INTRODUCTION
Extensive livestock activity is considered one of the main drivers of transformation and loss of biodiversity in the tropical rainforest (Etter and Zuluaga c2018). The rainforests have an estimated area of 713 million hectares in the entire tropics (Keenan et al. 2015) and are considered as one of the most diverse ecosystems on the Earth. Despite, the tropics are experiencing an accelerated loss of its natural rain forests, close to 11 % between 1990 and 2015. Particularly in Colombia, since the 1990s, large areas of natural forest have been converted to pasture for livestock (Etter et al. 2008, Armenteras et al. 2018, González Arenas et al. 2018); 6 147 000 hectares have been deforested, at an annual rate of 199 285 hectares between 2015-2017. Currently, only 58 714 000 hectares of natural forest remain (FAO c2020). The biodiversity, the stability of the global climate and livelihoods are seriously threatened, especially in the tropics. In Colombia, almost half of the ecosystems present conditions that threaten their integrity, and therefore their capacity to provide services to society (Etter et al. 2018). Despite the negative environmental impacts generated by livestock farming, this is a fundamental activity for the country's economy. This is the case of the municipality of Caucasia, which suffers from serious environmental problems generated by extensive cattle raising and mining. There are no reserves or protected areas that allow the conservation of biodiversity, and the remaining forests only represent 4 % of the territory (Alcaldía Municipal de Caucasia c2020: 2020-2023).
In recent decades, the SPS have been promoted as alternatives to reduce the environmental impact and pressure on forests, since they include a combination of multipurpose trees with improved pastures (Murgueitio et al. 2011, Calle et al. 2013, Chará et al. c2019). In these ecosystems, one of the key organisms for their ecological functions are the dung beetles, the main invertebrates involved in the removal and disintegration of dung in grazing areas. By manipulating and relocating organic matter, the dung beetles fertilize and improve the quality of the soil and prevent the reproduction of parasites that affect livestock (Nichols et al. 2008, Giraldo et al. 2011). Some studies have shown that SPS restores fauna and promote conservation of this group of insects (Arellano et al. 2008, Giraldo et al. 2011, Arellano Gámez et al. 2013, Montoya-Molina et al. 2015).
In this work, we evaluated how the transformation of a pasture to a SPS alters the diversity of dung beetles in a cattle farm in Caucasia municipality.
MATERIALS AND METHODS
Study area
The study was conducted in a livestock farm in the municipality of Caucasia, northern of Antioquia department (8°04'37'' North, 75°10'54'' West), over the central branch of the Colombian Andes, at 50 m height. As a tropical rainforest, the seasonality is determined by the regime of precipitation (Hoyos et al. 2018, Hoyos et al. 2019). At this location, we find a clear unimodal cycle of precipitation with two distinguishable seasons. The dry season extends from December to March with the minimum value in December. The rainy season extends from April to November with the maximum in June. Average meteorological conditions recorded by IDEAM (Instituto de Hidrología, Meteorología y Estudios Ambientales) during the sampling period were temperature of 28 °C, annual rainfall of 2445 mm, relative humidity of 75 %. The vegetation corresponds to Tropical Rainforest according to Holdridge classification. The terrain is moderately undulate in 85 % of its extension. In the hollows of the hills, some remnants of natural vegetation are preserved, as well as along the streams. In general, the landscape is homogeneous, dominated by conventional pastures with Brachiaria decumbens Stapf B, Brachiaria humidicola (Rendle), Andropogon gayanus Kunth, and Hyparrhe-nia rufa (Nees) as main pastures and with a few dispersed trees and continuous cattle grazing. Seven months before the investigation begin, six one-hectare plots of traditional pasture were transformed into a SPS. Three of the plots were selected, located in the middle of the traditional pastures, connected with small remnants of natural vegetation. Those plots are not joined to forest fragments where the samples were taken. The SPS ecosystem includes a combination of fodder plants, such as grasses and leguminous herbs, with shrubs and trees like Crescentia cujete L., Gliricidia sepium (Jacq.) Walp, Tithonia diversifolia Hemsl A. Gray, Gmelina arborea (Jacq.) Merr, Enterolobium cyclocarpum (Jacq.) Griseb, Albizia saman (Jacq.) Merr. (Height ranging between 0.5 and 1.5 m); occasional cattle grazing occurred there. Existing forest consisted of a secondary forest patch of less than eight hectares, surrounded by conventional pastures.
