INTRODUCTION
Forest edges appear due to discontinuity between two contrasting environments. Although boundaries and edge habitats are constitutive elements in natural landscape heterogeneity, the proportion of edge habitats has increased in tropical environments because of the increment of forest fragmentation (Laurance 2000, Fletcher Jr. et al. 2007, Chazdon 2014). The edge effects is understood as abiotic and biotic changes that take place at the boundaries of both forest patches and between adjacent ecosystems (Fletcher Jr. et al. 2007). Some deleterious consequences produced by forest fragmentation are determined by edge effects (Laurance et al. 2007, Haddad et al. 2015), therefore, the edge effect may modify physical gradients, species distribution, species composition, and species abundance (Laurance et al. 2007, Broadbent et al. 2008, Ries et al. 2017).
The relationship between bats assemblages and edge habitats has shown diverse responses, some of them contrasting (Meyer et al. 2016). Research made in fragmented landscapes suggest that the edge habitat seems to favor the establishment of opportunist species and affect trophic guilds in different ways (Reis and Muller 1995, Cosson et al. 1999, Schulze et al. 2000, Estrada and Coates-Estrada 2002, Klingbeil and Willig 2009). In contrast to these results, other investigations did not find significant differences between bat species richness and abundance between interior and edge habitats (Faria 2006, Meyer and Kalko 2008). However, Meyer and Kalko (2008) suggested that edge effects could have deleterious effects for some bat species considered as edge-sensitive, while other bat species could be benefited by edges and classified as edge-preferring. Other surveys indicated that the number of individuals and species richness of bats was higher in the edge than in primary forest but, species diversity was low in edges (Delaval and Charles-Dominique 2006). Sensitivity of bats species to the edge effects is also affected by the type of matrix and its contrast with the contiguous edge (Meyer et al. 2016). Significant changes in bat richness or composition were observed in landscapes with matrix highly contrasting to forest patches (e.g. water, Meyer and Kalko 2008), while in landscapes with low contrast between matrix and fragments there were no alterations at the bat community level (Gorresen and Willig 2004).
Modifications of vegetation structure are significant in forest edges where regenerating secondary forest and habitat disturbance is frequent (Faria et al. 2009). Studies on bat responses to second-growth vegetation in tropical environments show contrasting patterns (García-Morales et al. 2013, Meyer et al. 2016). For example, bat richness and abundance increase across succession gradients (Avila-Cabadilla et al. 2009), but this tendency is not evident in others (Castro-Luna et al. 2007). Regarding the trophic guilds, some studies indicate that frugivore and nectarivore bats are more abundant in secondary forests (Brosset et al. 1996, Castro-Luna et al. 2007, Willig et al. 2007), while foliage insectivores and gleaning animalivores tend to show low abundances in second-growth forests or disturbed habitats (Fenton et al. 1992, Brosset et al. 1996, Rocha et al. 2017).
The edge effects has been explored through comparisons of bat assemblages found in the forest interior against those found in the boundaries of forest fragments or continuous forest (Faria 2006, Meyer and Kalko 2008). However, there is scarce information about the relationship between bat assemblages throughout spatial gradients spanning the forest interior to the surrounding matrix (Delaval and Charles-Dominique 2006, Meyer et al. 2016). In addition, most of these studies were developed in tropical forests at low elevations and there is scarce information about edge effects or habitat disturbance on the bat assemblage structure in Andean forests (Pérez-Torres and Ahumada 2004, Montaño-Centellas et al. 2015). In contrast to lowland forests, Andean bat communities have fewer insectivorous species and are dominated by frugivorous and nectarivorous species where some genera are more diversified in the Andes than in lowlands (e.g. Sturnira and AnouraSoriano 2000). Therefore, understanding how Andean bat species could be affected by habitat transformation is crucial, especially because current Andean forest represents only 38 % of its original extent in Colombia (Rodríguez Eraso et al. 2013).
