos de Santa Ana was only higher in density, with effect sizes of 0.9 branches/m2 (CI: 0.8-1.0). Dominant species in Laguna de Cobre-Itabo, R. mangle and L. racemosa, are found mostly in 3 to 9 m height range (Fig. 1); while in Bajo de Santa Ana, A. germinans and L. racemosa predominate in 0 to 3 m. These results corroborate the observation that in Bajo de Santa Ana there are vegetation areas with high density of juvenile individuals of low stature, mainly of A. germinans.
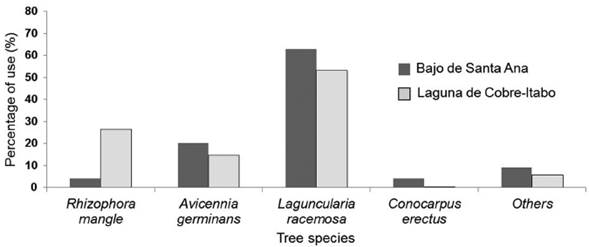
Figure 1 Distribution by height range of the mangrove species in two mangrove sites on the north coast of Havana, Cuba.
Foraging behavior of S. p. gundlachi: The most used mangrove species by S. p. gundlachi was L. racemosa, with more than 50 % of use at both mangrove sites (Fig. 2). A. germinans use was above 15 % at both sites, while R. mangle only had a high value in Laguna de Cobre-Itabo, with 26 %. Both mangroves sites presented a similar percentage of height range use by S. p. gundlachi (Fig. 3). The greatest used height range in the two mangroves sites were 2-3 m, 3-4 m, and 4-5 m, with values above 20 %. The foraging maneuver that predominated in both mangroves was the capture attempt on substrate (Bajo de Santa Ana (n=25): 2.90 capture attempt/min (CI: 1.97-3.93); Laguna de Cobre-Itabo (n=23): 3.6 capture attempt/min (CI: 2.15.6)), while the capture attempt in flight had minimal frequency (Bajo de Santa Ana (n=25): 0.26 capture attempt/ min (CI: 0.08-0.46); Laguna de Cobre-Itabo (n=23): 0.06 capture attempt/min (CI: 0-0.14)). The capture attempt rate on substrate did not statistically differ between both mangrove sites (Difference Observed: 0.24, P = 0.23).
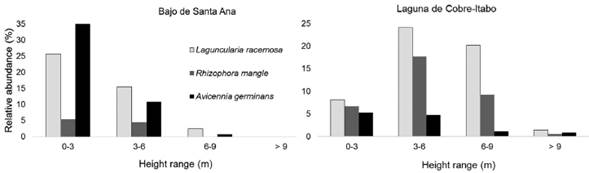
Figure 2 Percentage of use for mangrove species by forager Setophaga petechia gundlachi during the breeding season in Bajo de Santa Ana (n = 25) and Laguna de Cobre-Itabo (n = 23), Havana, Cuba.
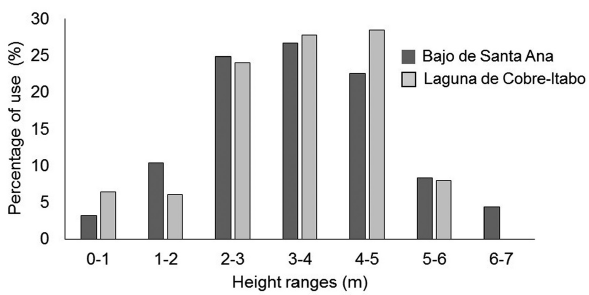
Figure 3 Percentage of use for height ranges by foraging Setophaga petechia gundlachi during the breeding season in Bajo de Santa Ana (n = 25) and Laguna de Cobre-Itabo (n = 23), Havana, Cuba.
Prey availability for S. p. gundlachi: In the analysis of potential prey, only nine of the ten groups of arthropods found were included: Araneae, Coleoptera, Diptera, Hemiptera, Hymenoptera, Insect larvae, Lepidoptera, Neuroptera and Orthoptera. Blattodea was excluded because it has not been previously reported as part of the S. petechia diet. Few individuals exceeded the maximum dimensions defined in this study as potential prey.
