INTRODUCTION
Molt is the regular process of feather growth (Howell et al. 2003) and constitutes an important and energetically expensive activity in the life cycle of birds (Foster 1975). Description of the extent and timing of molt patterns of birds is crucial to understand the trends and changes in their demography in reference to space and time (Hernández 2012), as well as the ecological, evolutionary, physiological, and phylogenetic factors that affect molt (Guallar et al. 2016). Research about molt and/or aging and sexing criteria in Neotropical landbirds is a growing research area (Rueda-Hernández et al. 2018), but further effort is needed in order to comprehend these aspects for most of them (Kennedy et al. 2018). Such studies have been conducted in remote places (such as Amazonian and Atlantic rainforests, montane forests, and volcanic islands), while only a few of them have been published on urban landscapes (González 1998, Bugoni et al. 2002, Cueva 2018).
Urbanization can affect the life history of birds, either directly by altering the ecological processes, physiology, behavior and morphology (Isaksson 2018), or indirectly by changing their predators, competitors and pathogens (Chace and Walsh 2006). Birds in urban areas are additionally affected by increased levels of human activity, leading to altered food type and food availability (Lancaster and Rees 1979, Ditchkoff et al. 2006) and resulting in many species being negatively impacted while others will benefit over time (Fokidis et al. 2008). Therefore, bird monitoring using bird banding techniques constitutes a mechanism to explore the potential effect of urban development on bird communities, their long-term survival, as well as to establish proper management policies in urban spaces (González 2004). The aim of this work was to study the molt patterns, as well as age and sex determination criteria of selected landbirds in an urban area of the central coast of Peru, in order to investigate the potential effects of urbanization on molt strategies, extent, and timing along the annual cycle of Neotropical birds.
MATERIALS AND METHODS
The study was carried out in the lower slopes of the "El Agustino" Hill (12°02'35.14" South, 76°59'30.82" West, 226 m), adjacent to the campus of Universidad Nacional Federico Villarreal (UNFV), El Agustino district, department of Lima, Peru. This urban area was settled on a desiccated-Subtropical desert (dd-S) (Holdridge 1962), whose water regime was characterized by an annual precipitation of 437 mm concentrated from June to August, and with an annual average temperature of 17o to 19 o C (SENA-MHI c2010). From March 2014 to February 2020, seven standard mist nets (12 m in length with 36 mm mesh) were used to capture birds around fruit crops and ornamental shrubs cultivated in introduced agricultural soil. Even though this area was restricted to general public, it was settled at a distance of 50 m away from faculty buildings. The nets were opened between 06:30 h and 12:30 h (GMT-5) during one day per month, ensuring a monthly effort of 36 net-hours. Mist-netting operations were performed on Sundays due to the low concurrence of people on campus. All birds were marked with a uniquely numbered aluminum leg band, processed and released following international standards (NABC 2001). Sex of birds was assessed by plumage coloration and the presence of breeding characters such as the brood patch (BP) and the cloacal protuberance (CP) following Pyle (1997b). Only vascularized and wrinkled BPs, as well as medium-sized and enlarged CPs, were considered in our analysis. Age was assessed, when possible, through identification of molt limits, plumage criteria, and extent of skull ossification (Pyle 1997a). Age was categorized according to the molt cycle-based aging system proposed by Wolfe et al. (2010), refined by Johnson et al. (2011) and based on the molt terminology of Howell et al. (2003) (Table 1). Feather-tract, molt extent terminology and color descriptions followed Pyle (1997b). Rectrices (rects), primaries (pp), secondaries (ss), and their coverts (covs) are considered as "inner" and "outer" depending on their position in relation to the body of the bird. Photographs of the body and open wings were taken to corroborate information collected in the field. Five standard measurements were taken (i.e. wing chord, tail length, bill length from nares to tip, tarsus length, and body mass) following Pyle (1997b); values presented are means ± standard errors (SE). Independent Student-s t-tests were performed to assess differences in the means between sexes using the JMP Pro version 13.1 (Sall et al. 2017). All tests were two- tailed, and p-values < 0.05 were considered significant (Zar 1999). Only species with > 5 individuals represented by each sex were considered in the sex-specific morphometric analysis (Kennedy et al. 2018).