Our goal was to describe changes in species richness, abundance, evenness, and species composition of trophic guilds of phyllostomid bats related to a matrix-edge-interior forest gradient in four sub-Andean forest fragments. Considering abiotic and biotic modifications that take place in edges such as reduced humidity, greater temperature variability, changes in forest structure and floristic composition (Murcia 1995, Didham and Lawton 1999, Laurance et al. 2002), we hypothesize that species richness, abundance, evenness, and species composition of trophic guilds of phyllostomid bats should be modified along the matrix-edge-forest interior gradient of the patches. We expect that species richness and abundance would be higher and evenness would be lower in the edges than in the interior of the patches, due to the increase of food resources associated with second-growth habitats typically found at the edges (Rocha et al. 2020). In contrast, we predict that richness and abundance would be lower in the matrix because of the low food availability in a hostile matrix such as pastures, except for sanguinivorous bats, which would be more abundant in this habitat in association to livestock commonly found there. Due to edges being biased toward pioneer shrubs and secondary species of plants (Laurance et al. 2002), we expect that shrub frugivorous bats would be more abundant in the edge, while canopy frugivores, foliage insectivores, and nectarivores would be more abundant in the interior of the fragments.
MATERIALS AND METHODS
Study area
Fieldwork was carried out in four sub-Andean forest fragments in the Encino municipality (Santander, Colombia), located on the western flank of the Eastern mountain range at elevations from 1600 to 2200 (06°10' N, 73°08' W). This region has a mean annual temperature of 21 °C and a total mean annual rainfall of 3111 mm with a bimodal regime. There are two rainy seasons (March-May and October-November) and two dry periods (June-August and December-February) (Solano et al. 2005). Andean and sub-Andean forests in this region are dominated by oaks (Quercus humboldtii Kotschy ex A. DC.) and are highly fragmented by agricultural and livestock activities. In Encino, sub-Andean forest is currently distributed in only 53 fragments with less than 100 ha that represents only 5.1 % of its original distribution (Otalora-Ardila and Lopez-Arevalo 2006).
Bat sampling
We selected four sub-Andean forest fragments, each of them less than 50 ha: Cachalu, Tapias, San Benito, and Pericos. Tapias and Cachalu fragments were considered as non-isolated fragments because the mean distance to the closest neighbor was less than 200 m, considering other fragments and riparian vegetation (Tapias: 94 m, Cachalu: 172 m). In the case of Cachalu fragment, this patch was close to the continuous forest of the Cachalu Biological Reserve (CBR), which is a private area that protects sub-Andean and Andean forests (Solano 2006). In contrast, San Benito and Pericos patches were considered isolated because the mean distance to the closest neighbor was higher than 200 m (San Benito: 874 m, Pericos: 378 m). All patches had a similar shape, edge density, and surrounding type of matrix (Otálora-Ardila and López-Arévalo 2006). Inside of the patches, selective logging at a small scale was frequently observed. Matrix was considered hostile for bats because it was constituted of open livestock pastures area.
Total sampling was made during 35 nights from March 2004 to July 2004 (nine nights for Pericos, Tapias and San Benito, and eight nights for Cachalu), using 16 mist nets set at ground level in each fragment. We established three sampling lines that covered a gradient from the forest interior to the matrix in each patch. Considering that edge effects on vegetation and bat responses at community-level and activity have been detected between 50 and 100 m (Laurance et al. 2002, Delaval and Charles-Dominique 2006, Jantzen and Fenton 2013), in each line we established four sampling stations at 75 meters between them: matrix, edge, 75m, and 150m. Matrix station was located 75 m from the edge to the pasture, edge station was placed in the patch border, 75m and 150m stations were placed 75 and 150 meters inside the patch, respectively. In each station, four mist nets were opened from 18:00 to 24:00, except on rainy or full moon nights. Sampling effort was calculated as m2 x mist net x hours (mnh) reaching a total effort of 83791 mnh. Sampling effort was similar between stations (matrix: 20884, edge: 20982, 75m: 20961, 150m: 20964). Captured bats were measured and marked in the wing membrane using tattoos and were released in the same place of capture. Some individuals were collected and deposited in the Mammals Collection of the Instituto de Ciencias Naturales at Universidad Nacional de Colombia (ICN).
