Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales
Print version ISSN 0370-3908
Rev. acad. colomb. cienc. exact. fis. nat. vol.37 no.144 Bogotá July/Sept. 2013
CIENCIAS NATURALES
* Programa de Pós-Graduação em Ensino, Filosofia e Historia das Ciências, UFBA/UEFS,Salvador, Brasil, diego.valderrama.bio@gmail.com
** Centro de estudios en Ciencias del Mar - CECIMAR, Universidad Nacional de Colombia, Sede Caribe, c/o INVEMAR, Calle 25 2-55, Rodadero Sur - Playa Salguero, Santa Marta, Colombia. sezeas@unal.edu.co
ABSTRACT
The North-West Gulf of Urabá, Colombia, harbors the southernmost Caribbean reefs, exposed to high turbulence and fluctuating turbidity and salinity. An annotated systematic check-list of sponges from this area is presented. A total of 77 demosponge species (class Demospongiae), 3 homoscleromorph species (class Homoscleromorpha) and 1 calcareous species (class Calcarea) were found to inhabit rocky shores and reefs, above 20 m in depth. Some species in Urabá bear siliceous spicules larger than in other Caribbean areas, probably owing to additional silicon input from heavy river discharge in the gulf. This work provides, additionally, the formal taxonomic description of 15 species, which are new records for the Colombian Caribbean.
Key words:Sponges, Porifera, Demospongiae, Calcarea, Caribbean,hipersilicified spicules.
RESUMEN
El nor-occidente del Golfo de Urabá, Colombia, abriga los arrecifes más meridionales del Mar Caribe, sometidos a altas turbulencias y condiciones fluctuantes de turbidez y salinidad. Se presenta una lista sistemática anotada de esponjas (Porifera) de esta área. Un total de 77 especies de la clase Demospongiae, 3 especies de la clase Homoscleromorpha y 1 especie de la clase Calcarea fueron encontradas en litorales rocosos y áreas arrecifales por encima de los 20 m de profundidad. Algunas especies en Urabá presentan espículas silíceas con dimensiones superiores a las registradas en otras áreas del Caribe, debido probablemente al aporte adicional de sílice de las grandes descargas de los ríos en el interior del Golfo. Este trabajo incluye, adicionalmente, la descripción taxonómica formal de 15 especies, que representan nuevos registros para el Caribe Colombiano.
Palabras clave: Esponjas, Porifera, Demospongiae, Calcarea, Caribe, espículas hipersilicificadas.
Introduction
The continental coast of Colombia in the Caribbean Sea has particular ecological and geological features that differ markedly from other intensively explored areas of the Caribbean (see Hajdu et al., 1995). This is especially true for the Gulf of Urabá, an area near the Colombia-Panama border that represents the southernmost portion of the Caribbean Sea. That area was part of the deep ocean corridor that connected the eastern Pacific to the Caribbean Sea before the rising of the Isthmus of Panama. Currently it is greatly influenced by large amounts of sediments and fresh water discharged by the Atrato River (Chevillot et al., 1993; Duque-Caro, 1990). Moreover, at the North-West margin of the Gulf, fringing and patch reefs flourish in conditions of fluctuating high turbulence, turbidity and salinity (Diaz et al., 2000). In spite of these environmental and geological features, the knowledge of the flora and fauna of the Gulf of Urabá is very poor, even compared to other areas of the Colombian Caribbean (Bula-Meyer & Schnetter, 1988, Alvarado, 1992; Zea, 1998).
The systematic study of sponges in Colombia is very recent, covering only the last three decades. In the Gulf of Urabá, surveys have been carried out on rocky shores and reef environments, less than 20 m in depth, along the coastline of Capurgana and Sapzurro towns, located in the North-West margin of the Gulf. The first sponge collections there were made during an expedition by Instituto de Investigaciones Marinas y Costeras - INVEMAR in 1977 (without the participation of the authors). This material was analyzed by Zea (1987) within a broader assessment of sponges from several areas of the Colombian Caribbean, describing 23 demosponge species for Urabá. Subsequent sponge sampling and ecological studies were carried out during a second expedition by INVEMAR in 1995, aimed at the description and characterization of the local reef areas (with the participation of S. Zea). As a result, Valderrama & Zea (2003) described patterns of sponge composition and abundance at 4 reef zones (1-17 m depth), recording the presence of 65 demosponge species and 1 calcareous species along belt transects (20 m2). Those and other species were cited or preliminarily described by Valderrama (2001). Although some specimens collected in the Gulf of Urabá have been included in the systematic revisions of some sponge groups [Valderrama et al. 2009 (redescribing 1 calcareous species of the genus Leucetta); Parra-Velandia, 2011 (redescribing several demosponge species of the genus Agelas)], most available material awaits further analysis and formal description.
The high diversity and abundance of sponges in the North-West of Gulf of Urabá (Valderrama & Zea, 2003) prompted additional sampling in 2004 (carried out by D. Valderrama) for natural products research at the Marine Natural Products Research lab (Universidad de Antioquia), which led to promising results (Galeano & Martinez, 2007; Martinez et al., 2007a, 2007b; Zabala et al., 2008). At this point we see the need for a synthesis and inventory of the sponge fauna from the area in order to support ongoing and future studies and to generate awareness in the region about the existence, need for conservation, and possible utilization of marine resources.