Sampling methods
Sampling was conducted bimonthly between April 2018 and January 2019 for a total of five in different seasons: dry (January), wet (June and August), and transition (April and November). In forest, SPS, and pasture, three transects were established, separated by at least 200 m, with five pitfall traps placed every 30 m per transect. The pitfall traps (Cultid-Medina and Medina 2015) were baited with 50 gr of a mixture of porcine and human excrement in a 3:1 proportion (Marsh et al. 2013). The small size of the SPS plots, 1 ha, led to a rather close distance between the traps. This number of traps and spacing was defined, based on Montoya-Molina et al. (2015) and Murillo-Ramos et al. (c2016). In each sampling 45 traps were left in the field for 48 h and were re-baited every 24 h. The total sampling effort in each ecosystem was 75 traps/48 hours. The collected specimens were fixed in 70 % ethanol for posterior identification and deposited at the Entomological Collection of the University of Antioquia (CEUA). During the handling of traps and samples, the necessary prophylactic measures were taken to avoid any type of contamination. To identify the specimens, we used the keys of (Medina and Lopera-Toro 2000, Genier and Kohlmann 2003, Peraza and Deloya 2006, Vaz-De-Mello et al. 2011, Solis and Kohlmann 2013, Sarmiento-Garcés and Amat-Garcia 2014). In addition, the specimens were also compared with those present in the CEUA and the Francisco Luis Gallego Entomological Museum of the National University of Colombia in Medellin (MEFLG). This collection has a standardization of morpho-species with a unique code, following the guidelines proposed at national level by the Instituto de Investigación de Recursos biológicos Alexander von Humboldt.
Data analyses
In the sampling design the three transects of each ecosystem are considered as replicas. Diversity was calculated as the effective number of species of order q (qD) based on Hill numbers (Jost 2006). This measure of diversity allows us to compare the magnitude of differences between communities. Diversity (qD) comprises diversity of order zero (0D) or species richness, diversity of order one (1D), or the exponential of Shannon's entropy index, and diversity of order 2 (2D) or the inverse of the Simpson's index. The observed and estimated true diversity indices (0D, 1D, 2D) were calculated using Hill numbers. True diversity considers the sample coverage as a measure of completeness of the inventory and represents the proportion of the community represented in the species in the sample.
The sample coverage value (Cn) fluctuates between O and 1; Cn « O indicates minimum completeness and Cn « 1 indicates maximum completeness (Chao and Jost 2012). The sample coverage and the estimation of the diversity orders were calculated with the SpadeR software (Chao et al. c2015). The magnitude of the difference (MD) was expressed as a percentage: % MD = 100 - [(qD sample 2_ x l00)/qD sample 1)]. Thus, when sample 1 is more diverse than sample 2, MD will be positive (+% MD); otherwise, it will be negative (-% MD). Therefore, % MD will vary from o (no change in the diversity of a given pair of samples) to (±) 100 % (completely different diversity) (Cultid-Medina and Escobar 2016). Values for diversity qD were calculated for each ecosystem and each season.
Rank abundance graphs, also known as Whittaker curves, were used to compare abundance patterns and species evenness among ecosystems. Non-parametric multi-dimensional scaling (NMDS) based on Bray-Curtis dissimilarity measures were used to visualize relationships between ecosystems. Statistical significance was determined between groups using a one-way ANOSIM using the PAST 3.x software (Hammer et al. 2001).