Data analyses
Species richness was defined as the number of species found in each sampling station (matrix, edge, 75m, and 150m). We used randomized (100x) sample-based species accumulation curves to verify inventory accuracy. Additionally, we used the non-parametric estimator Jack1 to assess the expected species number in each gradient sampling station because this estimator considers the movement heterogeneity of mobile animals such as bats (Brose and Martínez 2004). Accumulation curves and species richness estimators were obtained using EstimateS 9.1.0 software (Colwell c2013). Sampling effort was statistically similar between sampling stations (F= 0.0007, df= 3, P= 1) and between fragments (F= 0.0008, df= 3, P= 1). Bat abundance was defined as the number of individuals captured in each sampling station and each fragment. Recaptured individuals were not included in the calculations of relative abundance. Evenness was estimated with the inverse of the Simpson index (Magurran 1988) using iNEXT package (Hsieh et al. 2016). Each bat species was assigned to a trophic guild as follows: shrub frugivorous and canopy frugivorous according to Soriano (2000), and foliage insectivorous, nectarivorous and sanguinivorous following Kalko et al. (1996).
We analyzed the variation of species richness and abundance of bats using Generalized Linear Mixed-effects Models (GLMM) with a Poisson distribution. Response variables in these GLMMs were richness and abundance of bats, the gradient sampling stations (matrix, edge, 75 m, and 150 m) were understood as fixed factors and fragment was included as a random factor. When GLMMs indicated significant differences, we performed post-hoc Tukey HSD tests using the multcomp package (Hothorn et al. 2008). We compared the inverse of the Simpson index across the gradient sampling stations performing a Kruskal-Wallis test, and we made post-hoc comparisons using the Wilcoxon rank-sum test. To determine differences in the species composition of the trophic guilds across the gradient, we did a multivariate generalized linear model analysis with sampling stations and fragments as predictor variables using the mvabund package (Wang et al. c2019) for R software. These analyses were not performed for san-guinivores and foliage insectivores because these trophic guilds were represented by only one species. All analyses were conducted in the program R v.3.4.4 (R Core Team c2018). Values were expressed as mean ± s.d, and results were considered significant at P<0.05.
RESULTS
We captured a total of 566 individuals of 21 species belonging to the family Phyllostomidae in the four sampled fragments. We recaptured 27 bats of eight species along the gradient, but we did not recapture any bat across fragments. Carollia brevicauda (Schinz, 1821), Artibeus lituratus (Olfers, 1818), Dermanura glauca (Thomas, 1893), Platyrrhinus dorsalis (Thomas, 1900), and Desmodus rotundus (E. Geoffroyi Saint Hilaire, 1810) were the most abundant species (Table 1). The Jack1 estimator indicated high-level inventory completeness for the total sampling (> 78 % for total sampling, Supplementary Material Fig 1). In each sampling station, the phyllostomids species that were captured reached between 75 % and 91 % of total species (matrix= 75 %, edge= 89 %, 75m= 91 %, 150m= 83 %).
Table 1 Bat species and number of individuals captured in the matrix-edge-forest interior gradient in four Sub-Andean forest fragments in Encino (Santander, Colombia). Trophic guild is mentioned for each species.