The purpose of this study is to compile data on all sponge species known from the North-West Gulf of Urabá in a systematic species checklist, based on unpublished data and literature records (systematics and distribution). This includes relevant taxonomic notes, data on species distribution along local reef zones and the systematic description of 15 species that are new records from the Colombian Caribbean. Thus, this study adds new information for the inventory of the shal-low sponge fauna of the Colombian Caribbean, estimated at approximately 280 species (Zea, 1998).
Study area
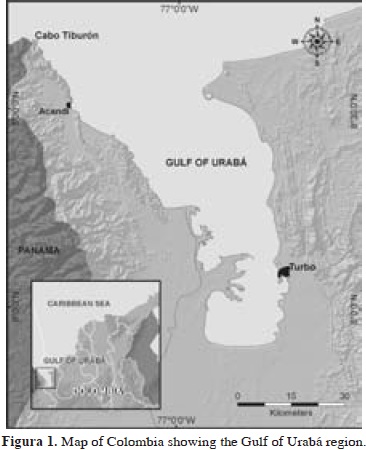
The Gulf of Urabá, located in the southernmost portion of the Caribbean Sea, is a N-S embayment, roughly 85 km long and 15-30 km wide. Fringing reefs and some isolated patch reefs are located at the North-West margin of the Gulf, from 8°35' N near the town of Acandí to the border between Colombia and Panamá at Cabo Tiburón, covering 12 miles of coast (Figure 1). That area is bordered by a limestone terrace that is strongly exposed to North-East Trade winds, which together with the discharge of the Atrato River to the south and several minor rivers, produce characteristic seasonal conditions of high turbulence and fluctuating turbidity and salinity in shallow waters. Sponge observations and sampling were carried out in 6 reef zones dominated by different coral-algal associations described in detail by Diaz et al. (2000).
Materials and methods
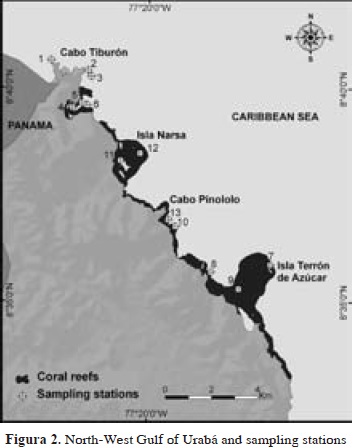
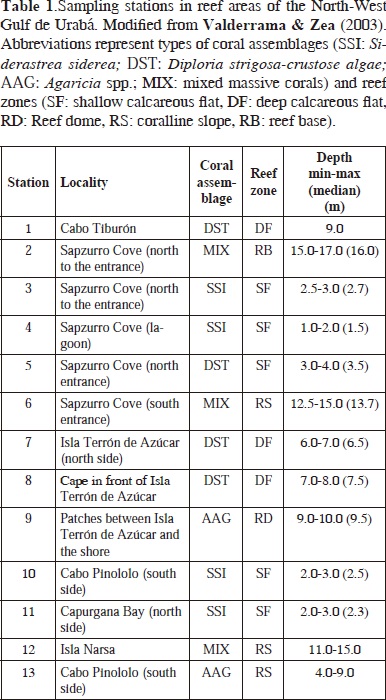
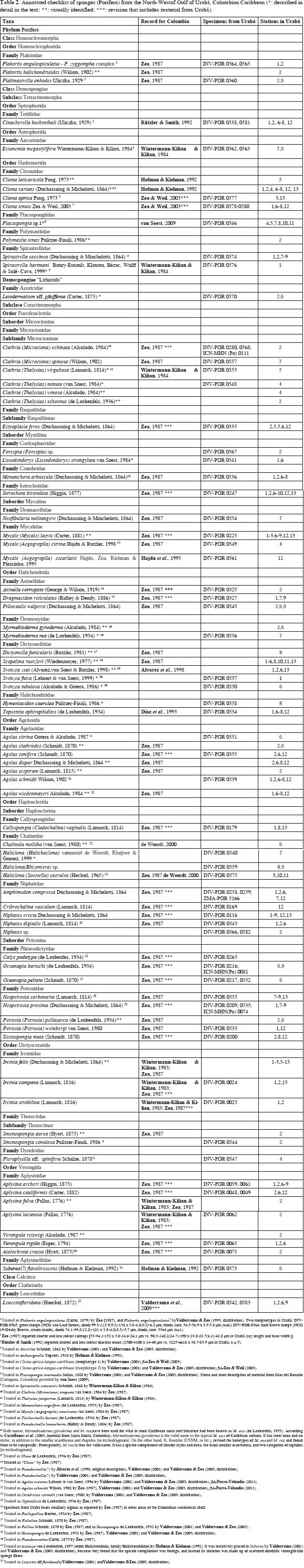
Sponge sampling was carried out between September 28th and October 2th 1995, during an expedition to the Gulf of Urabá by INVEMAR aboard the research vessel Ancón. Thirteen stations were surveyed by SCUBA, each spanning a plot of about 20 x 20 m (400 m2) of homogeneous coral cover, in depths between 1 and 17 m (Figure 2, Table 1). Species were visually identified in the field and fragments of those posing identification difficulties were collected for closer examination, on board and in the laboratory. Samples were fixed in 10 % formalin in seawater buffered with sodium borate (20 g l-1) and preserved in 70 % ethanol after 1-3 days. Permanent spicule preparations and tissue sectioning were performed following standard procedures (see Rützler, 1978; Zea, 1987). These preparations were analyzed under a Leitz Wetzlar compound microscope, identifying and measuring different spicule and spongin fiber types. Spicule types and skeletal organizations were drawn using a camera lucida.