The structure of the functional groups was evaluated in terms of ecological characteristics: i) according to their dung-processing behavior, such as paracoprids or tunnellers (T) endocoprids or dwellers (D), and telecoprids or rollers (R) (Hanski and Cambefort 1991), and ii) in terms of body size, small if < 10 mm or large if > 10 mm. This arbitrary classification has been used in previous studies with dung beetles (Escobar et al. 2008, Cultid-Medina and Medina 2015). The relative abundance of each functional group in each ecosystem was calculated to directly compare the communities.
RESULTS
Sample coverage was 99 % for the area and for each environment in the different seasons, indicating that sampling effort was adequate (Chao and Jost 2012). In total, 7446 beetles were collected and grouped into twelve genera, fifteen species, and seven morphospecies (Table 1). 68 % of species were captured in forest, 68 % in the SPS and 45 % in the pasture. The genera Scatimus, Trichillidium, and Canthidium were captured only in the forest, whereas the other genera were present in all ecosystems. The most abundant species were Onthophagus marginicollis (n = 4664, 62 %) captured mainly in the pasture and Can-thon aequinoctialis (n = 1398, 18.8 %) collected only in the forest. The abundances of the remaining 20 species were low and comprised 20 % of the community.
Table 1 Number of individuals collected for each species of dung beetle in each habitat in a tropical rainforest in Caucasia Colombia.
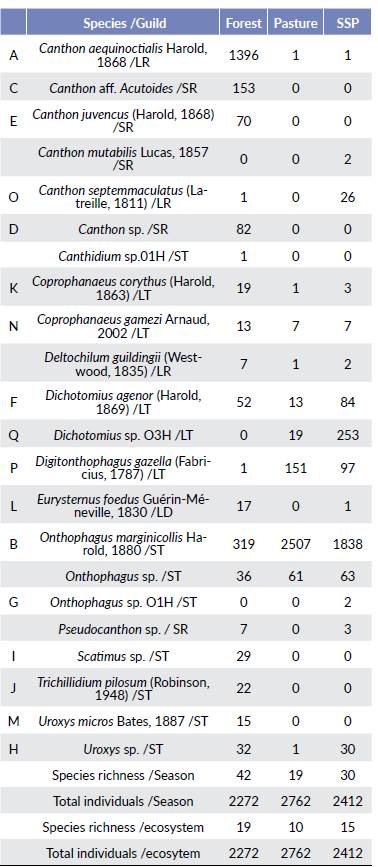
ST = small tunneller, LT = large tunneller, SR = small roller, LR = large roller, LD = large dweller, Tran = Transition, SPS = Silvopastoral systems
The rank abundance curves indicate that the number of species decreased from the most preserved site, the forest with thirteen species, to the most disturbed site, the pasture with five species (Fig.1). Species evenness was different between ecosystems; it was highest in the forest, whereas the lowest was observed in the pasture. The forest community was largely composed of species belonging to the Canthon genus (top portion of the curve), which were not observed in the SPS or pasture. C. aequinoctialis (A) was the most dominant species, followed by O. marginicollis (B). Even though O. marginicollis was common across all land use treatments, the few forest species that were also recorded in the SPS and pasture were at the tail of the curves. The relative abundance of O. marginicollis increased notably in the pasture and SPS, where it was the most dominant species. Even though Digitonthophagus gazella was not recorded in the forest, this species was the second and third predominant species in the SPS and pasture, respectively.