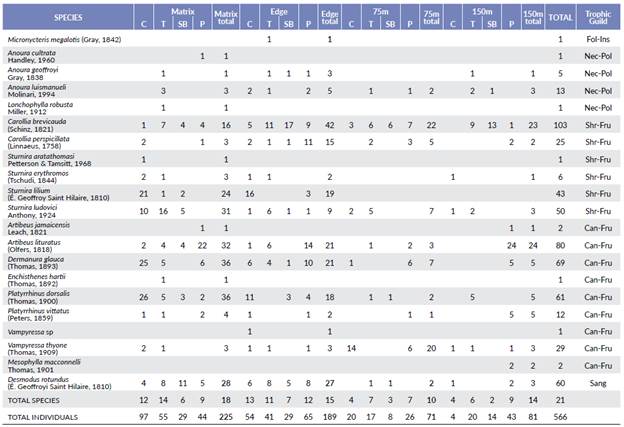
C= Cachalú, T= Tapias, SB= San Benito, P= Pericos. Fol-Ins= Foliage insectivorous, Nec-Pol= Nectarivorous-Polinivorous, Shr-Fru= Shrub frugivorous, Can-Fru= Canopy frugívorous, Sang= Sanguinivorous. Bold= Total individuals captured in each sampling station.

Figure 1 Ecological indexes of the bat diversity in the area. a. Number of species, b. number of individuals, c. inverse of Simpson index along the gradient matrix-edge-forest interior in four Sub-Andean forest fragments, and d. inverse of Simpson index in the four Sub-Andean forest fragments sampled in Encino (Santander, Colombia). Boxes represent the 25th and 75th percentiles, whiskers represent the 95 % confidence imits, and black lines within boxes represent medians. Black points represent outliers. Letters indicate significant differences (P < 0.05) according to the post hoc Tukey's all-pairwise comparisons for the number of species and number of individuals, and Wilcoxon pairwise comparisons for the inverse of Simpson index along the gradient and among fragments. C= Cachalu, P= Pericos, SB= San Benito, T= Tapias
Species richness was significantly different across sampling stations (Dev= 193, df= 43, Supplementary Material Table 1). Richness was similar between matrix and edge and between 75m and 150m sampling stations (Fig 1a, Supplementary Material Table 2). Matrix and edge species richness was significantly higher than at 75m and 150m (Supplementary Material Table 2).
Bat abundance was significantly different between sampling gradient stations (Dev= 501.2, df= 43, Supplementary material Table 1). Abundance was significantly higher in the matrix than in 75m and in 150m (Fig. 1b, Supplementary Material Table 2). The most abundant species found in the matrix were D. glauca, A. lituratus, and P. dorsalis, and at the edge were C. brevicauda and D. rotundus. At 75m, the most abundant species were C. brevicauda and Vampyressa thyone Thomas, 1909, and at 150m C. brevi-cauda and A. lituratus (Table 1, Figs. 2a-d).
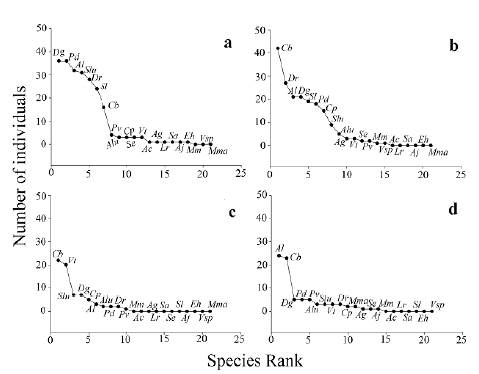
Figure 2 Rank abundance curves for captured individuals in a. matrix, b. edge, c. 75m, d. 150m in four Sub-Andean forest fragments in Encino (Santander, Colombia). Dg= D. glauca, Pd= P. dorsalis, Al= A. lituratus, Slu= S. ludovici, Cb= C. brevicauda, Pv= P. vittatus, Alu=A. luismanueli, Cp= C. per-spicillata, Se= S. erythromos, Vt= V. thyone, Ac= A. cultrata, Ag= A. geoffroyi, Lr= L. robusta, Sa= S. aratathomasi, Aj= A. jamaicensis, Eh= E.hartii, Mm= M. megalotis, Vsp= Vampyres-sa sp., Mma= M. macconnelli.
Species evenness was significantly different between sampling gradient stations (KW= 13.64, df=3, P = 0.003) and between fragments (KW= 9.81, df=3, P =0.02). Simpson diversity index was significantly higher at 75m and 150m than in matrix and edge (Fig. 1c). Evenness in San Benito was similar to Cachalu but was significantly higher than the evenness registered in Tapias and Pericos fragments (Fig. 1d). Evenness registered in Cachalu was like Pericos and Tapias (Fig. 1d, Supplementary Material Table 3).