RESULTS
Molt patterns, molt cycle-based age categories, and annual molt cycle for seven common resident species at an urban area of the central coast of Peru are summarized below (Tables 2-3, Fig. 1). Six out of the seven species exhibited a partial or eccentric incomplete preformative molts, and thus the presence of molt limits within wing covs or some flight feathers facilitated the recognition of formative- and definitive- plumaged individuals. Most species underwent complete prebasic molts, with two species suspending the definitive prebasic molt in a small portion (< 3.2 %) of the sample. Three species exhibited partial prealternate molts (Table 3). For three out of the seven species, selected measurements (Table 4) were found to be useful in distinguishing between sexes, with wing chord being the most common criteria to separate females from males (Table 5).
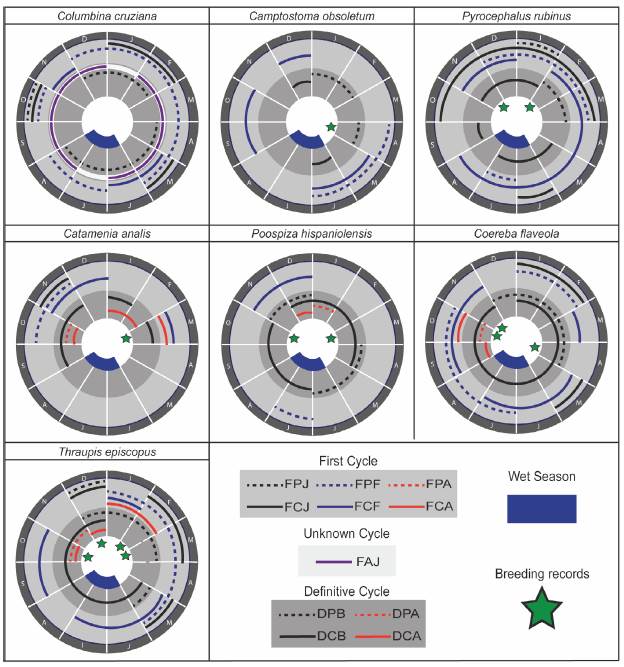
Figure 1 Annual molt cycle and breeding records for seven resident landbirds commonly found in urban areas from the coast of Lima, Peru; according to the representation format of Diaz et al. (2020). Age classes correspond to those from the W-R-P system (cycle code's definition in Table 1) and are grouped as those present in the first molt cycle and those present in the definitive cycles of the bird. Dashed and solid lines represent the distinct molt and plumage categories, respectively.
Table 1 Definitions of the molt-based age-classification system codes (WRP) (Wolfe et al. 2010) used in the present study.
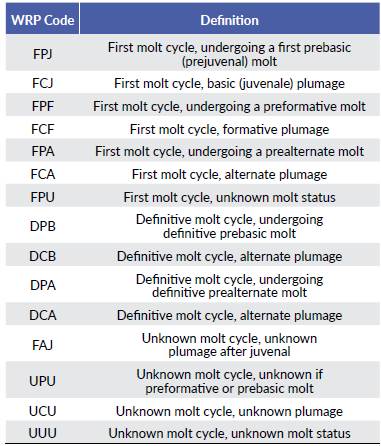
Table 2 Molt patterns and sample sizes for seven species of resident landbirds commonly found in urban areas from the coast of Lima, Peru. CBS = Complex basic strategy; CAS = Complex alternate strategy.
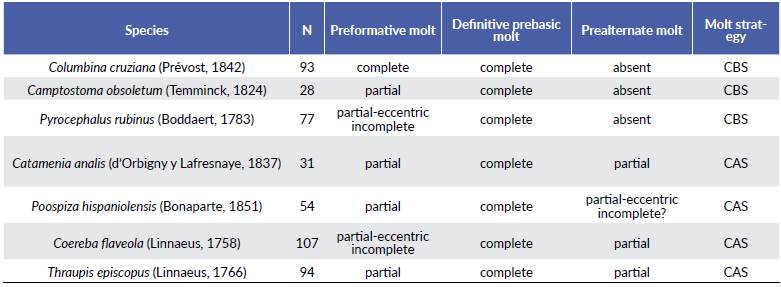
Table 3 Sample sizes of molt cycle-based age categories for sizes for seven species of resident landbirds commonly found in urban areas from the coast of Lima, Peru.