All information obtained was compared to previous sponge studies in the area and the latest literature on systematics and taxonomy of marine sponges. All specimens (including slides and vouchers) were deposited in the Porifera collections of the Museo de Historia Natural Marina de Colombia at INVEMAR (INV-POR), in Santa Marta, and the Museo de Historia Natural, Instituto de Ciencias Naturales, Universidad Nacional de Colombia [ICN-MHN (Po)], in Bogotá, Colombia. Institutional acronyms used in the text to refer to other sponge collections are as follows: Natural History Museum, London (BMNH), Instituto de Zoología de la Academia de Ciencias de Cuba (IdO), Museo e Istituto di Zoologia Sistematica, Università di Torino (MT-Por), Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts (MCZ), Museum National d'Histoire Naturelle de Paris (MNHN), Museo Civico de Storia Naturale "Giacomo Doria", Genoa (MSNG), Departamento de Zoologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro (UFRJ-POR), National Museum of Natural History, Smithsonian Institution, Washington (USNM), Peabody Museum, Yale University (YPM), Zoologisch Museum, Universiteit van Amsterdam (ZMA-POR).
Formal descriptions of new records for the Colombian Caribbean are provided. In general, each description includes:
(1) full synonymy [including holotype catalogue number and type locality (type loc.)] or reference to previously gathered synonymy, and additions; (2) material examined, including sample site and station number (st.n.), substratum, habitat, depth, sampling date and collector (coll.); (3) morphological description, including colors based on the Naturalist's Color Guide of the American Museum of Natural History (NCG, Smithe, 1975), spiculation [with measurements of n=25 spicules, unless otherwise noted, providing minimum-mean (standard deviation)-maximum sizes, usually length x width] and/or architecture (min.-max.); (4) distribution; (5) taxonomic remarks. Pictures in vivo and camera lucida drawings are also provided when available. Sponge classification follows the Systema Porifera (Hooper & van Soest, 2002).
Results and discussion
Diversity
A total of 77 demosponge species, 3 homoscleromorph sponge species and 1 calcareous sponge species have been found to date in the North-West Gulf of Urabá (including 1 species complex, 2 amphi-Atlantic species and 4 undescribed species). This represents 46 genera, 31 families, and 11 orders within 3 classes of the Phylum Porifera (Table 2). The species Calyx podatypa (de Laubenfels), although previously cited for Urabá (see Zea, 1987), was not found during the present study. Six additional morphotypes have also been found in Urabá but their identity is yet undetermined (see Valderrama, 2001).
This study shows the existence of some demosponge species in which spicule size and/or ornamentation are larger in Urabá than in other Caribbean areas (6 of 21 species for which data are available for comparison). This trend was evident in both length and width of part of the spicules of Plakinastrella onkodes (calthrops), Cinachyrella kuekenthali (oxeas), Leiodermatium aff. pfeifferae (oxeas) and Agelas citrina (acanthostyles). In fact, Zea (1987) detected this trend in at least 37 of 70 species from the Colombian Caribbean, comparing continental and oceanic populations (SW Caribbean), being more evident in populations from the southern coast of Colombia, especially from Urabá. These findings help to support the thesis that the presence of hypersilified skeletons in the Colombian continental shelf, is likely to be correlated to a high fluvial input of dissolved silica, discharged by the large rivers of the southern Caribbean and Central America (e.g., Atrato, Magdalena and San Juan rivers) (Zea, 1985, 1987).
Concomitantly, an increase in the production of ornaments and appearance of accessory siliceous elements was also detected in Urabá. For example, occurrence of a small size of spined tylostyles (recorded smooth elsewhere) in Clathria minuta and apperance of accessory sigmata in Niphates digitalis [recorded also in other continental areas of Colombia (Zea, 1987), but not elsewhere]. Similar findings were found in sponges experimentally exposed to environments with high concentrations of silica (references in Jones, 1979, see also Maldonado et al., 1999). Moreover, it has been demonstrated that the influence of silica concentration on spicule growth may influence not only spicule shape and size, but also the phenotypic expression of several spicule types which are available genetically for a certain sponge (Maldonado et al., 1999).
The sponge Svenzea tubulosa, however, showed a larger spicule size in width only (styles). Similarly, experimental studies with Spongilla lacustris and Suberites domuncula demonstrated that an increase in environmental silica is related to an increase in spicule width but not in length (Jones, 1979; Simpson et al., 1985). On the other hand, Weissenfels & Landschoff (1977) experimentally recorded in the fresh-water sponge Ephydatia fluviatilis normal values in spicule length, but not in width, in sponge individuals deprived of food, under normal environmental concentrations of silica. Temperature is another factor which may influence spicule growth. Experimental studies of its effect have been conducted with Microciona prolifera, being inversely correlated with spicule width, but little correlation with length (Simpson, 1978). In Colombia, for example, in some species those populations exposed to cold-water upwelling (in Santa Marta) show comparative smaller spicules than other continental areas (Zea, 1987).
Systematic descriptions of new records from the Colombian Caribbean
15 species of the Class Demospongiae are here formally recorded and described for the first time for the Colombian Caribbean. Of these, 2 species have names originating in the eastern Atlantic populations (presented as aff.) and 1 is a known but yet-unnamed species (presented as sp.). New species will be published elsewhere.