Trophic guilds were diverse: six shrub frugivores, nine canopy frugivores, four nectarivores, one foliage insectivore and one sanguinivore. Species composition of the shrub frugivorous guild was different along the sampling gradient (P = 0.04, Figs. 2a-d, Supplementary Material Table 4) and between fragments (P = 0.01, Figs. 3a-d, Supplementary Material Table 4). Only S. lilium (E. Geof-froyi Saint Hilaire, 1810) was significantly affected by the gradient, and there was a significant effect of fragment for C. brevicauda and S. ludovici Anthony, 1924 (Supplementary Material Table 4). In the case of canopy frugivores, the species composition was similar along the gradient (P = 0.14, Figs. Figs. 2a-d, Supplementary Material Table 4) but was different across fragments (P = 0.001, Figs. 3a-d, Supplementary Material Table 4). There was a significant effect of fragment only for A. lituratus (Supplementary Material Table 4). In contrast, the nectarivorous guild' species composition did not vary in the sampling gradient or between fragments (Supplementary Material Table 4). D. rotundus was the only sanguinivorous species captured, and it was registered in all sampling stations of the gradient across all fragments. Micronycteris megalotis (Gray, 1842) was the only foliage insectivorous species registered and was captured only on the edge of the Tapias fragment.
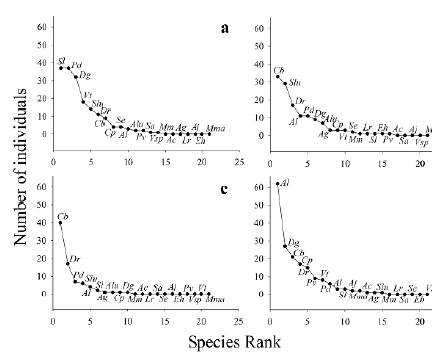
Figure 3 Rank abundance curves for captured individuals in four Sub-Andean forest fragments: a. Cachalú, b. Tapias, c. San Benito and d. Pericos, in Encino (Santander, Colombia). Dg= D. glauca, Pd= P. dorsalis, Al= A. lituratus, Slu= S. ludovici, Cb= C. brevicauda, Pv= P. vittatus, Alu=A. luis-manueli, Cp= C. perspicillata, Se= S. erythromos, Vt= V. thyone, Ac= A. cultrata, Ag= A. geoffroyi, Lr= L. robusta, Sa= S. ara-tathomasi, Aj= A. jamaicensis, Eh= E.hartii, Mm= M. megalotis, Vsp= Vampyressa sp., Mma= M. macconnelli
DISCUSSION
Our data show evidence that edge effects have a significant influence in Andean bat communities even at short distances from the fragment borders (~75 m), where bat species richness, abundance, evenness, and species composition of trophic guilds showed significant variation along the matrix-edge-forest interior gradient of the patches. The highest richness and abundance in the matrix can be related to the landscape configuration. In Encino's landscape, the matrix showed high contrast with forest fragments because it was an open area of habitat non-suitable for bats because it was mainly constituted by pastures that no offer food or refuge resources. In this landscape, the matrix can be used by bat species as a movement area and, therefore, the richness and abundance in the matrix increased. This agrees with the idea that not only the species within a specific habitat determine the local diversity, but that it may also be influenced by transitory or tourist species that come from surrounding areas (Moreno and Halffter 2001). High species richness and low evenness of bats found in the edges may be related to the indirect edge effects suggested by Murcia (1995). Indirect edge effects refer to changes in species interactions such as herbivory, parasitism, pollination, seed dispersion or invasions of generalist animals and plants (Valladares et al. 2006). In this study, shrub frugivores such as C. brevicauda and S. lilium and canopy frugivores such as A. lituratus, D. glauca and P. dorsalis were more abundant in the edges. High richness and abundance of these frugivorous species may be associated with the growth of plant species typical of initial succession stages and commonly found in the same locality studied (Camargo and Vargas 2006) and in other Andean forest edges (Berg et al. 2005, Cortés-Delgado and Pérez-Torres 2011, Lippok et al. 2014). Although we did not measure vegetation composition, qualitative observations indicated a high abundance of pioneer plants in the edges, such as those of the families Piperaceae, Cecropiaceae, Solanaceae, and Moraceae. These pioneer plants constitute the main food resource for generalist frugivores such as S. lilium, C. brevicauda, D. glauca, and P. dorsalis and they are mainly dispersed by these bat species in other tropical localities (Brosset et al. 1996, Kalko 1998, Giannini and Kalko 2004, Estrada-Villegas et al. 2010).