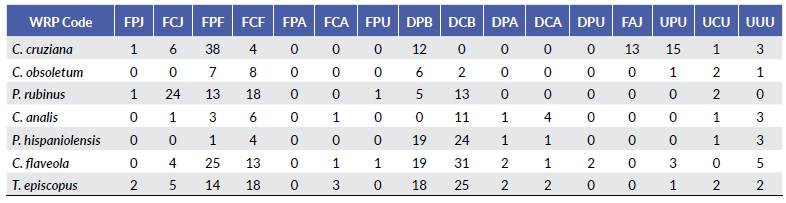
Table 4 Mean morphological measurements ± standard error for seven resident landbirds commonly found in urban areas from the coast of Lima, Peru. Length measurements are in millimeters; body mass in grams; sample size is indicated in parentheses.
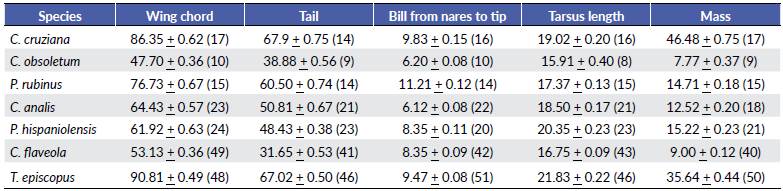
Table 5 Select mean morphological measurements ± standard error useful for classifying sex for four resident landbirds commonly found in urban areas from the coast of Lima, Peru. Length measurements are in millimeters; body mass is in grams; sample size is indicated in parentheses. df = degrees of freedom. P = p-value (a = 0.05).
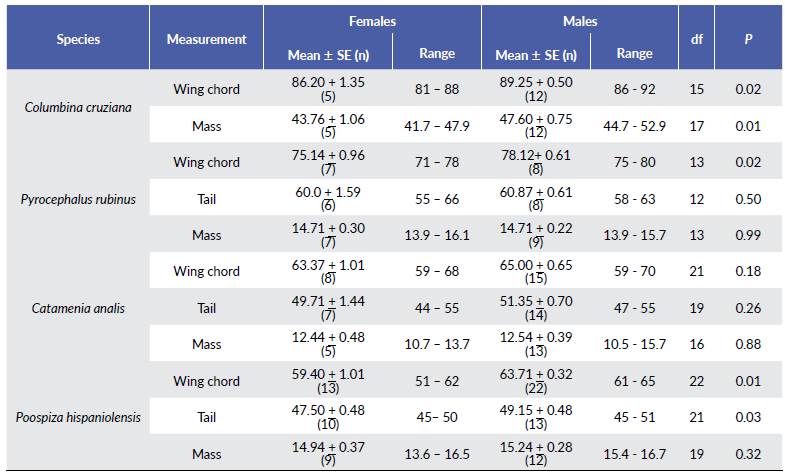
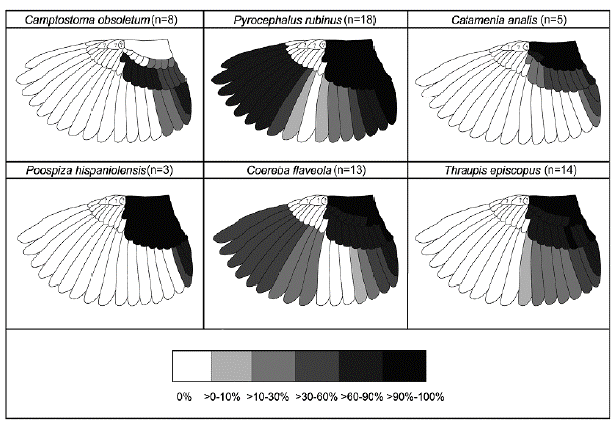
Figure 2 Representation of the frequency of feather replacement as consequence of partial or eccentric incomplete preformative molts for six resident landbirds commonly found in urban areas from the coast of Lima, Peru. The grayscale indicates the percentage of individuals (with available photographic information) that have replaced such feathers. Alula tracts were not included in the analysis.