Phylum Porifera Grant, 1836
Class Demospongiae Sollas, 1885
Order Hadromerida Topsent, 1894
Family Placospongiidae Gray, 1867
Genus Placospongia Gray, 1867
Placospongia sp.1. Fig. 3.
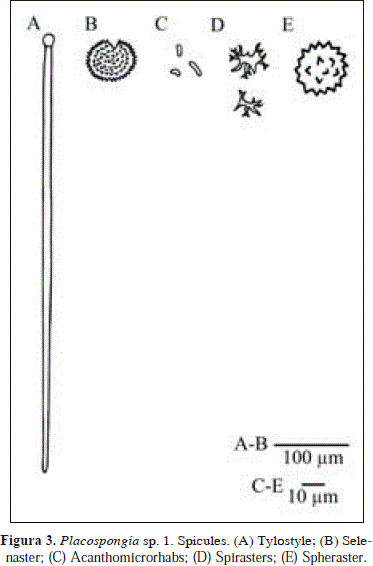
Synonymy fide van Soest, 2009: 11.
Placospongia carinata; Little, 1963: 56, fig. 25, 27; Hechtel, 1965: 62, pl. 7 (fig. 1); Alcolado, 1976: 6; Coelho & Mello-Leitão, 1978: 1; Pulitzer-Finali, 1986: 100; van Soest, 2009: 10 (unpublished specimens from the ZMA collection). Rua et al, 2006: 197; [NON P. carinata (Bowerbank, 1858)]; Muricy et al., 2011:67 (Brazilian records).
Placospongia intermedia; Lehnert & van Soest, 1998: 80; Alcolado, 2002: 60; Alcolado & Busutil, 2012: 68. [NON P. intermedia; de Laubenfels, 1936a: 454 (Caribbean coast) = Placospongia sp. 3 of van Soest, 2009]. [NON P. intermedia Sollas, 1888].
Material
INV-POR 0546: Sapzurro Cove (st.n. 4), on crevices between colonies of Siderastrea siderea, coralline flat of S. siderea, 3 m, 29 Sep. 1995, coll. S. Zea.
Description
Thinly encrusting, less than 2-3 mm in thickness. Color dark brown externally (NCG 22-Burnt Umber, 23-Raw Umber), orange internally (NCG 17-Spectrum Orange). External color remains after preservation in alcohol (brownish-red). Consistency firm but easy to break. Surface smooth to the touch, made up of polygonal plates, having a "veined" or furrowed appearance. In one of the furrows, it shows an oscule slightly elevated, 1.2 mm in diameter, white in alcohol.
Spicules: tylostyles, selenasters, acanthomicrorhabs, spirasters, spherasters.
Tylostyles, prominent tyles, bluntly rounded apices, 258-705.6(179.3)-903 x 7.6-12.4(2.4)-14.3 mm, tyle diameter, 10.9-16.6(2.9)-19 mm; selenasters, smaller ones tending to be bean-shaped and larger ones ellipsoidally rounded, 32.2-66.5(19)-85.1 x 19.6-52.6(19.3)-73.6 mm; acanthomicrorhabds, 4-7.1(1.5)-9.5 x 1.5-2.4(0.6)-4 mm; spirasters, few, tree-like 16.1-19.7(2.3)-23 mm (n=9); spherasters, rare, with short spines, 23.0-32.2 mm (n=3).
Distribution
According to van Soest (2009): Gulf of Mexico (Little, 1963), Jamaica (Pulitzer-Finali, 1986, Lehnert & van Soest, 1998), Brasil (Hechtel, 1976; Coelho & Mello-Leitão, 1978; Rua et al., 2006; also in Muricy et al., 2011) and Colombia (Cartagena). Additional records: Cuba (Alcolado, 1976; 2002), Guadalupe (Alcolado & Busutil, 2012). Colombia (Urabá). Rua et al. (2006) suggested that this species also occurs in the Pacific coast of Panama.
Comments
It has become customary to consider Placospongia specimens with "spirasters" as members of a cosmopolitan species: Placospongia carinata Bowerbank (1858). Nevertheless, van Soest (2009) has questioned this assignment for the material of the Caribbean authors [including citation of Lehnert & van Soest (1998) as P. intermedia], providing a short combined description of ZMA material from Colombia (Cartagena) and Grenada to aid future decisions about the status of the Caribbean populations. The material from Urabá examined here is broadly consistent with this description. However, larger sizes of acanthomicrorhabds (up to 15 x 2 mm) were not found in Urabá, only a smaller and wider type [4-7.1(1.5)-9.5 x 1.5-2.4(0.6)-4 mm vs. 6-8.6-15 x 1-2 mm]. Moreover, the two ectosomal and choanosomal tylostyle size categories suggested by van Soest (2009; also by Pulitzer-Finali, 1986) seem to overlap in Urabá, as a close re-examination of spicule slides showed rather continuous sizes between 199.5 x 7.1 mm to 903 x 14.3 mm [199,5-584,2(243,2)-903,0 x 4,8-10,8(3,2)-14,3 mm (n = 36)], being rarer those sizes below 500 mm in length [199,5-285,5(74,2)-432,3 x 4,8-8,3(3,1)-14,3 mm (n = 12) vs. 503,1-733,5(132,5)-903,0 x 6,5-12,0(2,5)-14,3 mm (n = 24)]. As cited by Hechtel (1965), some spherasters were also found in Urabá. A segregation of spiraster-like and amphiaster-like spicules could not be discriminated here due to low microscopic resolution.