Our data indicated that shrub frugivorous bats were more abundant in the edge supporting our prediction. However, the only shrub frugivorous species significantly affected by the gradient matrix-forest interior was S. lilium because this species was only captured in the matrix and the edge. These responses of shrub frugivorous bats to the matrix-edge-interior gradient were congruent with other studies focused on bat assemblages in lowland tropical fragmented forests in Guatemala, the Atlantic forest, and the Amazonia in Brazil (Schulze et al. 2000, Delaval and Charles-Dominique 2006, Faria et al. 2006, Klingbeil and Willig 2009), and it is also consistent with research developed in an Andean forest fragment in Colombia (Cortés-Delgado and Pérez-Torres 2011). Abundance of C. brevicauda and S. ludovici was significantly different between fragments, and this could be related to the type of perturbation occurring in the edge. These two bat species were more frequently captured in the edge of Tapias and San Benito fragments, where we observed some portions of the boundaries covered with shrubs of pioneer plants generating a less contrasting edge before the matrix. In contrast, the edges of Cachalú and Pericos fragments were abrupt and frequently cut down, therefore, the transition between forest and matrix was highly evident. A similar pattern was observed for frugivorous bat species in the edge of an Andean fragment, considered as a permeable edge because the presence of pioneer plants allows the bat presence in this portion of the fragment (Cortés-Delgado and Pérez-Torres 2011).
In contrast to our expectations, canopy frugivorous bats were more abundant in the matrix and the edge. This pattern agrees with other studies in lowland tropical forests affected by fragmentation such as the Atlantic forest and the Amazonian forests (Cosson et al. 1999, Gorresen and Willig 2004, Meyer and Kalko 2008, Klingbeil and Willig 2009). It is possible that canopy frugivores were less affected by the matrix-forest interior gradient because they are more mobile and can fly longer distances than the shrub frugivores (Meyer and Kalko 2008). The abundance of A. lituratus, a canopy frugivore, was significantly different among fragments and this could be related to spatiotemporal variation in the abundance of food resources. Most individuals of A. lituratus (77.2 %) were captured in Pericos fragment (Fig. 3d), where we observed several fig trees (Ficus sp.). These fig trees were bearing fruit during the sampling season and we captured several individuals of A. lituratus carrying figs, similarly to reports in other localities in Panamá and Venezuela (August 1981, Handley et al. 1991). Although our data indicated a higher abundance of canopy frugivorous at the matrix and edges, it is possible that this pattern could be biased by the fact that we used mist nets only at ground-level along the sampling gradient. Some studies using canopy mist netting in tropical lowland forests have detected more Stenodermatinae species at the canopy level (Kalko and Handley 2001, Pereira et al. 2010). Therefore, it is possible that abundance data for some species may be underestimated, particularly for the genus Plathyrrhinus and Vampyressa of which we captured few individuals.