The greatest prey biomass availability was found in the Laguna de Cobre-Itabo mangrove, with a value of 5.1 mg / 100 g of branch clipping (CI: 4.0-6.2), while Bajo de Santa Ana had a lower prey availability of 1.6 mg / 100 g of branch clipping (CI: 0.8-1.7) (Difference Observed: 3.9, P<0.01). The orders that contributed most to the biomass in Laguna de Cobre-Itabo, were Hemiptera with 70 %, followed by Araneae and Orthoptera with more than 10 % each. While in Bajo de Santa Ana, the orders that contributed the most were Araneae with 46 %, and Hymenoptera and the Insect larvae group with more than 9 % each. These biomasses differ in number of individuals and their size. In Bajo de Santa Ana, the size classes of 0.9 mm to 4.9 mm predominated (Fig. 4). On the other hand, in Laguna de Cobre-Itabo 5 mm to 6.9 mm, were the most represented classes.
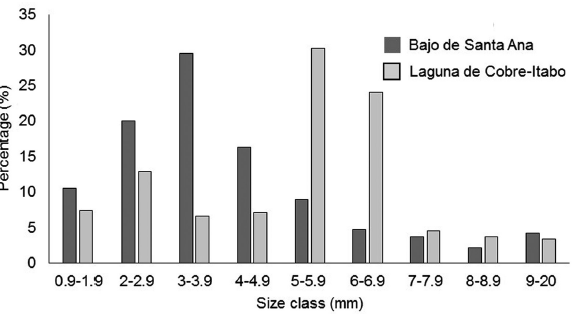
Figure 4 Percentage of individuals per size class of available prey for Setophaga petechia gundlachi during the reproductive season in Bajo de Santa Ana (n = 159) and Laguna de Cobre-Itabo (n = 813) of Havana, Cuba.
Among the mangrove species in Laguna de Cobre-Itabo, only the prey biomass values of L. racemosa and A. germinans were statistically different (Difference Observed: 4.3, P<0.01), with the highest and lowest values, respectively (Fig. 5). In Bajo de Santa Ana, L. racemosa also had the highest biomass, although it did not statistically differ from the biomass present in the other species (Difference Observed: 0.7, P=0.09).
DISCUSSION
In this work, prey availability for S. p. gundlachi was compared between two mangrove sites with different vegetation structure, as a measure of habitat quality. First, the impacts of deforestation on the vegetation structure which have been described by Guzmán et al. (2011) were characterized, for Bajos de Santa Ana mangrove. In the mangrove site in better condition, Laguna de Cobre-Itabo, was found the highest biomass of available prey for S. p. gundlachi. The mangrove species L. racemosa was the most used for foraging and offers a high biomass of available prey in Laguna de Cobre-Itabo mangrove.
Johnson and Sherry (2001) obtained a range of 3.5 to 16.9 mg / 100 g of branch clipping as the biomass available for insectivorous birds in eight ecosystems, both natural and anthropogenic, in Jamaica during the migratory season. The biomass value in Bajo de Santa Ana, 1.17 mg / 100 g of branch clippings (CI: 0.77-1.68), is below the minimum described for Jamaica (Johnson and Sherry 2001). In addition, the biomass value found in Laguna de Cobre-Itabo, 5.13 mg / 100 g of branch clipping (CI: 4.02-6.21), is close to the values found in mangroves of Jamaica, 4.59 and 3.5 mg / 100 g of branch clipping (Johnson and Sherry 2001). Both elements support the idea that the prey biomass value available in Bajo de Santa Ana is below that expected. This result may be associated to the differences between mangroves in vegetation structure. The disturbance described for Bajo de Santa Ana can influence directly on the arthropods, by diminishing the available microhabitats and affecting the vegetation microclimate (Hollander et al. 2015).
The capture attempt rate did not show differences among mangroves sites, although a lower value should be expected on the site with less prey availability (Hutto 1990, Morrison et al. 2010). The absence of differences could be because the capture attempt rate values include both failed and successful capture attempt. However, because the size of prey available was lower in Bajo de Santa Ana, in this mangrove, a similar number of capture attempts could imply a lower biomass obtained with respect to Laguna de Cobre-Itabo.