Family Spirastrellidae Ridley & Dendy, 1886
Genus Spirastrella Schmidt, 1868
Spirastrella coccinea (Duchassaing & Minchelotti, 1864) PL.1(A), Fig. 4.
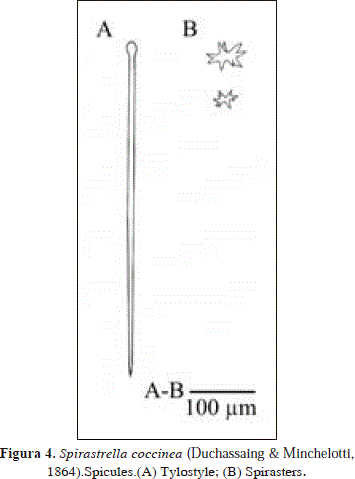
Synonymy in Wiedenmayer, 1977: 163. In addition:
Spirastrella coccinea, Pulitzer-Finali, 1986: 90, fig. 21; Kobluk & van Soest, 1989: 1210; Mothes & Bastian, 1993: 20, figs. 17, 18, 40; Lehnert & van Soest, 1998: 79; Lehnert & van Soest, 1999: 145, Alcolado, 1999: 121; Zea et al., 2009; Alcolado & Busutil, 2012; Muricy et al., 2011: 70 (Brazilian records). [NON S. coccinea of the authors cited by Hecthtel, 1965: 54 = S. hartmani Boury-Esnault et al., 1999, a valid species)].
Material
INV-POR 0574: Cabo Tiburón (st.n. 1), dead coral, calcareous terrace after cliff, 9 m, 28 Sep. 1995, coll. S. Zea.
Description
Thickly encrusting, 1.5-3.0 mm thick. Color red scarlet (NCG 14-Scarlet) in vivo and white in alcohol. Underwater it looks red with whitish scattered oscules. Consistency leathery. Smooth surface. Short, vein-like, whitish exhalant canals converge in oscules.
Spicules: tylostyles, spirasters.
Tylostyles, 426-550.8(83.9)-690 x 9-11.6(1.3)-12.9 mm; spirasters, 13.8-41.1(12.7)-57.5 mm.
Distribution
St. Thomas (Duchassaing & Michelotti, 1864), Bahamas (de Laubenfels, 1949; Wiedenmayer, 1977; Pulitzer-Fi nali, 1986; Zea et al., 2009), North Carolina (Wells et al., 1960), Gulf of Mexico (Apalachee Bay, Little, 1963), Puerto Rico (Wiedenmayer, 1977), Dominican Republic (Pulitzer-Finali, 1986), Guadalupe (Alcolado & Busutil, 2012), Bonaire (Kobluk & van Soest, 1989), Brazil (Fernando de Noronha Archipelago, Mothes & Bastian, 1993; Alagoas state, Muricy et al., 2011), Jamaica (Lehnert & van Soest, 1998, 1999), Cuba (Alcolado, 1999), Colombia (Urabá).
Comments
The material examined from Urabá fits with recent descriptions of Spirastrella coccinea (Duchassaing & Minchelotti, 1864) (see Wiedenmayer, 1977). This species occurs sympatrically with an orange morphotype, which is assigned here to S. hartmani Boury-Esnault et al., 1999 described below, as it bears smaller tylostyles (297-477 x 6.5-12.9 mm) and spirasters that reach smaller sizes (down to 5.8 mm) than S. coccinea (red morphotype).
Spirastrella hartmani Boury-Esnault, Klautau,Bézac, Wulff & Solé-Cava, 1999
PL. 1(B), Fig. 5.
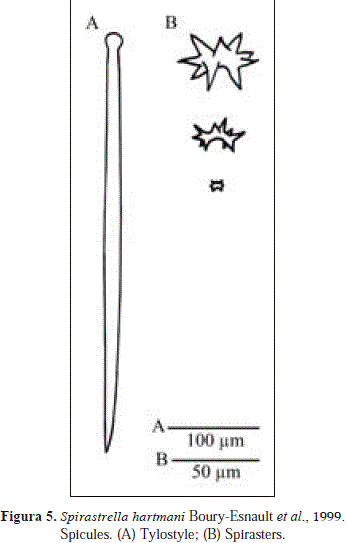
Synonymy in Wiedenmayer, 1977: 162 (as Spirastrella cunctatrix). In addition:
Spirastrella hartmani Boury-Esnault et al., 1999: 46 (holotype: MNHN-NBE-D.1469; YPM 21026 and 21027; type loc.: San Blas Island, Panamá); Muricy et al., 2008: 60; Zea et al., 2009; Muricy et al., 2011: 70 (Brazilian records); Hajdu et al., 2011: 98; Moraes, 2011.
Spirastrella coccinea; of the authors cited by Hechtel, 1965: 54 [NON S. coccinea (Duch. & Mich., 1964), a valid species; NON S. coccinea; Dickinson, 1945 =S. sabogae Boury-Esnault et al., 1999].
Spirastrella cunctatrix; Wintermann-Kilian & Kilian, 1984: 130; Pulitzer-Finali, 1986: 90, fig. 21; Alcolado, 1999: 121 [NON S. cunctatrix Schmidt, 1968 and other authors from the Mediterranean, a valid species].
Material
INV-POR 0576: Cabo Tiburón (st.n. 1), dead coral, calcareous terrace after cliff, 9 m, 28 Sep. 1995, coll. S. Zea.