The sanguinivorous trophic guild was represented by only one species, D. rotundus, and in agreement with our prediction, this species was more abundant in the matrix and edge, than at 75m and 150m. High abundance in the matrix and the edge could be related to the presence of livestock in these two types of habitats. In line with our results, this species is more abundant in intermediate successional-stage habitats (Avila-Cabadilla et al. 2009) and in fragments surrounded by pastures (Estrada and Coates-Estrada 2002). Among foliage insectivores, we captured only one individual of M. megalotis. The low abundance of foliage insectivores and the absence of animalivorous species could be associated with their reluctance to inhabit altered habitats, being considered edge-sensitive species (Gorresen and Willig 2004, Meyer and Kalko 2008). In contrast to our prediction, nectarivorous species were more abundant in matrix and edge. The edge effects seem to not negatively affect the abundance of nectarivorous bats, where probably the current size of sampled fragments still allows the persistence of these species and maintenance of the pollination process. Similar patterns were suggested for bird assemblages, where alteration of the pollination processes was more drastic in fragments smaller than 10 ha (Murcia 1995).
Overall, we found that richness, abundance, evenness, and composition of trophic guilds were affected along the gradient sampled, suggesting that edge effects have significant influence even at short distances from the border (~75 m). Although bats are highly mobile organisms, other studies have shown that edge effects modified bat richness, abundance, and diversity at distances less than 100m. For example, research carried out in lowland forests indicates that bat species richness and abundance were significantly different between the forest edge and the contiguous 50 m inside the forest (Delaval and Charles-Dominique 2006). Similarly, the activity of insectivorous bats was significantly higher within 20 m of the edge and stabilized up to 40 m (Jantzen and Fenton 2013). Additionally, we did not have any recapture between fragments, but we recaptured 16 individuals along the gradient, and eleven were recaptured at the same site of their original capture, suggesting that few bats moved regularly among the sampled stations. The total recapture rate (4.8 %) was similar to those reported in other mountain agroecosystems (Williams-Guillén and Perfecto 2010), and lower than in other Andean (Montaño-Centellas et al. 2015) and lowland landscapes (Bianconi et al. 2006, Zortéa and Alho 2008).
In spite of the highly fragmented nature of the locality studied, we captured bat species considered rare and, based on our best knowledge, this is currently one of the localities with the highest number of phyllostomids bats species (21) reported among Colombian Andean localities (e.g., 6 to 20 species, Cadena et al. 1998, Pérez-Torres and Ahumada 2004, Numa et al. 2005, Estrada-Villegas et al. 2010, Rodríguez-Posada 2010, Cortés-Delgado and Pérez-Torres 2011, Mora-Beltrán and López-Arévalo 2018). In agreement with other studies in fragmented localities, bat species richness was similar to that registered in small forest patches immersed in an agriculture mosaic (Numa et al. 2005). Our data suggest that sub-Andean forest patches sampled in Encino still preserve some structural elements of vegetation that may support high bat species richness even when these patches are immersed in a non-suitable matrix. Similarly, Sáenz Jiménez (2010) suggests that the diversity and structure of Andean forests in Encino and nearby areas, which are dominated by oaks, offer food resources, roosts and, microhabitat conditions suitable especially for small mammals. In this sense, these fragments might constitute an important habitat for bat assemblages especially because these sub-Andean forests have experienced a drastic fragmentation and currently represent only 5 % of their original coverage (Otálora-Ardila and López-Arévalo 2006). Although we show here that bat richness is still high despite the edge modifications, we did not consider other variables such as activity patterns or bat behavior that might be affected by disturbance gradients. Although phyllostomid bat species may persist in transformed landscapes (Law et al. 1999, Estrada and Coates-Estrada 2002, Bernard and Fenton 2007), we must consider that if habitat loss and isolation between forest remnants increase, some bat species probably will be threatened. However, in highly fragmented localities, small patches might function as a network of forest islands that promote the maintenance of vertebrate diversity. Thus, the gradient studied has allowed maintaining an important portion of the biodiversity of bats in the Andean region and probably of other components of its biodiversity. However, effective conservation strategies should be promoted focused on maintaining adequate amounts of mature forests, and at the same time, promoting successional processes that minimize the effect of a hostile matrix.