Croaking Ground Dove (Columbina cruziana; n=93). FPJ: complete. FCJs appeared superficially like older birds but were duller and scaled with pale-buffy tips along the upperparts, including upperwing covs. They also lacked the chestnut bar on lesser secondary covs characteristic of older birds, and their primary covs were tinged buffy along the rachis and tips when fresh (Appendix S1a of the supplementary material). Juveniles were also characterized by their pale iris and bill, and narrower rects compared to older birds (Appendix S1b, c of the supplementary material). FPF: complete. FPFs resembled FCJs when molt had just started or resembled FCFs when molt was nearly complete (Appendix S1d of the supplementary material). DPB: complete. After following complete molts, FCFs and DCBs were not distinguishable and were aged as FAJ (Appendix S1e of the supplementary material). FAJs were characterized by the absence of molt limits in flight feathers or covs, black wing feathers, white iris, and a bright yellow bill with black tip (Appendix S1b of the supplementary material). However, some FCFs were identified by the presence of retained juvenile feathers, including 1-2 outermost primary covs, outer pp (p8-10), or inner ss (s2-5) (Appendix S1d of the supplementary material). Individuals of unknown age included categories UPU, UCU, and UUU. It was difficult to sex FCJs and FPFs, except for very few (21 %) of the latter when the preformative molt was about to finish. Among the rest of the post-juvenile birds, males (58.6 %) appeared to have more extensive gray color in the head and with pinkish brown underparts, whereas females were browner throughout (27.6 %). Breeding characters: CP and BP were poorly developed and thus unreliable for sexing. Southern Beardless-Tyrannulet (Camptostoma obsoletum; n=28). FPF: partial. Includes most body feathers, none to some inner (0-4) median covs, none to all greater covs, but no lesser covs. None to all terts, some ss (s5-9), but no rects were replaced as a consequence of this molt (Fig. 2). FCFs had molt limits among greater coverts, between greater coverts / primary coverts, and among ss. Replaced (formative) secondary covs were distinctively darker, wider and buff-edged compared to the retained ones (juvenile) (Appendix S2a of the supplementary material). Moreover, retained (juvenile) rects were narrow and relatively abraded in this age category (Appendix S2b of the supplementary material). DPB: complete. DCBs had no molt limits and exhibited gray head, pale yellowish under-parts, olive gray back, yellowish green rump, dark remiges with olive edging, and rounded rects with yellowish white edges and tips (Appendix S2b, c of the supplementary material). Individuals of unknown age included categories UPU, UCU, and UUU. Sexes were similar in all plumages.
Breeding characters: One individual with a vascularized BP was recorded in April.
Vermilion Flycatcher (Pyrocephalus rubinus; n =77). FPJ: complete. FCJs had overall dull brown plumage with buffy tips along upperparts and upperwing covs (Appendix S2d of the supplementary material). They also had whitish underparts with dull brown streaks along the breast and sides. Dark morph FCJs had dark brown plumage with faint buffy color tips, especially along the back and upperwing covs (Appendix S2e of the supplementary material). FPF: partial to eccentric incomplete. It included most body feathers, all secondary covs, some ss (s6-9), and all rects as consequence of partial molts. Some pp (p2-10), ss (s1-9), all rects, but no primary covs can be replaced as consequence of eccentric incomplete molts (Fig. 2). FCJs and FPFs could not be sexed in early stages in the cycle; however, FPFs that were about to finish their molt were reliable sexed on individuals of color morph. FCFs were identified by the presence of molt limits, which occurred between greater covs and primary covs, and even between pp and ss as a consequence of an eccentric replacement (Appendix S2f of the supplementary material). DPB: complete. DCBs had no molt limits and exhibited broader wing feathers and rects compared to younger birds (Appendix S2g of the supplementary material). DCB males exhibited black upperparts, and bright red head and un-derparts; whereas DCB females exhibited grayish brown upperparts, whitish breast, and streaked underparts with variable amount of red tones along the belly and flanks. DCBs of dark color morph occurred in both sexes and were difficult to sex in the hand. Individuals of unknown age included categories UCU, and FPU. Breeding character: Two individuals with medium-sized CP were recorded in November and February. One individual with a vascularized BP in November.