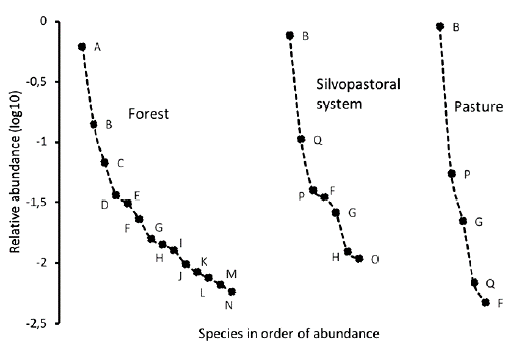
Figure 1 Comparative rank-abundance curves between ecosystems. Letters for each species are the same as those given in Table 1
The forest was the only ecosystem with exclusive species: C. aff. acutoides, Canthon juvencus, Canthon sp., Scati-mus sp., Trichillidium pilosum, and Uroxys micros, while nine species were recorded across all the three ecosystems, and ten were common in the SPS and in the pasture (Table 1). That is, all the registered species of the pasture were found in the SPS. The forest beetle community is distinct from the pasture and SPS communities (Fig. 2), the ANOSIM test showed differences between Forest-pasture (R = 0.464, P < 0.001) and between forest-SPS (R = 0.250, P = 0.001), the pasture and SPS communities overlapped and did not show statistic differences (R = -0.019, P = 0.5).
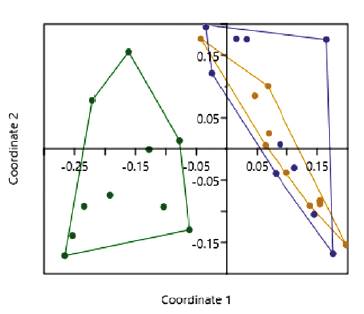
Figure 2 Non-parametric multi-dimensional scaling (NMDS) analysis of the dung beetle across ecosystems. Each dot represents a sample. The colors represent: forest (green), pasture (brown), and SPS (blue).
Five functional groups were detected in both the forest and the SPS, while only three were recorded in the pasture (Fig. 3). Three species of large rollers dominated the forest (61 %). Compared to pasture, the dominance of small rollers decreased in the SPS by 13 %, the proportion of large tunnellers increased 16.8 % and large rollers 1 %. In addition, the wealth in each guild increased by one or two species, except of the large diggers where the number was equal.
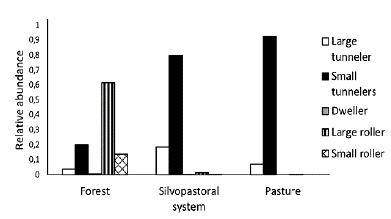
Figure 3 Relative abundance of the five functional groups of dung beetles, large tunnellers, small tunnellers, large rollers, small rollers, and dwellers, in different environments.
Diversity (qD)
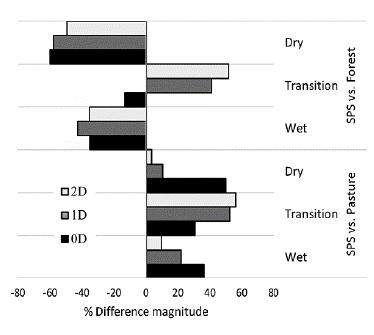
When all climatic periods were included, the diversity in the SPS (°D = 15, 1D = 2.57, 2D = 1.68) was greater than that in the pasture (°D = 10, 1D = 1.52, 2D = 1.20) and less than in the forest (°D = 19, 1D = 4.40, 2D = 2.47). The calculation of the magnitude of the differences (MD) indicates that the SPS presents a species richness 33 % higher than the grassland, 48 % and 28 % higher in diversity 1D and 2D respectively. With respect to the forest, the SPS reached 79 % of the species richness (0D) and 58 % of the forest 1D diversity; conversely, the pasture only reached 53 % of the richness and 34.5 % of the forest's diversity 1D.
Along all periods, the diversity (0D, 1D, 2D) in SPS was higher than in pasture and lower than in forest (Fig. 4) The only exception took place during the transition period, when the SPS was the most diverse in terms of 1D and 2D. In the pasture and in SPS the lowest values of diversity qD were presented in the dry season. The magnitude of the SPS vs pasture differences shows that the SPS was 50 % richer in species than pasture in the dry season; in the other seasons, at least 30 % richer in species (Fig. 4). For 1D and 2D, the SPS was almost 50 % more diverse than pasture in the transition period, in the other periods the magnitude of the differences was less than 20 %. The greatest difference in diversity with respect to the forest (SPS vs Forest) occurs in the dry period, where the SSP reaches 40 % of 0D, 42 % and 51 % of the diversity, 1D and 2D, respectively, while the pasture reaches lower percentages in all orders of diversity (20 % of the wealth, 38 % and 49 % of 1D and 2D, respectively).
