Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales
Print version ISSN 0370-3908
Rev. acad. colomb. cienc. exact. fis. nat. vol.36 no.140 Bogotá July/Sept. 2012
ZOOLOGÍA
ABSTRACT
A history of some details the author's research in eastern Colombia is presented for the last 27 years since 1977 in Vichada, Vaupés, Amazonas and most recently in Meta up to the present. Some comments are made about each research project in the context of primatology and some hypotheses and ideas of the author are presented about species, populations and other groups of mammals in the country. Especially discussed are aspects of Cebus albifrons and its relation to Sapajus (=Cebus) apella (a new taxonomic change) and the primates Calli cebus lugens, Alouatta seniculus, Lagothrix lagothricha, Cacajao melanocephalus, Callicebus caquetensis and Callicebus ornatus.
Key words: primatology, Colombia, Thomas Defler
RESUMEN
Se relata algunos detalles de la historia de las investigaciones del autor en el oriente de Colombia durante 27 años de trabajo en Vichada, Vaupés, Amazonas y más recientemente en el Meta, desde 1977 hasta el presente. Se hace comentarios sobre cada investigación, ubicándola en el contexto de la primatología y se destacan algunas hipótesis e ideas del autor en cuanto a la naturaleza de las especies, poblaciones de primates y otros grupos de mamíferos en el país. Especialmente se discute aspectos de los primates Cebus albifrons y su relación con Sapajus (=Cebus) apella (nuevo cambio taxonómico) , y los primates Callicebus lugens, Alouatta seniculus, Lagothrix lagothricha, Cacajao melanocephalus, Callicebus caquetensis y Callicebus ornatus.
Palabras claves: primatología, Colombia, Thomas Defler
Introduction
It seemed the culmination of my life to come to Colombia 36 years ago in order to work as a Peace Corps volunteer primatologist with the former INDERENA. The choice of Vichada as my first objective was a long process of consultation with professor Jorge Ignacio Hernández-Camacho, my mentor and my last professor; we talked about many aspects of Colombia, including field work in primatology, possible species present, research tactics, local relations and personal security, even before we decided upon the Faunistic Territory of El Tuparro in eastern Vichada (declared a National Park in 1982). In December, 1976 I made a preliminary trip to El Tuparro and the area seemed to be a magnificent point to begin my Colombian career, even though my original plan had been to work in the Colombian Amazon. But this would come later.
El Tuparro, the Llanos Orientales and Vichada
At the time there was not much information about primates in El Tuparro. Profesor Hernández-Camacho suggested a long list of possible primates, but nobody had reconnoitered that very large territory for primates; there were many regions that had not been visited by park officials. Only some primate collections by INDERENA employees had identified four species in the western border, defined by Hormiga Creek. These were Sapajus apella (earlier known as Cebus apella, but separated from Cebus by Lynch-Afaro et al. 2012; Solari et al., en press), Callicebus torquatus lugens, Alouatta seniculus; and Cebus albifrons had been identified in the eastern regions of the reserve.
Two aspects had called themselves to my attention when I first arrived in El Tuparro: Cebus albifrons was a very poorly-known species at the time and Sapajus apella (known to be often sympatrically distributed with Cebus albifrons in the Amazonian forest to the south) was in fact parapatrically distributed with relation to Cebus albifrons---that is one species seemed to strictly replace the other species in El Tuparro. Because of these facts I decided to study the ecology and behavior of Cebus albifrons and to learn more details about the distributions of these two species that had been considered before to be congeneric (Defler, 1979a, 1979b, 1980, 1982, 1985a, 1985b). The group that I studied lived in a forest that clothed a prominent inselberg (monadnock, bornhardt, pan de azucar) close to the administrative center, overlooking the Orinoco River. In this region, inselbergs were a common feature of the landscape, great gneiss granitic islands of rock surrounded by savanna. The runoff from rain usually maintained a substantial forest around its base, where three species of monkey roamed.
To me at that time, the most important ecological questions were densities and species richness in the east of the country, as well as actual distributions, since there existed and still exist so many unknown facts about El Tuparro. Most of these problems I discussed with professor Hernández-Camacho in our nocturnal Bogotá chats over cuba libres. Many unknowns had already been posed by him in his very important publication with the North-American veterinarian Robert W. Cooper, on the non-human primates of Colombia (Hernández-Cooper & Cooper, 1976), the primate article most often cited in the field of primatology at that time. I came to believe that the ecological relations between Cebus (Sapajus) apella and Cebus albifrons in the eastern parts of the country needed to be elucidated, especially since these relationships changed to the south in the closed-canopy forest.
Making life more interesting, a healthy population of the giant otter Pteronura brasiliensis existed within the Territorio. This was a mammal species that had always interested me. So I resolved to include a census of its presence in the region during the various years that I traveled the rivers of El Tuparro studying the distributions of Sapajus apella y Cebus albifrons and other primates in heretofore unknown corners of El Tuparro. (Defler, 1985a,b).
Callicebus torquatus, Alouatta seniculus, Aotus sp.
In 1978 I moved to the western side of the Territory in order to study primates in a type of forest that I hadn't known before, the typical gallery forest of the llanos, growing along Hormiga Creek. This forest contained populations of Callicebus lugens (known as C. torquatus at that time) as well as populations of Alouatta seniculus, Aotus sp. and Cebus (Sapajus) apella.
I was able to gather basic ecological data on Callicebus torquatus, including densities, home range sizes, group sizes and other details of these interesting monkeys. Parallel to this effort I added to my knowledge of Alouatta seniculus, a very widely distributed Colombian primate but of variable home range and group size. These data became valuable when I was able to compare the home ranges and group sizes of the species across several forest types, thus suggesting a preferred habitat for this species (more on this below). I collected the same type of information on the Cebus (Sapajus) apella of the forest.
In Tapón I experimented with techniques of censusing primates and I clarified some details that should be used when censusing. This seems valuable when we consider the great effort that has been made in censusing primates throughout South America (Defler, 1983, Defler & Pintor, 1985). In 1982, while I was working on the western part of El Tuparro,the Territorio Faunístico El Tuparro was declared a Parque Nacional Natural.
Having calculated densities for various primate species throughout El Tuparro, I decided to pursue as a long-time project the calculation of primate densities in a transect from northern Vichada to southern Amazonia. This project was pursued intermittently during the next twenty years and the results of which I now publish in this journal (Defler, en prensa). Census sites included the source of the Bita river in Vichada, the two extremes of El Tuparro, Lake Taraira in Vaupés, the Puré River (located inside PNN Río Puré, Amazonas) and the Purité River beside the Amacayacu National Park, Amazonas (Fig. 1).
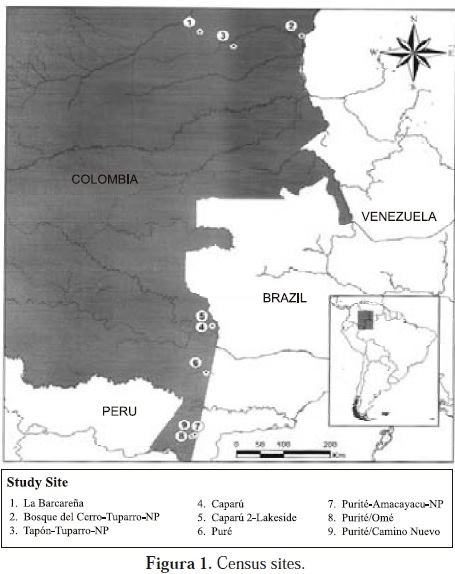
The basis of these transect censuses from the Llanos Orientales to the Amazon allow three hypotheses to be tested.
1. According to censuses of other organisms, including primates in other places outside of Colombia, primate richness should increase inversely with a decrease in latitude (Terborgh, 1992; Huston, 1994; Rosenzweig, 1997; Peres & Janson, 1999).