Band-tailed Seedeater (Catamenia analis; n=31). One FCJ looked like a female but was duller with dusky streaks along the head, underparts, and upperparts. FPF: partial. It included most body feathers, some (6 inners) to all lesser and median covs, and none to all greater covs. None to all terts and rects were replaced as consequence of this molt (Fig. 2). FCFs had molt limits among secondary covs (including lesser, median or greater covs), ss, and rects. FCF females exhibited a variable mixture of pale brown (juvenile) and buffy brown (formative) feathers with dusky streaks along upperparts, and underparts except on central abdomen. Moreover, formative females showed blackish secondary covs with distinct buffy edging, contrasting in wear and color with other retained covs. FCF males exhibited pale gray feathers (formative) that mix with the retained pale brown (juvenile) feathers along the head and upperparts, and grayish white underparts tinged buff with faint streaks along the breast and sides. Formative males also had distinct secondary covs, being black with a greyish edging and white tips. Moreover, the greater covs had a buffy-gray and whitish coloration at the leading edge of each feather (Appendix S3a of the supplementary material). No FPAs were recorded. One FCA had three generation of feathers and two molt limits as a consequence of the preformative and first prealternate molt. It replaced some of the body feathers including the head, lores, breast, back, scapulars, all lesser covs, and two inners median covs as consequence of the prealternate molt (Appendix S3b of the supplementary material). No rectrices were replaced. No DPBs were recorded. DCBs had no molt limits (Appendix S3c of the supplementary material). DCB females showed a buffy brown coloration with faint dusky streaks along the underparts; while, DCB males exhibited blackish lores, gray (or gray tinged with buffy) upperparts, and chestnut undertail coverts. Both sexes exhibited rects with a large white patch of variable extent on the middle of the inner webs. DPA: partial. It followed a similar replacement pattern as evidenced in FCA, but also included none to all median and greater covs (Appendix S3d of the supplementary material). DCAs showed molt limits among secondary covs (median and greater covs), ss, and rects. In both sexes, alternate feathers had a similar color pattern but glossier than the basic ones. Individuals of unknown age included categories UCU, and UUU. Breeding characters: One bird with large-sized CP was recorded in March.
Collared Warbling-Finch (Poospiza hispaniolensis; n=54). FPF: partial. It included most body feathers, all secondary covs, none to all terts, and some (two inners, two outers) to all rects Fig. 2). FCFs had molt limits between greater covs / primary covs, and among ss. FCF females resembled older birds but were duller, and with a variable amount of dusky streaks along underparts. They also showed distinct secondary covs with a buffy edging, and buffish white tips. One FCF male also resembled older birds but was duller and with pale brown tones along its face, crown, and upperparts. It also exhibited distinct secondary covs, being black with a greyish edging and flat white tips. Additionally, greater covs showed a buffy and white coloration at the leading edge of each feather. DPBs: complete. DCBs were had no molt limits and exhibited uniform wing covs and remiges that do not differ in wear or quality (Appendix S3e of the supplementary material). DCB males exhibited a well-defined white supercilium and throat, black auriculars and lores, gray (or gray tinged with buffy) upperparts, and a black band across the breast bordered by a gray color gradient along their sides until the belly level. DCB females showed similar plumage pattern, but the gray and black were replaced by brown feathers. Moreover, DCB females lacked the breast band and showed none to very few streaks along their under-parts. One male individual displayed a suspended/arrested prebasic molt. Such bird retained some inner secondaries (s4-6), and the outermost primary (p9) with its respective primary cov. DPA: partial. Although we captured one DPA male following a partial molt (Appendix S3f of the supplementary material), one DCA male exhibited molt limits between median and greater covs, and among pp and ss as consequence of an eccentric incomplete molt. It replaced some body feathers including the head, breast, back, scapulars, all lesser and median covs, but no greater covs. Moreover, the two visible outermost pp covs, some pp (4-9), all terts, but no rects were replaced as consequence of this molt. Individuals of unknown age included categories UCU, and UUU. Breeding characters: Two individuals with medium-sized CP were recorded in October and March.