In addition, the disturbance in Bajo de Santa Ana can affect the availability of foraging habitat for S. p. gundlachi. For both mangroves' sites, the intermediate height of the vegetation layer is most used to foraging, intervals of 2 to 5 m. However, in Bajo de Santa Ana about 20 % of the vegetation is below 2 m due to deforestation (Guzmán et al. 2011, Márquez 2013), and A. germinans species is the most affected. These areas with low vegetation, exhibit less foliage availability to forage, and create open spaces where predation risk may be higher and foraging efficiency lower (Morrison et al. 2010).
It is important to highlight that L. racemosa was the most used for foraging and offers a high biomass of available prey in Laguna de Cobre-Itabo. The high biomass of arthropods in this species can be attributed to temporal correspondence of sampling months with peak flowering season of L. racemosa, (June and July) (Menéndez et al. 2006), which increases the resources availability for arthropods. Previous work of S. p. gundlachi in Florida, has described the breeding areas as corresponding to mangrove vegetation, with predominance of A. germinans, and with the presence of other species such as R. mangle and C. erectus (Prather and Cruz 1995). For another subspecies resident in Puerto Rico, its reproduction is reported in wetland areas with predominance of A. germinans and R. mangle, all nests being found on mature A. germinans trees (Vincenty et al. 2009). So, this would be the first evidence of the importance of L. racemosa mangrove for a tropical subspecies of S. petechia.
Salgado-Ortiz et al. (2008) describe that during the breeding season, S. p. bryanti may invest more energy in defending the territories and maintenance. Therefore, food availability can be a key resource in this stage of its life cycle. In addition, the prey availability is an important cue for the selection and size establishment of territories in tropical insectivorous birds (Newmark and Stanley 2016). For migratory subspecies, it is believed that in the process of habitat selection, several of the habitat characteristics that are considered by the bird, like shrubs density (Quinlan and Green 2012) and the proximity to water reservoirs (Frydendall 1967), are related to food availability. Considering the statements above, it is expected that prey availability is a good measure of habitat quality during the reproductive season of S. p. gundlachi, and that the low values of prey available in Bajo de Santa Ana can affect the time and energy expended on defending territories and nests, so may have negative repercussions on nesting success and subsequently on the populations of this subspecies.
Márquez (2013) found similar density of S. p. gundlachi in both mangroves' sites. The lack of association between bird density and prey availability could be due to a negative correlation between density and other important population parameters, such as survival and reproductive success (Johnson 2007). Bock and Jones (2004) describe how this may occur, mainly in conditions of human disturbance, as the bird's ability to identify and occupy the best habitats for their reproduction is affected. These elements are generally associated with ecological traps (Robertson and Hutto 2006). These results indicated the unreliability of bird population density as habitat quality indicator for this subspecies under the conditions studied.
In the breeding season, another important element for habitat selection and reproductive success is the nesting site availability, which was not considered in this work. This variable may be important since the nesting sites selection in this species is a determinant for predation, nest parasitism and on annual productivity (Quinlan and Green 2012). In addition, factors such as predation and parasitism are more intense for tropical subspecies (Salgado-Ortiz et al. 2008), such as S. p. gundlachi. On the other hand, these interactions become more complex in urban landscapes where both native and non-native predators exist (Kristan et al. 2007). Taking this into account, future studies should consider the nesting site availability, as a factor that could be used as a measure of habitat quality for S. p. gundlachi in the mangroves studied.
These results provided evidence of how the changes in mangrove vegetation structure can influence a critical factor such as the food availability, which can have implications for the conservation of populations of S. p. gundlachi on the coast of Havana. All the above highlights the importance to continue other studies in these areas, emphasizing measures of habitat quality like reproductive success for S. p. gundlachi, and its link with prey availability and environmental disturbances. This subspecies presents a habitat restriction to the mangrove ecosystems for its breeding; therefore, it is essential to know how its populations respond to the loss and modification of this habitat, for its future conservation. Increasing our knowledge, would help management strategies to ensure that the future urban development of this area is compatible with biodiversity conservation.