2. Some of the highest species richness known should be found in the exuberant forest of the Colombian Amazon since Colombia is the country with the highest species richness of birds (Hilty & Brown, 1986; Renjifo et al., 2002; Stevenson, 2001)
3. According to variable density results from low to high densities, the data on densities should provide indications on preferred habitats for a species, accepting that high density habitats are preferred habitats (Johnson, 2007).
Methods
Vichada
Using al azar observational methods (Altmann, 1974) I collected data on all aspects of Cebus albifrons in a forest surrounded the Cerro Rocoso in El Tuparro as described in Defler (1979a,b).
Using observations of Alouatta seniculus I calculated densities and group make-up of groups of Alouatta (Defler, 1981). In Tapón gallery forest I collected demographic data on Ca llicebus torquatus as described in Defler (1983). I collected al azar data on Sapajus apella during several years (Defler, 1982) and experimented with census techniques, as described in Defler and Pintor (1985). I developed a five kilometer trail and did strip census of primates in a forest along an upper tributary of the Bita River in 1985 (Defler, in press). I collected population data on the Pteronura brasiliensis of El Tuparro, using a small unmotorized canoe traveling downriver (Defler, 1982, 1984, 1986).
Methods used for studying Cebus albifrons can be found in Defler (1979a, 1979b), for the distribution and state of the population of Pteronura brasiliensis in Defler (1982), for the distribution of Cebus (Sapajus) apella and C. albifrons in the región of El Tuparro in Defler (1985), for densities of Alouatta seniclus en El Tuparro en Defler (1981) and for methods of censusing primates en El Tuparro in Defler & Pintor (1985).
Densities in El Tuparro were determined via direct counts since the study forests were clearly delineated from the savanna, allowing specific areas to be covered [Defler & Pintor, 1985]. In the Hato La Barcareña in northern Vichada calculations were made by repeated censuses along a 5 km trail, calculation of perpendicular distances to the groups observed and calculation of a detection distance for each species (Robinette et al,1974; Defler & Pintor, 1985; Peres, 1999).
Methods used at the Estación Biológica Caparú in Vaupés for studying the general ecology of Lagothrix lagothricha can be found in Defler (1987, 1989), for the time budget of Lagothrix lagothricha Defler (1995), for the diet of Lagothrix lagothricha in Defler y Defler (1986), and for the use of space and for locomotion in Defler (1996) and in Defler (1999). Additionally, study methods for the Cacajao melanocephalus research can be found in Defler (1999, 2001).
Research methods on density of primates have been described in various places; my method involved repeated censusing of 4-5 km transects from the edge of the lake into the forest, where 264 km were accumulated and where the group's numbers of individuals and distance perpendicular to the trail were registered. Various methods of calculating density were applied; in the case of Sapajus apella there were sufficient observations to be able to use the program DISTANCE (Peres, 1999a; Defler, en prensa).
Vaupés
After five years of work in Vichada, I developed a research plan to study the ecology of Lagothrix lagothricha and Cacajao melanocephalus in the Colombian Amazon. I established myself in a station that I developed a kilometer north of the shore of the unpopulated Lake Taraira in southern Vaupés, this the most extensive river-lake found in the Colombian Amazon (Defler, 2009). During the 16 years spent at this station my wife and I received many primate orphans that were sent to us by INDERENA and by prívate citizens who charged us with an attempt to reintroduce the primates to the forest. Also, during this time a series of primate censuses were accomplished during 2 ½ años.
The development of the Estación Biológica Caparú (1°04'25.65"S, 69°31'15.71"W) for Amazonian biological research was a personal plan dating back to 1982, impelled by my curiosity about the primate species Lagothrix lagothricha and Cacajao melanocephalus, two species that at the time were very poorly known. With the help of two Yucuna Indigenous men whom I had known from a previous trip that I made to the Mirití-Paraná to reconnoiter the Amazon in 1981, I identified a region in southern Vaupés department with these two species and six other species or primates (Aotus vociferans, Saimiri sciureus, Cebus albifrons, Sapajus apella, Callicebus lugens, Saguinus leucopus). The area included the river-lake Lake Taraira (1°08'35.55"S, 69°29'21.63"W, 56 msnm), a 24 km long ancient cut-off meander of the lower Apaporis river. The area's topography was made up of a complex of igapó (seasonably flooded forest with black water), Pleistocene river terrace and PlioPleistocene hills (Carvajal-L. et al, 1976; Palacios et al., 2009).
The Apaporis River shows evidence of tremendous floods at the end of the Pleistocene when the glaciers melted and sent extreme volumes of water down the river courses of eastern Colombia. These floods formed extensive river terraces about 11-15 m above the average high water marks of today (Van der Hammen et al. 1992a, 1992b, Carvajal- L. et al, 1976; Ibarra et al., 1976). Currently these ancient river terraces are covered by high and distinctive forests, particularly of Oenocarpus bataua, Euterpe sp. y Mauritica flexuosa and trees important to Cacajao and Lagothrix such as the carquero (Eschweilera sp.) and the very tall yetcha (Micrandra spruceana) (Carvajal-L. et al, 1976; Ibarra et al., 1976; Defler & Defler, 1996; Rangel-Ch. et al, 2008).
I studied a group of Lagothrix lagothricha from 1984-1989), especially for spatial use, time budget and diet as described in Defler (1987, 1989a, 1989b, 1990a, 1995, 1996a, 1996c, (1996). During 1994-1998 I studied the ecology of Cacajao melanocephalus around Lake Taraira (Defler, 1991, 1999, 2001; Barnett et al., in press). I also carried out a series of primate censuses during the first 2 ½ years of residence.
Amazonas
After leaving Vaupés in 1998 because of pressure from the guerrillas, I searched for a place in the upper Purité River beside Amacayacu National Park in order to continue primate research.
Along the Purité River on a hill site above the flooding river and outside the park I developed a new research site, the Estación Ecológica Omé. Posterior archaeological research by professor Marcote of the Instituto de Ciencias Naturales determined this was an ancient inhabited site of indigenous people dated from 670-1350 BP (+/50 years) (3°32'07.92"S, 69°53'26.75"W - 104 msnm) (Morcote Ríos, 2008) . I selected the area because of its isolation from negative influences in the region, but it proved to be so isolated that it has been difficult to access because of the necessity of traveling various days on the Amazon and Ica-Putumayo rivers, then traveling upriver on the Purité river to enter Colombian again. This station was developed during brief two month visits that have allowed a large series of primate censuses and some research on the use of space of the primate Callicebus torquatus lucifer (=C. lucifer according to other authors)(Defler, in press).
Generally when moving from place to place all observations of primates were registered using the most exact coordinates possible, given the maps available. It was not until 1995 that it was possible to begin registering observations using the new GPS system.
Results and discussion
Since Cebus albifrons had been observed in the field only very briefly before beginning my study, any new natural history observation was valuable (Defler, 1979 a,b). I first realized that the groups were very large in comparison with the numbers reported in the literature. My study group was made up of 35 animals and the neighboring groups were similar, illustrating perhaps a type of ecological release (Tabla 1) (where in the absence of some competitors the densities rise (according to Peres & Dolman, 2000) or "ecological release", (defined by Terborgh & Faaborg, 1973, as "an expansión or increase in density, distribution or commonly observed behavior when a species is observed under different intensities of competition"). The putative effects of competition with S. apella have been implicated in limiting Cebus albifrons group sizes.
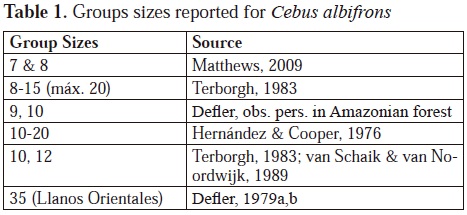
Studying this species in a forest limited by savanna was a great help, since the limits of the home range for any group are much clearer in comparison with studies in continuous forest without border limits. The home range in El Tuparro was about 120 ha for the defended territory of the group, the first territory that had been calculated for this species. Ecological densities were the highest reported in the literature with much lower densities in closed canopy forest.