Description
Thickly encrusting, 1.5-3.0 mm thick. Color orange (NCG 132c-Orange Rufous) in vivo and light brown (NCG 39-Cinnamon) in alcohol. Consistency leathery. Surface smooth to the touch, showing branching surface canals converging toward scattered oscula, elevated in a vein-like pattern.
Spicules: tylostyles, spirasters.
Tylostyles, straight, thicker at the middle and thinner below the tyle, 297-477 x 6.5-12.9 mm (n=7); spirasters, 5.8-62.1mm (n=6).
Distribution
According to Wiedenmayer (1977): Dry Tortugas and West coast of Florida, Gulf of Mexico (South-West of the Apalachee Bay), Jamaica, Bahamas (also in Pulitzer-Finali, 1986; Zea et al., 2009), Bermuda, North Carolina. In addition: St. Thomas (Boury-Esnault et al., 1999), Colombia (Santa Marta, Wintermann-Kilian & Kilian, 1984; Urabá), Cuba (Alcolado, 1999), Brazil (Muricy et al., 2008; Muricy et al., 2011; Hajdu et al., 2011; Moraes, 2011).
Comments
Spirastrella hartmani Boury-Esnault et al., 1999, was cited from Santa Marta, Colombia but not described in detail by Wintermann-Kilian & Kilian (1984, as Spirastrella cunctatrix). The material examined from Urabá broadly fits with recent descriptions of S. hartmani [see Wiedenmayer, 1977 (as S. cunctatrix), Boury-Esnault et al., 1999]. Further comments in S. coccinea above.
Demospongiae "Lithistids"
Family Azoricidae Sollas, 1888
Genus Leiodermatium Schmidt, 1870
Leiodermatium aff. pfeifferae (Carter, 1873)
Pl. 2, Fig. 6.
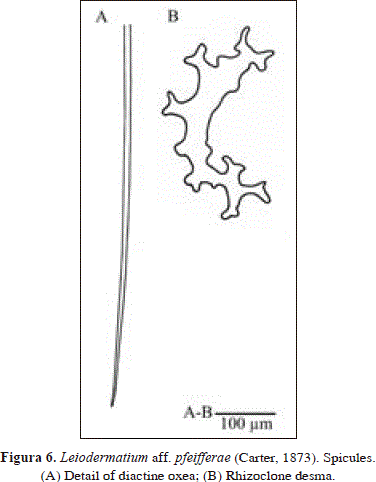
Synonymy in Sollas, 1888: 319 (as Azorica pfeifferae). In addition:
Leiodermatium pfeifferae; Rützler, 1986: 126, fig. 34; Alcolado, 2002: 59; Muricy et al., 2011: 142 (Brazilian records); (?) Kelly-Borges & Valentine, 1995 (Oceania).
Material
INV-POR 0570: Sapzurro Cove (st.n. 2), under a pagoda-like coral overhang, reef base, 18 m, 28 Sep. 1995, coll. J.A. Sánchez.
Description
Massive, flabellate, forming a shallow horizontal plate, about 1.5 cm high, 4 cm wide, with walls 3 mm thick, with undulated margins. It presents a small area of attachment to the substratum. Color cream-white alive. Consistency stony hard. Microhispid surface at exposed areas. Inconspicuous openings. The skeleton consists of a regular reticulation of rhizoclone desma spicules. The ectosome is formed by perpendicular tufts of oxea more than 1 mm high.
Spicules: rhizoclone desmas, diactine oxeas.
Rhizoclone desmas, non tuberculated, some with bifid zygomes, 166-485 x 19-28.5 mm (n=5); smooth desmas as developmental stages; very long (broken in slide) diactine oxeas, fragments up to 1406 x 12.9 mm.
Distribution
Eastern Atlantic: according to Carter (1876) and Sollas (1988), from Madeira to the coast of Portugal and the Cape Verde Islands. Western Atlantic: according to Sollas (1888): Amboina, Bermuda (also in Rützler, 1986), Brazil (Bahia; also in Muricy et al., 2011), Cape St. Vincent. Additionally: Cuba (Alcolado, 2002), Colombia (Urabá). Indo-Pacific: Oceania (Kelly-Borges & Valentine, 1995).
Comments
The habit of the specimen from Urabá is very similar to that drawn by van Soest & Stentoft (1988) as Leiodermatium lynceus Schmidt, 1870. However, those authors report smaller oxea (190-230 x 1 mm vs. broken fragments up to 1406 x 12.9 mm in Urabá) and a smooth surface (hispid in Urabá). The habit of L. pfeifferae (Carter, 1873) is also very similar but the type was described as a large sponge (dimensions: 29 x 23 cm), covered externally by tubercles (fide Sollas, 1888) that are absent in the specimen from Urabá (dimensions: 4 x 1.5 cm). In spite of these differences, they are thought to be conspecific as both wall thickness (about 3 mm) and oxea dimensions (cf. Carter, 1876 and Sollas, 1888, 750-1814 x 8.5 mm in L. pfeifferae) are very similar. Moreover, Sollas (1888) reported inconspicuous openings and hispidation of 500 mm, similar to the one reported here (approx.1 mm in height). In addition, the appearance of the rhizoclone desmas is very similar, especially, in regard to the occurrence of bifid zygomes. Although recent authors on the Caribbean (see Rützler, 1986) use the name L. pfeifferae for their material, this assignment is tentative until its conspecificity with eastern Atlantic populations, the area in which the species was originally described, is confirmed.