Discussion and Concluding Remarks
The species recorded in this study represent 6 % of these found in Colombia (Medina et al. 2001), and most of the species (70 %) are also commonly found in the Caribbean dry forest (Noriega et al. 2013). Comparing the richness of species with other investigations of livestock areas, in Caucasia four species were not recorded than in the tropical rainforest in Córdoba (Murillo-Ramos et al. c2016), and twelve species less than in Cesar and Atlántico (Montoya-Molina et al. 2015, Rangel-Acosta and Martínez-Hernández 2017). Species richness was lower, likely because of the simplicity of landscape and grasslands that can limit the presence of certain groups of beetles. The evaluated forest covers less than 8 hectares and is strongly influenced by the surrounding pastureland, in addition cattle eventually enters the forest affecting its structure. However, the forest constitutes the only refuge for beetles. A 27 % of the species were only found there, highlighting the importance of these environmental zones to maintain the connectivity with other areas for the conservation of these species.
In the Neotropics, the beetle community is modulated by arboreal coverage (Halffter and Arellano 2002) and it has been concluded that the lower the degree of structural diversity of the vegetation, the lower the species richness and the greater the dominance of a few beetle species (Estrada et al. 1998, Nichols et al. 2007). Among the ecosystems studied, the forest is the only one with tree cover and structural diversity of vegetation, in the SPS these characteristics are incipient because the trees have not grown enough (trees have sizes less than 1.5 m), and in the pasture the tree cover is almost null. This explains why the forest beetle community is the most species-rich, statistically diverse, and different with the pasture and the SPS, and in turn, why these last two communities are similar to each other, with a great dominance of a few species (even the dominant species is the same, O. marginicollis). The abundance of these species is associated with the habitat's characteristics: open areas, with high an-thropic disturbance (Cultid-Medina and Medina 2015). In the SPS, all the species of the pastureland are also present. However, there were modifications in the relative richness and abundance of species that led to a lower dominance of O. marginicollis, greater equity in the community, and in general greater diversity as will be discussed later. In habitats with little or no tree cover, in addition to their low species richness, the distribution of abundance in the trophic guilds is dramatically altered, with a hyper-abundance of small-bodied species that are suggested to be more resistant to habitat disturbance (Nichols et al. 2007, Escobar et al. 2008, Gardner et al. 2008). This fact is clearly observed in the pasture community and, to a lesser extent, in the SPS where there is a slightly different trend; the abundance of small tunnellers decreased by 13 % and they colonized species of different guilds and body sizes, big tunnellers like Dichotomius agenor, Dichotomius sp.03H and big rollers like Canthon septemmaculatus, this last guild is considered as a positive indicator in the natural restoration processes (Cultid-Medina and Medina 2015). The integration of the big tunnellers and rollers, with small-bodied species adapted to open areas, promotes the relocation of the excrement with the benefits that this entails for the environmental sustain-ability in the cattle areas (Escobar et al. 2008, Nichols et al. 2008, Giraldo et al. 2011). The large-bodied beetles can tunnel into compacted soils and therefore enhance the work of the smaller beetles that depend on the terrain conditions for nesting (Giraldo et al. 2011). Finally, it can be said that the transformation of the pasture into an SPS system expanded the guild of dung beetles.