Cebus albifrons and Sapajus apella
I wanted to know more about the ecological interaction that Cebus albifrons has with S. apella, which is evidently the strongest competitor of C. albifrons. A question that I posed was "What type of interaction do these two species have in their contact zone in El Tuparro?" where they are essentially parapatric in distribution, where one species replaces the other. So I decided to study the distribution of the two species in the región and undertook a series of voyages by canoe, motor bike and on foot to see if the distribucions were truly parapatric. The result of this was more than 2000 km of travels (1500 km by canoe), where I found a strict separation of the two species in the north along the Tomo River, but in the south of the park, especially along the Tuparro River, the separation was not so clear, especially on the left bank of the Tuparro where I found groups of Cebus albifrons integrated into a population of Sapajus apella (sympatry) and ultimately lower down on the Tuparro river I found a forest with the two species sympatrically distributed just before the beginning of a pure Cebus albifrons population to the east. Along the same river C. albifrons used flooded forests and Sapajus apella (observed closest to the river on the south bank), did not use flooded forests (Defler, 1985a). Fig. 2.
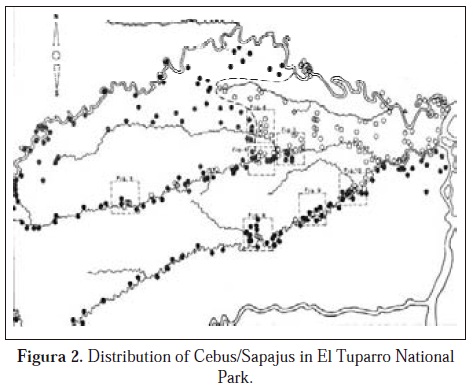
The population of Cebus albifrons studied in El Tuparro is isolated from the principal population that extends southward from the south bank of the Vichada River. With field work in that region, It was possible to prove that Sapajus apella and Cebus albifrons are sympatric south of the Vichada River and that Cebus albifrons exists in very low densities and in groups much smaller than those in El Tuparro (Bennett et al. 2001; Terborgh, 1983; Haugaasen & Peres 2005, 2009; Defler, 2010; Lynch-Alfaro et al, 2012).
Lynch-Alfaro et al. (2011, 2012) sugggest the "gracile" Cebus (i.e. Cebus albifrons, C. capucinus and C. olivaceus) originated in the Amazon while the "robust" Cebus (that is the Sapajus) originated in the Atlantic forest (Mata Atlantica) and invaded Amazonia about 400.000 years ago. The last common ancestor of Cebus and Sapajus has been calculated at about 6 MA. Considering the ecological dominance of Sapajus in many parts of Amazonia (measured by the densities shown by Peres, 1993, 1997a, b; Haugasen & Peres, 2005, 2009) and the much higher densities in parts of the Colombian and Venezuelan llanos and the success of C. capucinus in the Chocó and Central America, this author suggests some other origin for Cebus, perhaps on the periphery of Amazonia in forests less humid and associated with sabanas and dry forests. I suggest that if the gracile Cebus originated in the Amazon they would be ecologically dominant to Sapajus, which is clearly not the case. The isolated population of Cebus albifrons in NE Vichada with parapatric Sapajus apella to the west and to the south suggests to me that Sapajus apella arrived after Cebus albifrons and that the more recent species was able to out-compete Cebus albifrons. This accords well with the ecological dominance of S. apella in Amazonia where the two species are sympatric.
Cebus albifrons and Cebus olivaceus in Venzuela are genetically related species and have many similarities, suggesting that they have common ancestors. Perhaps C. olivaceus comes from C. albifrons and they are sister groups with C. capucinus (Lynch-Alfaro et al., 2012; Ruiz-Garcia et al. 2010). It is possible that the llanos could be a principal factor in permitting the persistence of these two species and that their presence in the forests to the south might be the result of an invasion from the savannas during a glacial maximum when there were many more savannas and an Amazonia much more fragmented. This hypothesis is supported by molecular studies showing that various subpopulations of Cebus albifrons exist in the Amazon within what we generally see as Cebus albifrons (Ruiz-Garcia et al., 2010; Lynch Alfaro et al., 2012).
During my stay in El Tuparro professor Hernández-Camacho asked me to collect several examples of Cebus albi frons, an unwelcome task for me, since I wasn't accustomed to collecting. But this was part of my education as a biologist, since my education outside of Colombia had not included the importance of study collections. Another project accomplished with professor Hernández-Camacho was the establishment from this collected material of a neotype for Cebus albifrons, since there were various problems with the original description of von Humboldt which was not supported with a holotype and which impeded comparison with other taxa that would allow the construction of an adequate taxonomy for the species (Humboldt & Bonpland, 1812; Defler & Hernández-Camacho, 2002). The problem of an adequate taxonomy for Cebus albifrons, however, was not solved by the establishment of a neotype, since we are now flooded by new molecular data that shows how complex the evolution of Cebus albifrons has been (Boubli et al., 2012; Ruiz-Garcia et al., 2010; Lynch et al., 2012).
Attempts to study Sapajus apella in Tapón were not as successful as the study of Cebus albifrons. This was due, I think, to previous collections of primates in this forest by INDERENA employees that rendered the primates (especially the Sapajus) very timid. I was never able to habituate groups of Sapajus, whereas it was very easy to habituate the C. albifrons from eastern El Tuparro. Nevertheless, it was possible to distinguish several species differences between the two that have been helpful in understand their competition (Defler, 1982).
Callicebus
In western El Tuparro a study of some population characteristics of the then denominated Callicebus torquatus lugens (now recognized as Callicebus lugens) elucidated group sizes (3.5), territory sizes (14.2 ha) and other details. This taxon is now recognized as Callicebus lugens because of the discovery in Brazil of the karyotype for this taxon, the lowest karyotype known among the primates (2n=16) (Bonvicino et al., 2002). However, we now have a dilemma in Colombia. In order to confirm that what was denominated before as Callicebus torqutus lugens (sensu Hershkovitz, 1990) corresponds to the taxon Callicebus lugens with a karyotype 2n=16, we have to determine the karyotypes of Colombian animals, work that is difficult because of Colombian legislation, resulting in many difficulties applying for official permission.
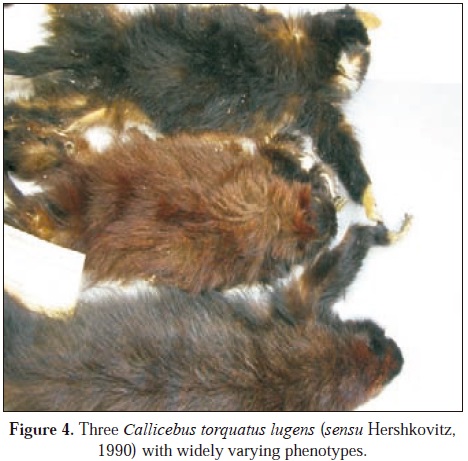
(1812) defined lugens as a completely black animal ("d'un beu noir et un peu relevé: ce pelage est d'une teinte uiforme sur le corps entier, à léxception de la face, du col et des mains de devant.") with the exception of the face, neck and hands. Hernández-Camacho y Cooper (1976) distinguished only Callicebus torquatus lugens and C. t. medemi and Hershkovitz (1963) maintained that it was not possible to distinguish lugens from lucifer with the diagnostic characters that had been published. Nevertheless, Hershkovitz (1990) later distinguished between lucifer and lugens, placing the two in Colombia, separated by the Caquetá river and distinguishing them using the bands of color on the hairs of the neck and sides of the body, lugens with dark brown o blackish color with hairs of uniform color o weak bands of pheomelanin and lucifer with hairs of the neck and sides of the body colored brown or reddish brown with hairs strongly banded (with pheomelanin) (Hershkovitz, 1990).