Order Poecilosclerida Topsent, 1928
Suborder Microcionina Hajdu, van Soest & Hooper, 1994
Family Microcionidae Carter, 1875
Subfamily Microcioninae Carter, 1875
Genus Clathria Schmidt, 1862
Subgenus Thalysias Duchassaing & Michelotti, 1864
Clathria (Thalysias) virgultosa (Lamarck, 1814) PL 1(C), Fig. 7.
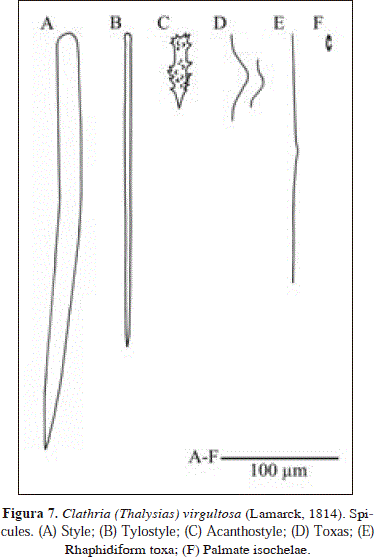
Synonymy in Hooper, 1996: 411. In addition:
Microciona juniperina; Alcolado, 1976: 5.
Thalysias juniperina; Rathe Peralta, 1981: 17.
Clathria (Thalysias) virgultosa; Lehnert & van Soest, 1998: 87, fig. 16.
Clathria virgultosa; Alcolado, 1999: 122; 2002: 64; Zea et al., 2009.
Clathria clathrata; Alcolado, 1976: 5 (fide Alcolado, 2002: 64)
Material
INV-POR 0553: Isla Terrón de Azúcar (st.n. 7), calcareous algae, dead sides of coral, calcareous terrace, 6-7 m, 30 oct. 1995, coll. S. Zea.
Description
Thickly encrusting. Red in color (NCG 12-Geranium) with dark purple tones (NCG 8-Carmine). Consistency rubbery and elastic. Surface uneven, with low tubercules surrounded by vein-like branching surface canals, white in color, converging towards the scattered oscules. Ectosome contracts out of the water. The specimen is covered in part by the sponge Monanchora arbuscula (Duchassaing & Michelotti). A massive individual was also seen in the field, approx. 10 cm thick.
Spicules: styles, tylostyles, acanthostyles, toxas, rhaphidiform toxas, palmate isochelae.
Styles, thick, curved, smooth heads, 278-344.4(34.7)-399 x 9.5-17.6(3.3)-23.8 mm; tylostyles, straight, fusiform, wide range of sizes, tyles conspicuous in smaller sizes, but less conspicuous in larger ones, 124-298.8(70.8)-375 x 2.4-5.7(1.9)-9.5 mm; acanthostyles, spines highly dispersed or absent on neck, 54.1-66.2(5.8)-77.1 x 9.2-13.3(3.1)-20.7mm; toxas, 28.8-69.2 (15.1)-89.7 mm; rhaphidiform toxas, 181-281.2(96.9)-551 mm (n=12); palmate isochelae, 11.2-17.8(1.4)-20.1 mm (n=19).
Distribution
According to van Soest (1984): St. Thomas, Florida, Guadaloupe, Puerto Rico, Cuba (also in Alcolado, 1976; 1999), Yucatán. In addition: Bahamas (Zea et al., 2009), Dominican Republic (Rathe Peralta, 1981), Colombia (Santa Marta, Wintermann-Kilian & Kilian, 1984; Urabá), Jamaica (Lehnert & van Soest, 1998).
Comments
Clathria (Thalysias) virgultosa (Lamarck, 1814) was cited from Santa Marta, Colombia but not described in detail by Wintermann-Kilian & Kilian [1984, as Thalysias juniperina (Lamarck, 1814)]. The material examined from Urabá is broadly consistent with the description of van Soest [1984, as Rhaphidophlus juniperinus (Lamarck, 1814)]. Nonetheless, toxa are not as small (8-42.5-76 in St. Thomas and Florida vs. 28.8-69.2-89.7 mm in Urabá).
Clathria (Thalysias) minuta (van Soest, 1984). Fig. 8.
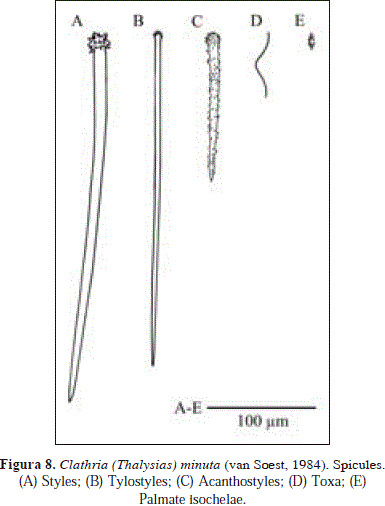
Synonymy in Hooper, 1996: 410 and Muricy et al., 2011: 147. In addition:
Clathria minutus; Alcolado, 1999: 122.
Clathria (Thalysias) ?minuta; Zea et al., 2009.
Material
INV-POR 0548: Sapzurro Cove (st.n. 4), crevices between colonies of Siderastrea siderea, reef flat of S. siderea, 2-3 m, 29 Sep. 1995, coll. S. Zea.
Description
Thinly encrusting, 1 mm thick, 2-3 cm in diameter. Color scarlet red (NCG 14-Scarlet). Consistency soft, fragile. Oscules are not apparent. The specimen consists of tiny fragments.
Spicules: styles, tylostyles, acanthostyles, toxa, palmate isochelae.