Bananaquit (Coereba flaveola; n =107). FCJs resembled older birds but were duller and with more gray tones in face and crown, and with olive tones in the back and the upper secondary covs. FPF: partial - eccentric incomplete. It included most body feathers, none to all lesser and median covs, and at least one to all greater covs as consequence of partial molts. Moreover, some pp (p4-9) and ss (s4-9), none to all rects, but no primary covs were replaced as consequence of eccentric incomplete molts (Fig. 2). FCFs had molt limits between secondary covs / primary covs, or even sometimes among pp and ss in case of an eccentric pattern. Formative secondary covs contrasted in size and appearance with the retained ones that were worn, tapered, and washed brown with faint olive-edging (Appendix S4a of the supplementary material). FPA: partial. One FCA had three generation of feathers and two molt limits as a consequence of the preformative and first prealternate molt. It replaced some body feathers, including the head, breast, back, scapulars, all lesser covs, some median covs, and three inners greater covs as consequence of the first prealternate molt (Appendix S4b of the supplementary material). No rectrices were replaced. DPB: complete. DCBs had no molt limits and exhibited well-defined white supercilium, bright yellow underparts, and dusky gray upperparts. DCBs also exhibited uniform gray covs and remiges that do not contrasted in wear or color between both type of feathers (Appendix S4c of the supplementary material). Two individuals displayed suspended/arrested prebasic molts. One individual retained all terts and three inner primary covs whereas the other individual retained some inner secondaries (s3-6), and the three outermost primary covs. DPA: partial. One DCA had a molt limit among greater covs. It replaced some body feathers including the head, breast, back, all lesser and median covs, and the innermost greater cov. Moreover, some inner rects on left side (r3-5), and no terts were replaced (Appendix S4d of the supplementary material). Individuals of unknown age included categories FPU, DPU, UPU, and UUU. Sexes were similar in all plumages. Breeding characters: Three individuals with medium-sized CP were recorded in October and April, whereas three individuals with large-sized CP were recorded in September and January. Five individuals with vascularized BP were recorded in October, November and April, whereas one individual with wrinkled BP was recorded in October.
Blue-gray Tanager (Thraupis episcopus; n =94). We captured at least two introduced races in our study site, the race quaesita from norwestern Peru and another race probably from western or central Amazonia (subspecies caerulea, major, urubambae, or coelestis). Individuals of these groups were differentiated by the amounts of blue and grey shading in plumage, and color of the upper wing covs. Besides such differences, both races exhibited similar molt patterns that are described below. FPJ: complete. FCJs in both races resembled older birds but were duller, lacked contrast between head and back, and had washed blue (quaesita) or light blue (Amazonian race) coloration on lesser covs. FPF: partial. It included most body feathers, all lesser covs, none to all median covs, and at least one to all greater covs. Moreover, some ss (s1-9), and none to some rects (1-3 inners, one outermost), but no primary covs or pp were replaced as consequence of this molt (Fig. 2). FCFs had molt limits among secondary covs (including median or greater covs), and ss. Formative secondary covs contrasted in size and appearance with the retained ones that were worn and duller. Lesser covs exhibited a blue (quaesita) (Appendix S5a of the supplementary material) or whitish coloration (Amazonian race), while median and greater covs were dark bluish (quaesita) or bluish-green (Amazonian race). No FPAs were recorded. FCAs had three generation of feathers and two molt limits as a consequence of the preformative and first prealternate molt. It replaced some body feathers including the head, breast, back, scapulars, all lesser covs, but no median or greater covs as consequence of the prealternate molt (Appendix S5b of the supplementary material). DPB: complete. DCBs had no molt limits and exhibited uniform pale gray (quaesita) (Appendix S5c of the supplementary material). or bluish grey (Amazonian race) head and un-derparts, and darker bluish gray upperparts. DPA: partial. It followed a similar replacement pattern as evidenced in FCA. DCAs had molt limits between lesser and median covs, or among median covs. First and definitive alternate feathers had a similar color pattern but glossier than the formative and basic ones (Appendix S5d of the supplementary material). Individuals of unknown age included categories UPU, UCU, and UUU. Sexes were similar in all plumages. Breeding characters: Three individuals with medium-sized CP were recorded in October, February and March, whereas one individual with large-sized CP were recorded in February. One individual with vascularized BP were recorded in February.