After examining more than 100 skins of this complex from the two putative taxons, this author finds a range of color characteristics from both sides of the Caquetá river, at times the same, at times different, that makes it impossible to assign many specimens to the taxons as defined Hershkovitz (1990). For example, figure 3 and figure 4 show specimens that supposedly are assigned to one of the two taxons, but which on examining them, results impossible to place in any one taxon. Recently my colleague, the geneticist Marta Bueno of the Universidad Nacional de Colombia has determined the karyotype of a specimen from the north bank of the Amazon River above Leticia. This karyotype (2n=20) does not seem to differ from karyotypic characteristics determined for other Callicebus torquatus (Egozcue et al., 1969; Benirschke & Bogart, 1976). The specimen is definitely not C. lugens and must be placed in the C. torquatus complex. As we do not detect a genetic difference from other C. tor quatus, we assign this Colombian Callicebus to a subspecies C. torquatus lucifer. Basically I suggest that C. torquatus lucifer and C. t. medemi are found in southern Colombian and that their karyotype is 2n=20. The possibility that specimens north of the Caquetá River are Callicebus lugens must await karyotypic designation, although at the moment we treat these animals north of the Caquetá River as Callicebus lugens.

Identical data was collected in the Hormiga Creek forest on Alouatta seniculus to that of Cerro Rocoso, and they were similar densities but with different group sizes (5.75 individuals Tapón, 6.8 individuals in Cerro Rocoso) and different home range size (13.3 ha in Tapón, 23.75 in Cerro Rocoso) and densities (23 individuals/km2 for Tapón; 27 individuals/ km2 for Cerro Rocoso) (Defler, 1981).
An experiment examining how to best census primates clarified various important points. Especially it pointed out the difficulties of censusing a far-ranging species such as Sa pajus in narrow forests that skewed the range of the animals from an idealized circle or oblong so that they were no longer detected al azar, thus inflating the densities of the animals (Defler & Pintor, 1985).
Lagothrix lagothricha lagothricha, Cacajao melanocephalus
My research in Vaupés (1983-1995) generated some of the first data on Lagothrix lagothricha and taught me about the effects of the poverty of eastern Colombia. Our data on precipitation at the research station in southern Vaupés includes more than 14 years and shows an average of 3950 mm per year, well above the precipitation for the majority of known Colombian Amazonian sites (Palacios et al. 2009). Various correlations with species richness in both plants and animals show an increase in species richness up to about 2.500 mm of annual precipitation, after which species richness levels off or declines (Gentry, 1988; for plant diversity; Kay et al., 1997, for primary production; Peres & Janson, 1999; for neotropical primates). The number of primate species en southern Vaupés is 8 or 9 at only two degrees south of the equator; this is a moderate species richness compared to primate communities south of the Amazon river (with annual precipitations below 2.000 mm [Leticia has a multi-annual average of 3194 mm, according to SIATAC (2012) and precipitations shows a tendency to rise towards the north, towards the research station Estación Biológica Mosiro Itajura-Caparú at the coordinates 1°04'27.41" S, 69°31'01.73" W (92 m altitude] . Species richness south of the Amazon river reach 10-14 species, the highest of the neotropics. Calculations of densities of primates at Mosiro Itajura-Caparú are very low in comparison to data from Brazil, Ecuador and Peru and it is worthwhile making some comments here.
Hypotheses regarding an increase in species richness up to the equator are not valid for Colombian primates. Eastern Colombia is low in primate species richness, but it is also low in densities or biomass of primates compared with the large data base published by Peres, (2005, 2007); Peres & Janson, (1999); Defler, en prensa; Palacios & Peres, (2005) as well as data in Colombia from the Duda River (Stevenson, 1996). Low biomass true for all of the eastern Colombian Amazon into the Colombian trapezium and the conservation of communities having such low densities comes with special problems, especially when certain species are heavily hunted preferentially (e.g. tapir, Tapirus terrestres, woolly monkey Lagothrix lagothricha, tayasuids Tayassu peccari y T. tajacu, deer Mazama spp.) (Peres & Palacios, 2007). Communities in Eastern Colombia are sensitive and can be easily perturbed by excessive hunting. Table 2 compares eastern Colombia primate species richness and densities with other selected sites in South America.
There are many species that cannot maintain themselves with sustained hunting (e.g. the cracids , the atelids, tapir) and automatically when there are low densities these species are unable to replace themselves compared to a forest with high species densities (which ultimately also may not be able to sustain populations with concentrated hunting). The moving of indigenous settlements from one place to another is often because the prey species have become very rare or extinct and the community requires a prey base that provides more protein. With a large enough forest and a small enough human population these moves allow the previous prey bases to recover. But with less forest and more people (for example in the Chocó), prey bases do not recover. (Robinson & Redford, 1991; Robinson & Bennett, 2000a, 2000b) and certain animals become more scarce and endangered (for example, Ateles geoffroyi).
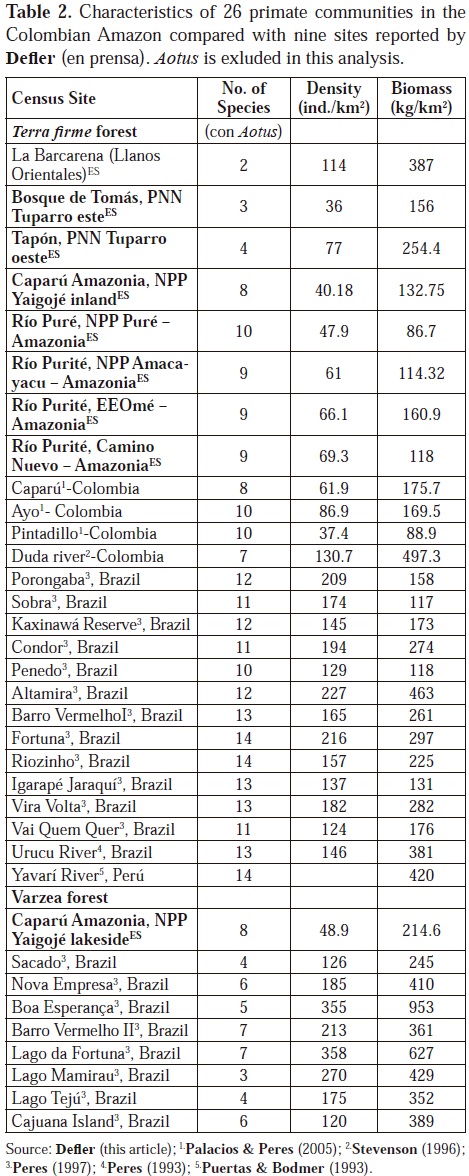
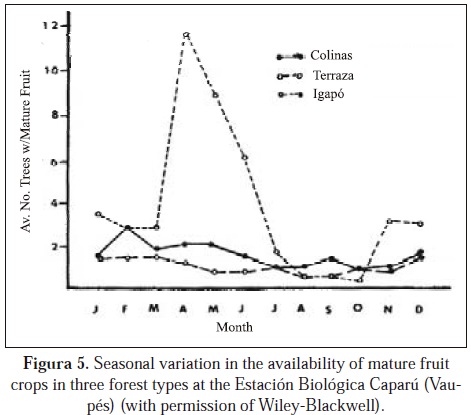
Low densities of primates (and other animals) in Eastern Colombia can be explained by soil leaching due to high precipitation and to low productivity of plants due to absent nutrients (Kay et al., 1997; Peres, 2008). High precipitation leaches soils, continuing to impoverish them and northward from Leticia (at 20000 mm) the precipitation continues increasing, reaching 4000 mm in southern Vaupés (Palacios et al., 2009). Numbers in many fruit crops at the Estación Biológica Mosiro Itajura-Caparú are very low, even though the plant diversity is high (although how plant diversity at this research station compares to plant diversities in other parts of the Amazon has not been measured) (Defler, unpublished data). Fruit set is limited by deficiencies in nitrogen, phosphorus, potasium, calcium and an abundance of aluminum (Jordan, 1985). Defler (in press) show soil data for eastern Colombia for primate census sites (except for PNN El Tuparro) and extremely low values are evident in these Eastern Colombian forests. In contrast, the llaneron forests with much higher primate biomass also show much higher levels of soil nutrients and lower levels of aluminum. The only site that does not show tendencies for low primate biomass is the igapó of the Estación Biológica Mosiro Itajura-Caparú with the highest seasonal biomass found in any Colombian Amazonian site (214.6 kg/km2), even though the site has extremely high levels of aluminum and the same general low levels of other nutrients equal to terrestrial sites. Though research must be done, the hypothesis is that the high biomass of the igapó is due to a seasonal pulse in production of fruit biomass, itself due to a seasonal influx in the flooding water of enough nutrients to sustain a one-time fruit pulse that attracts the primates (Lagothrix, Saimiri) (Figure 5). Added to the fruit pulse in igapó is a young leaf pulse (Alouatta, Lagothrix) and a concentration in the production of the favorite Cacajao fruit Eschweilera spp. (Figure 6).