True diversity suggests that SPS is more diverse than pasture, which confirms the findings of previous researchers (Arellano et al. 2008, Giraldo et al. 2011, Montoya-Molina et al. 2015, Gomez-Cifuentes et al. 2019). When comparing the magnitude of the change in diversity, with other SPS in the Caribbean (Montoya-Molina et al. 2015), the increase was greater and in a shorter time (17 months vs. three years). While in the SPS the 1D diversity was 2.1 times greater than the pasture (in the Caribbean was 1.57 times). In terms of species richness, the SPS reaches 79 % of forest richness and is 33 % richer than grassland, in contrast, in the Caribbean it reached 61 % of forest richness and is 36 % richer than grassland. Probably the greatest increase of diversity in the present research is related to the lower species richness in the forest, which makes the SPS reaches a very high percentage of its richness in a short time. In addition, due to rainfall conditions, restoration of lowland tropical rainforest is probably more accelerated than in dry forest, in such a manner that the diversity increases in shorter time. In the processes of natural restoration, it is expected that in a short period (one to three years) the restored systems will reach between 25 % and 50 % of the beetle diversity of the reference system and the colonization of species typical of the forest such as large and small predators, will occur (Cultid-Medina and Medina 2015). The SPS reached in all expressions of diversity qD more than 55 % of the diversity of the forest and the large roller tend to increase slightly. However, the results should be treated with caution when comparing with the forest because it does not meet the proposed requirements for a reference ecosystem, due to its area (Cultid-Medina and Medina 2015) and, consequently, the increase in diversity could be overestimated. Therefore, the comparison of the SPS with the grassland can better reflect the changes in diversity. To verify the continuity of the trend observed in the present results, it would be appropriate to carry out continuous monitoring of the sites. The SPS tends to be more diverse than pasture in all seasons, mainly in the transition season, likely because temperature and soil moisture are more favorable than in the dry period; other authors have found similar results in areas of cultivation (Rangel-Acosta and Martínez-Hernández 2017). In the dry season the magnitude of change in diversity was smallest with respect to abundant (1D) and dominant species (2D), reflecting that the SPS is also affected during this season, however the reduction was not as drastic as in the pasture, being this one of the most important aspects to highlight in this work. It could be thought that in the SPS the effects were less severe because there is more plant coverage on the ground. Leaf litter and plants help maintain humidity and create favorable conditions that allow beetles to remain even when rainfall was zero. It has been found that soil cover (Montoya-Molina et al. 2015) and soil moisture (Sowig 1996) are variables that affect beetle richness and diversity. On the other hand, in pasture, the soil cover was almost completely lost during the drought, due to the dehydration of the grasses. In compact and dry soils, it is difficult for beetles to dig their tunnels (Janzen 1983, Escobar 1997) and in the pasture more than 90 % of the community corresponds to small tunnellers, so most of them are exposed to high temperatures and dehydration. In addition, the availability of food is reduced by low rainfall, the sun dries out the excrement, and the beetles cannot use it (Halffter and Halffter 1989). At this season, only O. marginicollis and D. gazella, species that are slightly abundant. It is noteworthy that in both pasture and SPS the only species that increased its abundance during the drought was D. gazella, probably because of its Indo-African origin and its biological characteristics. As an invasive species (Amat-Garcia et al. 2009) probably has greater capacity to adapt to extreme conditions, making it more resilient than other species. In the forest, unlike the grassland and SPS, the diversity was not affected by the drought. Tree cover maintains adequate microclimate conditions for beetles and food availability, important factors that modulate community structure (Halffter 1991, Hanski and Cambefort c1991). Similar results are described for the semi-deciduous forests of Mexico (Andresen 2005). It is expected, that as structural diversity and tree cover increase of the SPS with the growing trees, the effects of drought will be even more mild and the diversity increase.
The transformation of traditional extensive livestock farms into SPS led to changes in the composition and structure of the beetle community, making the SPS more diverse than the pasture environments in all seasons even in the dry period, when conditions are most adverse for beetle survival. This research suggests the importance of transforming conventional pastures into silvopastoral systems as a measure to preserve and increase the diversity of forest dung beetles and their relations with other organisms that are vital in diminishing the environmental impact in cattle areas of the country.