DISCUSSION
Molt patterns for our target species were similar to those reported for related Nearctic and other Neotropical taxa studied so far (Rueda-Hernández et al. 2018). For example, C. cruziana exhibited complete preformative and prebasic molts as shown by other columbids (Pyle 1997b, Johnson and Wolfe 2017, Bosque et al. 2018), whereas C. obsoletum, P. rubinus, C. analis, P. hispaniolensis, C. flaveola and T. episcopus exhibited partial or eccentric incomplete preformative molts and complete prebasic molts as reported in other tyrannids, thraupids and Nearctic taxa previously included among thraupids (e.g. the genus Piranga) (Pyle 1997a, b, Guallar et al. 2009, Gómez et al. 2012, Pyle et al. 2015, Díaz and Hernández 2018). These might suggest the phylogenetic conservation of molt patterns across temperate and tropical taxa (Ryder and Wolfe 2009); however, despite taxonomic affinities, there are environmental variables (i.e. abrasive habitats, solar exposure, ambient temperature) that may strongly influence molt patterns among Neotropical species (Willoughby 1991, Greenberg and Marra 2005, Kiat et al. 2019). For example, even though the presence of prealternate molts in temperate zone species of North America appear to be more common in Nearctic-Neotropical migrants than in residents, the presence of prealternate molts in resident thraupids has been suggested to be due to prolonged exposure to sunlight, especially for birds inhabiting open or scrubby habitats (Moreno-Palacios et al. 2017, Díaz et al. 2020). All thraupids studied here did also exhibit partial to eccentric incomplete prealternate molts. However, our results for C. flaveola and T. episcopus contrasted with previous studies that have suggested the existence of complete preformative molts (Johnson and Wolfe 2017) and even the absence of prealternate molts (Gómez et al. 2012) for conspecific populations in other neotropical regions. These two species are considered exotic species introduced in Lima for illegal trade possibly from Amazonia or northern Peru (Guillen and Barrio 1995). The high relative abundance of these species in various green areas of the city would suggest that these species have already found their ecological niche in the urbanity of Lima (González 1998, Nolazco 2012). Such a niche shift would not only have modified its interspecific interactions but also might have influenced variation on some of its life-history traits, including the molt patterns. Unfortunately, variation in molt patterns between urban and non-urban populations of these and other invasive species yet requires further investigation.
Timing of molt for our target species was similar to other Neotropical related taxa so far studied. In the case of C. cruziana, the absence of discrete preformative molt periods might suggest that some individuals follow their own molt cycle likely determined by fledging time, as reported for C. passerina Linnaeus, 1758 in an arid coast of Venezuela (Bosque et al. 2018). However, the tendency for individuals to molt in the dry season have been also proposed for another three ground-dove species inhabiting seasonal savannas of central Venezuela (Bosque et al. 2004). In the case of C. obsoletum, P. rubinus, P. hispaniolensis, C. flaveola and T. episcopus, these species evidenced discrete preformative/prebasic molt periods mostly during the dry season as documented for other resident tyrannids and traupids inhabiting semiarid scrubs of coastal Chile (Pyle et al. 2015). However, these species showed low molt synchrony likely influenced by the urban nature of the habitat. Despite the similarity in seasonality, P. rubinus and C. flaveola evidenced extensive molt periods compared to what have been reported in other neotropical thraupids from previous studies (Pyle et al. 2015, Moreno-Palacios et al. 2017). These extended periods can be explained by the fact that both species can reproduce throughout most of the year, based on breeding records in other urban areas of the city of Lima (González 1998, 2004, iNaturalist c2020). In the case of C. analis, more evidence is needed in order to infer molt periods for this species. Previous studies for a closely related species (i.e. Pipraeidea bonariensis Bonaparte, 1838) in a montane desert scrub at the highlands of the same region showed preformative/ prebasic molt also occurring mainly during the dry season (early April to late November) (Diaz et al. 2018). Lastly, the few instances of prealternate molts among the traupids studied here point to periods soon after the wet season and within 2-3 months before prebasic molt; however, more information is needed to establish a proper timing of prealternate molts.