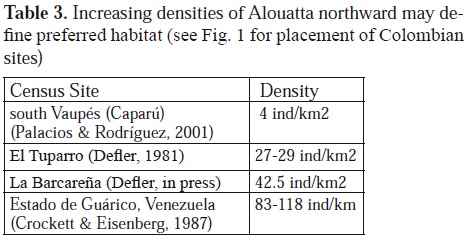
Some species show very interesting tendencies of densities. Besides Cebus albifrons, briefly discussed above, densities of Alouatta seniculus are some of the lowest densities in southern Vaupés, with ecological densities of around 3.85 individuos/km2, based on research of Palacios & Rodríquez (2001). If the same site were calculated for crude density (i.e. an area of forest including both ecological density and forest without individuals) densities for A. seniculus in this regions would be much lower. High densities reported for central Venezuela of 83-118 individuals/km2 in Guárico State at Hato Masaguaral are the highest known so far (Crockett & Eisenberg, 1987). Two places separated by 150 km in the Parque Nacional Natural El Tuparro have 30 individualss/ km2 at each site, and are similar to densities calculated in various other studies in the country. This author suggests that A. seniculus is found in an environmental cline where the environmental conditions for A. seniculus improve from the south to the north from Amazonia towards the center of the Venezuelan llanos. Other censuses taken in the north of Vichada resulted in the highest densities for this species so far known in Colombia of 42.5 individuals/km2 (Defler, in press). Peres (1997b) suggests that the species is sensible to changes in the quality of leaves in the closed canopy forests where he censused. More fertile forests must provide better hábitat for A. seniculus, which is more commonly found in the interior of forests to south of the Amazon river, but these conditions are not commonly found in Eastern Colombia.
Looking at these densities for A. seniculus, perhaps we can identify the preferred hábitat for the species as well as for Cebus albifrons, concluding that the sites with the highest densities define preferred habitat (Table 3). There might be some problems with this interpretation, given that reproduction, survival and abundance may not all be positively correlated so that demographic data is needed to assure that there is high survival rate within the high density population (Johnson, 2007); some populations of birds and rodents at lower densities have been shown to have higher survival rates than high density populations Van Horne, (1983). However, the majority of studies define preferred habitat using positive correlations with population densities, although demographic information should be included to measure survivability. Following this argument, can we not conclude something about the origin of such species if we know something about their preferences? For a first attempt on the problem of preferred habitat, areas of high density suggest a lot.
Although my research on Cacajao melanocephalus leaves much to be accomplished, despite the time I dedicated to it, I have recuperated from the destruction of my notes and library by the guerilla in 1998 that led to my leaving the Estación Biológica Mosiro Itajura-Caparú, and I have been able to publish some results. I managed to publish details such as densities of Cacajao, that like A. seniculus are ecological densities around the Taraira Lake of southern Vaupés (Defler, 2001). This is very definitely a special type of habitat of 48-50 km of lakefront igapó that provide habitat especially for these two species. Recently I have participated in writing another pair of articles about Cacajao to be included in a book on the Pitheciidae (Barnett et al. in press-a, in press-b).
I invested several years in the research on Lagothrix lagothricha, partly because of the huge home range that they utilized in southern Vaupés and their low density, due to habitat poverty. My study group of 24 animals utilized 760 h, which is one of the largest calculated. In contrast, groups on the Duda River used around 169 ha with densities of about 28 animals/km2. These data show the extremes between two habitats, one very productive on the Duda river, another quite poor on the Apaporis river (Defler, 1987, 1989, 1995, 1996, 1999; Defler & Defler, 1996). Even smaller home ranges have been found by Pablo Stevenson's group from the University of Los Andes for Lagothrix lagothricha that have not been published.
Although I left the Estación Biológica Mosiro Itajura-Caparú in 1998 because of the guerilla, I am happy to know that the station has continued to function under the administration of Conservation International Colombia and that many young Colombians have been able to finish their thesis projects on primates and other fauna and flora (e.g., Alarcón-Nieto & Palacios, 2009). With a little luck and the support of others we hope that these activities continue into the future.
Amazonas
After leaving the Estación Biológica Mosiro Itajura-Caparú in 1998 I decided to search for another more isolated site to continue research in the field. During the search for a secure area, isolated from security concerns I arrived at the the Purité River, a border river of southern Amacayacu National Park. This place is extremely isolated, requiring six days of travel from Leticia (partly through Brazil) to arrive. Here I was able to construct three small buildings and to accomplish primate censuses at four sites along the Purité river, obtaining results that fully agree with other eastern Colombian censuses of very low population densities of primates (Defler, in press). Also, I was able to begin research on the use of space by the primate Callicebus lucifer (=Callicebus torquatus lucifer). However, the use of this new station (Estación Ecológica Omé) is logistically very complicated, and I have searched for yet another site, closer to Bogotá for my research.
Meta and prívate reserves
Due to the difficulties of managing the Estación Ecológica Omé and the total cost of a trip there I discovered a solution that allows more continued primate research in the field. I discovered the existence of a private reserve Las Unamas in Meta, made up of gallery forest and a very large 1.300 ha fragment that earlier was a part of a very wide gallery forest connected to the Camoa River, to the east of San Martin, Meta. This reserve forms part of one of the original homesteads (Haciendas) of this part of the llanos. The forest has been protected for many years (although there is some poaching). The primate fauna is made up of five species: Sapajus apella, Alouatta seniculus, Saimiri sciureus, Calli cebus ornatus and Aotus brumbacki. Of these Callicebus ornatus requires the most attention due to being classified by the IUCN and Colombia as Vulnerable (VU). I choose to study population densities and preferences of habitat. There are indications that Callicebus ornatus can utilize degraded forests and it is common to find groups close to human populations as long as they are not disturbed. Because some studies have calculated extremely high populations in small fragments, it may be that many individuals have escaped forest being cut and have become refuges in nearby fragments (Mason, 1966; Wagner et al., 2010). I hope to identify some habitat preferences of these primates and to indicate what natural population densities are. In this case, I hypothesize that the highest populations known do not correlate with the most preferred habitat, since these high populations are clearly related to habitat disturbance.
belongs to a complex of small primates of Callicebus that are highly interesting, especially because they are monogamous, a reproductive system not common among the primates (Defler, 2010). These small monkeys defend small territories and they are basically frugivores, supplementing with a quantity of small invertebrates such as spiders and orthopterans.
Callicebus has become a strong interest of mine. I have described a new species from southern Caquetá with two colleagues of mine, C. caquetensis (Defler et al., 2010). All indications suggest that this species is highly endangered due to destruction of its habitat (Garcia et al., 2010). We have fortunately been able to receive financial support from two Canadian oil companies that will permit two years of research for various persons in our group. The research will include aspects of the species' ecology and its molecular phylogeny.
Colombia continues to offer me many opportunities for primate research and I continue with this effort until I am no longer physically able to do so. This brief resume cannot include many details of my research that actually demand a book, a Project that is also in course.
Acknowledgements
I particularly thank the many Colombians who have given me support in my research during so many years of effort. I include especially professor Jorge Hernández-Camacho, Colombia's deceased source of knowledge of so much Colombian biota and my mentor during our time of association. I became a better Colombian biologist, having been associated with him. I also thank José Vicente Rodríguez Mahecha of Conservación Internacional Colombia and before that of INDERENA, who gave me physical and psychological support for many difficult efforts in the east of the country and also to my dear friends, the professors Carlos Moreno Benavides y Amanda de Moreno who offered me the stability and intellectuality of their home and the security of open arms. I thank my compadre Sr. Hector Castillo, a good friend, who has always supported my life and activities in Leticia, helping me to resolve difficult logistics problems. As well, I thank Sr. and Sra. Pedro y Consuelo Pinzón who provided a home in La Pedrera and helped resolve so many logistical problems there for my trips to Caparú. I thank professor Moises Wasserman of the Universidad Nacional de Colombia who as Decano of the Facultad de Ciencias listened to me and understood my crie de coeur and did what was necessary to continue my association with the UNC. I thank the Universidad Nacional de Colombia both in Leticia and in Bogotá for providing an academic home and support for my research endeavors. I thank Russell Mittermeier, President of Conservation International for various grants to myself and to so many other field researchers and to Anthony Rylands. Who always shared his knowledge when I needed it. I thank Sara Bennett for company and her dedication to the many orphan primates what arrived at Caparú, and that continue to appear in Amarayacu doe to human cruelty.
I particularly thank INDERENA, the wildlife Conservation Society, Conservation International, Margot Marsh Foundation for Biodiversity, National Geographic, The Woolly Monkey Foundation, International Primate Protection League, Conservación Internacional Colombia, Fundación Natura, Ohio Biodiversity Alliance, Louiseville Zoological Garden, Primate Action Fund (Conservation International), World Wildlife Fund USA, for the various financial grants given to me throughout the years.
I am especially thankful to Conservación Internacional Colombia for adopting and continuing maintenance of La Estación Biológica Mosiro Itajura - Caparú in Vaupés for the future generations of Colombians who will be able to accomplish research in the Colombian Amazon using this infrastructure.
I give particular thanks to the Universidad Nacional de Colombia (in Bogotá and in Leticia), the Departament of Biology and the Facultad de Ciencias for having supported me in my research and for having provided a congenial intellectual home and appreciated colleagues who have provided me with an academic circle of dedicated biologists that has helped me to obtain my academic objectives in research and teaching. I also thank the active people of the Asociación Primatológica Colombiana, who have provided the primatologist-colleagues to make this life's voyage interesting.
Bibliography
Alarcón-Nieto, G. & E. Palacios (editors) 2009. Estación Biológica Mosiro Itajura-Caparú: Biodiversidad en el territorio del Yagojé-Apaporis. Conservación Internacional de Colombia, Bogotá [ Links ].
Altmann, J. 1974. Observational study of behavior: sampling methods. Behaviour 49(3):227-266. [ Links ]
Barnett, A., T.R. Defler, M. Oliveira, H. Queiroz & B.M. Bezerra in press. Cacajao ouakary in Brazil and Colombia: Patterns, Puzzles and Predictions. In. The Pitheciidae. Springer, New York. [ Links ]
Barnett A., M. Bowler, B.M. Bezerra & T.R. Defler in press. Ecology and behaviour of uacaris (genus Cacajao). In. The Pitheciidae. Springer, New York. [ Links ]
Bennett, C.L., S. Leonard & S. Carter 2001. Abundance, diversity, and patterns of distribution of primates on the Tapiche River in Amazonian Peru. Am J Primatol 54:119-126. [ Links ]
Benirschke, K. & M. H. Bogart 1976. Chromosomes of the tan-handed titi (Callicebus torquatus, Hoffmannsegg, 1807). Folia Primatologica 25: 25-34. [ Links ]
Bonvicino, C. R., V. Penna-Firme, F. F. Nascimento, B. Lemos, R. Stanyon & H. N. Seuánez 2002. The lowest diploid number (2n=16) yet found in any primate: Callicebus lugens (Humboldt, 1811). Folia Primatologica 74:141-149. [ Links ]
Boubli, J. P., A. Rylands, I. P. Farias, M. E. Alfaro & J. Lynch-Alfaro. 2012. Cebus phylogenetic relationships: a prliminary reassessment of the diversity of the untufted capuchin monkeys. Am J Primatol 74(4):381-393. [ Links ]
Carvajal-L, F.J., F.N. Posada-A., L.C. Molina-M., A. Delgado-F., L.E. Acero-D., O. Araújo-M. & F. Rodríguez-M. 1976. Bosques. Pp. 225-322, En: Anónimo, editor. La Amazonia Colombiana y sus Recursos. Proyecto Radargrametrico del Amazonas. República de Colombia, Bogotá D.F. [ Links ]
Crockett, C. M. & J. F. Eisenberg 1987. Howlers: variations in group size and demography. Pp. 54-68, In: B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham & T. T. Struhsaker, editors. Primate Societies. The University of Chicago Press: Chicago. [ Links ]
Defler, T.R. 1979a. On the ecology and behavior of Cebus albifrons in eastern Colombia: I. Ecology. Primates 20:475-490. [ Links ]
Defler, T.R. 1979b. On the ecology and behavior of Cebus albifrons in eastern Colombia: II. Behavior. Primates 20:491-502. [ Links ]
Defler, T.R., 1980. Notes on interactions between the tayra (Eira barbara) and the white-fronted capuchin (Cebus albifrons). J Mammal 61:156. [ Links ]
Defler, T.R. 1981. The density of Alouatta seniculus in the Llanos Orientales of Colombia. Primates 22:564-569. [ Links ]
Defler, T.R. 1982a. A comparison of intergroup behavior in Cebus albifrons and Cebus apella. Primates 23:383-392. [ Links ]
Defler, T.R. 1982b. the giant otter by canoe. WWF Monthly Report. Dec. Project 3038:345-348. [ Links ]
Defler, T.R. 1983. Some population characteristics of Callicebus torquatus lugens (Humboldt, 1812)(Primates: Cebidae) in eastern Colombia. Lozania 38:1-9. [ Links ]
Defler, T.R. 1983. Some population characteristics of Callicebus torquatus lugens (Humboldt, 1812)(Primates: Cebidae) in eastern Colombia. Lozania 38:1-9. [ Links ]