We did not have enough recaptures to estimate molt duration, but we could still infer relevant information from recapture data for some of the species under study. With respect to the preformative molt, the evidence of a captured individual of C. cruziana that was still undergoing its preformative molt after 112 days of being captured for the first time (molting rate of 1.9 feathers per month, assuming no interruptions of the primary molt) opens the possibility of the existence of protracted complete molts for this species. In fact, it has been estimated that adult primary molt in other ground-dove species might take as long as 150-200 days to be completed, with a mean rate ranging from 1.5 to 2 feathers per month (Bowman 2002, Bosque et al. 2004). We also recaptured an individual of P. rubinus still undergoing its preformative molt after 141 days of being captured for the first time, a considerably longer period compared to what has been previously reported for other populations of this species in North America (Butler 2013). Given the molt extent and the timing in which in dividuals of this species were recorded undergoing preformative molts, our most parsimonious explanation is species is suspending molt probably to escape from the driest peak of the season (January to April), and then activating it again some weeks or months after this event. Such hypothesis could also be the case for C. flaveola that also exhibited long-terms of individuals undergoing the preformative molt. However, we cannot rule out the possibility that these species are exhibiting protracted preformative molts as suggested for other neotropical landbirds (Pyle et al. 2015, Moreno-Palacios et al. 2017). With respect to the prebasic molt, we recaptured individuals of P. hispanio-lensis and C. flaveola still undergoing their prebasic molts after 35 days, and from 50 to 57 days of being capture for first time, respectively. It is known so far that the duration of prebasic molts in Neotropical resident passerines can range approximately from 59 days (e.g. Volatinia ja-carina Linnaeus, 1766) to 310 days (e.g. Pithys albifrons Linnaeus, 1766) (Johnson et al. 2011, Silveira and Marini 2012, Moreno-Palacios et al. 2018), while in temperate regions the molt of resident species ranges from 42 to 105 days (Newton 1966, Lind 2001, De la Hera et al. 2009, Butler 2013). Finally, and with respect to the duration of the complete molt cycle, we captured two formative-plumaged individuals of P. rubinus after 303 and 360 days of being initially captured in their juvenile plumage. We also captured an individual of C. flaveola undergoing its definitive prebasic molt after 362 days of being captured undergoing its definitive prebasic molt from a previous molt cycle. These events corroborate that the molt cycle of Neotropical birds typically match to an annual cycle, as have also been proposed for temperate birds (North America and Europe) (Howell et al. 2003, Johnson and Wolfe 2017). Further study is needed in order to estimate average durations of the different molt stages of Neotropical birds in urban and non-urban landscapes.
The sex-specific morphometric analysis showed that females of C. cruziana, P. rubinus, C. analis, and P. hispaniolensis were smaller than males. Selected measurements showed significant differences between sexes (except for C. analis), being the wing chord the most common criteria to distinguish female from males (Table 1). Previous studies among Neotropical passerines have evidenced that significant differences in morphometric measurements (e.g. wing chord, tail length, or mass) between sexes can vary across species, but being in general males larger than females (Montalti et al. 2004); although exceptions such as the reversed sexual dimorphism in size can occur (Pyle et al. 2015). Differences in sexual dimorphism in size can be attributed to ecological polymorphism and sexual differences in niche utilization (Selander 1966, Montalti et al. 2004). Our species without sexual dichromatism, C. obsoletum, C. flaveola, and T. episcopus could only be sexed by the presence of breeding characters. Thus, sex-specific analysis for such species was not feasible due the limited number of breeding individuals (< 7 %). This added to the relatively small number of captured juveniles, might suggest that our urban study area does not constitute a main breeding site for these species, or there have been occurred misidentifications of non-breeding individuals among breeding individuals. Similar proportions of captured breeding individuals for some commonly studied species have been reported in another banding station at a nearby urban location (8 km SE from our study site) (Salvador pers. comm.), which makes less likely the possibility of having committed misidentification of non-breeding individuals. It is also important to note that the proportion of captured breeding individuals might be subjected to several other factors, such as the sample effort, habitat size and structure, and niche utilization, to cite some examples (Dunn and Ralph 2004).
In conclusion, except for C. flaveola and T. episcopus, our study of urban birds did not show variation in molt strategy and extent compared to what has been reported for the analyzed species from previous publications. Molts occurred mostly during the dry season, but with low synchrony among species likely influenced by the urban nature of the habitat. Additionally, some species exhibited extensive molt periods potentially explained by the fact that these species can breed throughout the year and/or the existence of suspended or protracted molts. We emphasize the importance of increasing our knowledge about molt patterns and morphometrics for a better understanding of the ecology and evolution of urban birds. We also recommend making studies evaluating the effect of urbanization upon other bird life-history traits such as abundance, body condition, longevity, and survival rate of these species.