Defler, T. R, 1984. Associations between giant river otter (Pteronura brasiliensis) and fresh water dolphins (Inia geoffrensis). J Mammal 64:692. [ Links ]
Defler, T.R. 1985a. Contiguous distribution of two species of Cebus monkeys in El Tuparro National Park, Colombia. Am J Primatol 8:101-112. [ Links ]
Defler, T.R. 1985b. Those crafty, capricious, clever capuchins. Animal Kingdom 89:16-21. [ Links ]
Defler, T. R, 1986. The giant river otter in El Tuparro National Park, Colombia. Oryx XX:87-88. [ Links ]
Defler, T. R. 1987. Ranging and use of space in a group of woolly monkeys (Lagothrix lagothricha) in the NW Amazon of Colombia. Int J Primatol 8: 420. [ Links ]
Defler, T. R. 1989a. Recorrido y uso del espacio en un grupo de Lagothrix lagothricha (Primates: Cebidae) mono lanudo o churuco en la Amazonia Colombiana. Trianea (Acta científica y tecnológica, INDERENA) 3: 183-205. [ Links ]
Defler, T. R. 1989b. Wild and woolly. Animal Kingdom September/October. [ Links ]
Defler, T. R. 1990. Salves y lanudos. Ecológica 4. [ Links ]
Defler, T. R. 1995. The time budget of a group of wild woolly monkeys (Lagothrix lagothricha). Int J Primatol 16(1): 107-120. [ Links ]
Defler, T. R. 1996a. Aspects of the ranging pattern in a group of wild woolly monkeys (Lagothrix lagothricha). Am J Primatol 38: 289-302. [ Links ]
Defler, T. R. 1996c. The IUCN conservation status of Lagothrix lagothricha lugens Elliot, 1907. Neotropical Primates 4(3): 78-80. [ Links ]
Defler, T. R. 1999a. Fission-fusion behavior in Cacajao melanocephalus ouakary. Neotropical Primates 7(1): 5-8. [ Links ]
Defler, T. R. 1999b. Locomotion and posture in Lagothrix lagothricha. Folia Primatologica 70(6): 313-327. [ Links ]
Defler, T.R. 2001. Cacajao melanocephalus ouakary densities on the lower Apaporis River, Colombian Amazon. Primate Report 61 (November, 2001):31-36. [ Links ]
Defler, T. R. 2003. Densidad de especies y organización espacial de una comunidad de primates: Estación Biológica Caparú, Departamento del Vaupés, Colombia. In: F. Nassar & V. Pereira (eds.), Primatología del Nuevo Mundo. Fundación Araguatos, Santa Fe de Bogotá, pp. 21-37. [ Links ]
Defler, T. R. 2009. Fundación de la Estación Biológica Caparú. En: G. Alarcón-Nieto & E. Palacios, editores. Estación Biológica Mosiro Itajura-Caparú: Biodiversidad en el territorio del Yagojé-Apaporis. Conservación Internacional Colombia, Bogotá. pp. 41-51. [ Links ]
Defler, T. R. en prensa. Aspectos para la conservación de los primates colombianos: ¿cuál es el futuro? In: Defler, T.R., Stevenson, P.R., Bueno, M.L., Guzman, D., editores. Primates colombianos en peligro de extinción. Bogotá [ Links ]
Defler, T. R. en prensa. Species richness, densities and biomass of ten primate communities in eastern Colombia. Esta revista. [ Links ]
Defler, T. R. & D. Pintor. 1985. Censusing primates in a forest of known primate density. Int J Primatol 6(3):243-260. [ Links ]
Defler, T. R & S. B. Defler 1996. The diet of a group of woolly monkeys (Lagothrix lagothricha lagothricha) in southeastern Colombia. Int J Primatol 17(2):161-190. [ Links ]
Defler, T. R. & J. I. Hernández-Camacho. 2002. The true identity and characteristics of Simia albifrons Humboldt, 1812: Description of neotype. Neotrop Primates 10(2):49-64. [ Links ]
Defler, T. R. & M. Bueno. 2007. Aotus diversity and the species question. Primate Conserv 22:55-70. [ Links ]
Defler, T. R., M. L. Bueno & J. I. Hernández-Camacho. 2001. Taxonomic Status of Aotus hershkovitzi: Its Relationship to Aotus lemurinus lemurinus. Neotrop Primates 9(2):37-52. [ Links ]
Defler, T. R., M. L. Bueno & J. Garcia 2010. Callicebus caquetensis: A new and critically endangered primate from southern Caquetá, Colombia. Primate Conserv 25:1-9. [ Links ]
Garcia, J., T. R. Defler & M. L. Bueno 2010. The conservation status of Callicebus caquetensis (Pithecidae, Platyrrhini), a new species in southern Caquetá Department, Colombia. Neotrop Primates 17(2):37-46. [ Links ]
Gentry, A. 1988. Tree species richness of upper Amazonian forests. Proc Nat Acad Sci USA 85:156-159. [ Links ]
Haugaasen, T. & C. A. Peres. 2005. Primate assemblage structure in Amazonian flooded and unflooded forests. Am J Primatol 67:243-258. [ Links ]
Haugaasen, T. & C. A. Peres. 2009. Interspecific primate associations in Amazonian flooded and unflooded forests. Primates 50:239-251. [ Links ]
Hernández-Camacho, J. & R. W. Cooper, Jr. 1976. The non-human primates of Colombia. Pp. 35-69, En: R.W. Thorington, Jr. & PG Heltne, editores. Neotropical primates: field studies and conservation. National Academy of Science Press, Washington, D.C. [ Links ].
Hernández Camacho, J. & T.R. Defler. 1985. Some aspects of the conservation of non-human primates in Colombia. Primate Conserv 6:42- 50. [ Links ]
Hershkovitz, P. 1963. A systematic and zoogeographic account of the monkeys of the genus Callicebus (Cebidae) of the Amazonas and Orinoco River basins. Mammalia 27(1):1-80. [ Links ]
Hershkovitz, P. 1990. Titis, New World monkeys of the genus Callicebus (Cebidae, Platyrrhini): a preliminary taxonomic review. Fieldiana (Zoology New Series). 55:109 pp. [ Links ]
Hilty, S. L. & W. L. Brown 1982. A guide to the birds of Colombia. Princeton University Press, Princeton NJ. [ Links ]
Humboldt, A. von & Bonpland. 1811. Recueil d´observations de zoologie et d´anatomie comparés, fait dans l´océan Atlantique, et dans la mer du nouveau continent et dans la mer de sud pendant les anneés 1799, 1800, 1801, 1802 et 1803. 1. Levrault Schoell, Paris. pp. 319-321. [ Links ]
Huston, M. A. 1994. Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press: Cambridge. [ Links ]
Ibarra, C., J. Morelo, J. Briceño, A. Cortés, B. de Motta, C. Luna, F. Garavito & C. Pulido 1976. Suelos. Pp. 93-216, En: Anónimo, editor. La Amazonia Colombiana y sus Recursos. Proyecto Radargrametrico del Amazonas. República de Colombia, Bogotá D.F. [ Links ]
Johnson, M. D. 2007. Measuring habitat quality: a review. The Condor 109:489-504. [ Links ]
Jordan, C. F. 1984. Nutrient cycling in tropical forest ecosystems. John Wiley & Sons, New York. [ Links ]
Kay, R. F., R. H. Madden, C. van Schaik & D. Higdon. 1982. Primate species richness is determined by plant productivity: implications for conservation. Proc Nat Acad Sci USA 94:13023-13024. [ Links ]
Lynch-Alfaro, J. S., J. P. Boubli, L. E. Olson, A. DiFiore, B. Wilson, G. A. Gutierrez-Espeleta, K. L. Chiou, M. Schulte, S. Neitzel, V. Ross, D. Schwochow, M. T. T. Nguyen, I. Farias, C. H. Janson & M. E. Alfaro. 2011 onli ne; 2012. Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. J Biogeogra 39: 272-288. Online 18 Oct. 2011 [ Links ]
Lynch-Alfaro, J. S., J. S. Silva; A. B. Rylands. 2012. How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. Am J Primatol 74:273-286. [ Links ]
Mason, W. A. 1966. Social organization of the South American monkey, Callicebus moloch: a preliminary report. Tulane Studies in Zoology 13:23-28. [ Links ]
Matthews, L. J. 2009. Activity patterns, home range size and intergroup encounters in Cebus albifrons support existing models of capuchin socioecology. Int J Primatol 30:709-728. [ Links ]
Morcote-Ríos, G. 2008. Antiguos habitantes en ríos de aguas negras. Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá [ Links ].
Palacios, E. & A. Rodríquez 2002. Ranging pattern and use of space in a group of red howler monkeys (Alouatta seniculus) in a southeastern Colombian rainforest. Am J Primatol 55:233-251. [ Links ]
Palacios, E. & C. Peres 2005. Primate population densities in three nutrient- poor Amazonian terra firme forests of south-eastern Colombia. Folia Primatologica 76:135-145. [ Links ]
Palacios, E., A. Rodríguez & G. Alarcón-Nieto 2009. Aspectos físicos y biológicos del bajo Río Apaporis y la Estación Biológica Mosiro Itajura-Caparú. Pp. 29-40. In: G. Alarcón-Nieto, E. Palacios, editores. Estación Biológica Mosiro Itajura-Caparú: Biodiversidad en el territorio del Yagojé-Apaporis. Conservación Internacional Colombia, Bogotá [ Links ].
Peres, C. A. 1993. Structure and spatial organization of an Amazonian terra firme forest primate community. J Trop Ecol 9: 259-276. [ Links ]
Peres, C. A. 1997a. Primate community structure at twenty western Amazonian flooded and unflooded forests. J Trop Ecol 13:381-405. [ Links ]
Peres, C. A. 1997b. Effects of habitat quality and hunting pressure on arboreal folivore densities in neotropical forests: A Case Study of Howler Monkeys (Alouatta spp.). Folia Primatologica 68(3-5):199-222. [ Links ]
Peres, C. A. 1999a. Effects of subsistence hunting and forest types on the structure of Amazonian primate communities. Pp. 268-283, En: J.G. Fleagle, C. Janson & K.E. Reed, editors. Primate Communities. Cambridge University Press, Cambridge. [ Links ]
Peres, C. A. 1999b. General guidelines for standardizing line-transect surveys of tropical forest primates. Neotropical Primates 7:11-16. [ Links ]
Peres, C. A. 2008. Soil fertility and arboreal mammal biomass in tropical forests. Pp. 349-364. In: W. Carson & S. Schnitzer, editores. Tropical Forest Community Ecology, Blackwell Scientific, Oxford. [ Links ]
Peres, C. A. & C. Janson 1999. Species coexistence, distribution, and environmental determinants of neotropical primate richness: A community- level zoogeographic analysis. Pp. 55-74. In: J.G. Fleagle, C. Janson, K. E. Reed, Editors. Primate Communities. Cambridge University Press, Cambridge. [ Links ]
Peres, C. & P. M. Dolman 2000. Density compensation in neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122;175-189. [ Links ]
Peres, C. A. & E. Palacios 2007. Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: implications for animal-mediated seed dispersal. Biotropica 39(3):304-315. [ Links ]
Puertas, P., Bodmer, R. E. 1993. Conservation of a high diversity primate assemblage. Biodiversity Conserv 2: 586-593. [ Links ]
Rangel-Ch., J. O., T. van der Hammen, N. Espejo, N. E. & I. Romero 2008. Cambios en la vegetación y en el clima durante los últimos 60.000 años en el valle inferior del Río Caquetá, Amazonia, Colombiana. Pp.165-215, In: J. O. Rangel Ch. Colombia: Diversidad Biótica VII: Vegetación, palinología y paleoecologia de la amazonía colombiana. Universidad Nacional de Colombia, Bogotá [ Links ].
Renjifo, L. M., A. M. Franco-Maya, J. D. Amaya-Espinel, G. H. Kattan & B. López-Lanús. 2002. Libro rojo de aves de Colombia. Panamericanas Formas e Impresas, Bogotá [ Links ].
Robinette, W. L., C. M. Loveless & D. A. Jones 1974. Field tests of strip census methods. J Wildl Manage 38(1):81-96. [ Links ]
Robinson, J. G. & K. H. Redford 1991. Sustainable harvest of neotropical forest mammals. Pp. 415-429. In J.G. Robinson & K.H. Redfored, editors. Neotropical wildlife use and mammals, The University of Chicago Press, Bogotá [ Links ].
Robinson, J. G. & E. L. Bennett 2000a. Carrying capacity limits to sustainable hunting in tropical forests. In J.G. Robinson & E.L. Bennett, editors. Hunting for sustainability in tropical forests. Columbia University Press, New York. [ Links ]
Robinson, J. G. & E. L. Bennett, editors 2000b. Hunting for sustainability in tropical forests. Columbia University Press, New York. [ Links ]
Rosenzweig, M. L. 1997. Species diversity in space and time. Cambridge University Press, Cambridge. [ Links ]
Ruiz-Garcia, M., M. I. Castillo, C. Vásquez, K. Rodriguez, M. Pinedo- Castro, J. Shostell & N. Leguizamon. 2010. Molecular phylogenetics and phylogeography of the White-fronted capuchin (Cebus albifrons; Cebidae, Primates) by means of mtCOII gene sequences. Mol Phylogenet Evol 57:1049-1061. [ Links ]
Schwarzkopf, L. & A. B. Rylands 1989. Primate species richness in relation to habitat structure in Amazonian rainforest fragmentes. Biol Conserv 48: 1-12. [ Links ]
Siatac 2012. Sistema de Información Ambiental Territorial de la Amazonia Colombiana - Coordinado por SINCHI. <siatac.sic.net.co> downloaded16 junio 2012. [ Links ]
Solari, S., Y. Muñoz-Saba, J. V. Rodríguez-Mahecha, T. R. Defler, H. E. Ramírez-Chaves y F. Trujillo, 2012. Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozoología Neotropical. In press [ Links ]
Stevenson, P. 1996. Censos diurnos de mamíferos y algunas aves de gran tamaño en el Parque Nacional Natural Tinigua, Colombia. Univ Sci 3(1-2): 67-81. [ Links ]
Stevenson, P. 2001. The relationship between fruit production and primate abundance in Neotropical communities. Biol J Linn Soc 72:161-178. [ Links ]
Terborgh, J. 1983. Five New World Primates: A Study in Comparative Ecology. Princeton, New Jersey, Princeton University Press. [ Links ]
Terborgh, J. 1992. Diversity and the tropical rainforest. Scientific American Library, New York. [ Links ]
Terborgh, J.& J. Faaborg 1973. Turnover and ecological release in the avifauna of Mona Island, Puerto Rico. The Auk 90(4):759-779. [ Links ]
Thomas, O. 1914. On various South-American mammals. Annals and Magazine of Natural History, series 8, 13:345-363. [ Links ]
Van der Hammen, T., J.F. Duivenvoorden, J.M. Lips, L.E. Urrego & N. Espejo. 1992a. Late Quaternary of the middle Caquetá River area (Colombian Amazonia). J Quaternary Science 7(1):45-55. [ Links ]
Van der Hammen, T., L.E. Urrego, N. Espejo, J.F. Duivenvoorden & J.M. Lips. 1992b. Late glacial and Holocene sedimentation and fluctuations of river water level in the Caquetá River area (Colombian Amazonia). J Quaternary Science 7(1):57-67. [ Links ]
Van Horne, B. 1983. Density as a misleading indicator of habitat quality. J Wildl Manage 47(4):893-901. [ Links ]
Van Schaik, C.P. & M.A. van Noordwijk. 1989. The special role of male Cebus monkeys in predation avoidance and its effect on group composition. Behav Ecol Sociobiol 24(5): 265-276. [ Links ]
Wagner, M., F. Castro & P. R. Stevenson 2010. Habitat Characterization and Population Status of the Dusky Titi (Callicebus ornatus) in Fragmented Forests, Meta, Colombia. Neotrop Primates 16(1):18-24. [ Links ]
Recibido: 3 de julio de 2012
Aceptado para publicación: 5 de septiembre de 2012.