Styles, slightly curved with densely spined heads, 309-430.8(69.4)-603 x 5.7-10(1.4)-11.9 mm; tylostyles, straight with microspined heads, 143-309.2(66.5)-387 x 2.4-4.3(0.5)-5.7 mm, acanthostyles, entirely spined, 80.8-121.6(34.7)-183 x 4.8-10.5(1.9)-14.3 mm; toxa, not abundant, 43.7-79.4(14.5)-97.8 mm (n=20); palmate isochelae, 16.7-18.3(0.6)-19.6 mm.
Distribution
Bahamas (Zea et al., 2009), Curaçao (van Soest, 1984), Northeast (Fernando de Noronha Archipelago) to Southeastern Brazil (Arraial do Cabo, Hooper, 1996; Muricy et al., 2011), Cuba (Alcolado, 1999), Colombia (Urabá). Also reported from Tropical West Africa (van Soest, 1993).
Comments
Unlike the original description of Clathria (Thalysias) minuta (van Soest, 1984, as Raphidophlus minutus), the specimen from Urabá does not bear a small category of smooth tylostyles (147-191.5-258 x 1.5-2.1-2.5 mm). Moreover, its microspined acanthostyles show a wider size range (142.5-309.2-387.1 x 2.4-4.3-5.7 mm), reaching much smaller sizes than the original (294-322.6-361 mm). These discrepancies may correspond to a geographical variation in spicule size and shape, as the assumedly greater concentration of dissolved silica in the Gulf of Urabá, produced by the Atrato River discharge, may increase the production of spicule ornaments, as well as, the appearance of accessory siliceous elements.
Suborder Myxillina Hajdu, van Soest & Hooper, 1994
Family Coelosphaeridae Dendy, 1922
Genus Lissodendoryx Topsent, 1892
Subgenus Lissodendoryx Topsent, 1892
Lissodendoryx (Lissodendoryx) strongylata van Soest, 1984 Fig. 9.
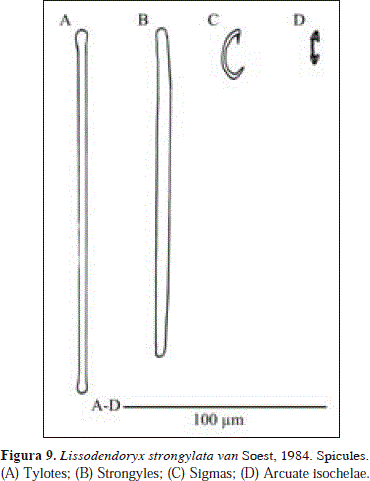
Synonymy:
Lissodendoryx strongylata van Soest, 1984: 58, pl. V 4-5, fig. 21 (holotype: ZMA POR. 3508; paratype: ZMA POR.3509; type loc.: Piscadera Baai, Curaçao).
Material
INV-POR 0541: Cabo Tiburón (st.n. 1), growing within beds of the algae Amphiroa spp., calcareous terrace after cliff, 9 m, 28 Sep. 1995, coll. S. Zea.
Description
Exhalant fistules, approx. 4.0-5.5 mm wide, with walls 0.5 mm thick. Color lilac in vivo, white-transparent when preserved in alcohol. Fistules are papyraceous, fragile, easy to tear. They were observed emerging within beds of algae (Amphiroa spp.). It is not clear if they arose from an encrusting or massive base.
Spicules: tylotes, strongyles, sigmas, arcuate isochelae.
Tylotes, elongated heads, barely perceptible, similar in shape to strongyles but thinner, 171-177.7(9)-200 x 2.4-4.3(0.5)-4.3 mm; strongyles, 152-165.8(8.1)-181 x 4.3-5.2(0.5)-5.7mm; sigmas, 23-26.3(2)-29.9 mm; arcuate isochelae, 16.1-19.2(3.2)-27.6 mm.
Distribution
Curaçao (van Soest, 1984), Colombia (Urabá).
Comments
The material examined here differs from the original description of Lissodendoryx strongylata van Soest, 1984, in terms of habit (fistulose vs. thick masses of amorphous shape in the holotype) and color (lilac vs. brick-red in the holotype), characteristics which, together with spiculation (especially the possession of strongyles instead of straight styles), distinguish Lissodendoryx strongylata from other congeneric species (cf. van Soest, 1984; Zea & van Soest, 1986). Nevertheless, the close relationship between this species and the material of Urabá is evident by the possession of the same spiculation. It is possible then that the holotype was an amorphous mass of dark skin that had lost its transparent fistules, such as those of the specimen of Urabá. Unfortunately, the nature of the sponge base that supports the fistules of the Urabá specimen (massive or encrusting) is unknown.
Lissodendoryx strongylata is here assigned to the subgenus Lissodendoryx Carter, 1882, despite of the strongylote nature of its choanosomic spicules [contrary to the styloids typical for the subgenus (cf. van Soest, 2002)]. The remaining spicule complement, including ectosomal tylotes, sigmas and arcuate isochelae as microscleres, and lack of a smaller category of echinating acanthostyles, are characteristic of this subgenus. The combination of ectosomal tylotes and choanosomal strongyles in L. strongylata is a feature that seems not to occur in any of the five subgenera proposed by van Soest (2002) for the genus Lissodendoryx.
Order Halichondrida Gray, 1867
Family Desmoxyidae Hallmam, 1917
Genus Myrmekioderma Ehlers, 1870
Myrmekioderma rea (de Laubenfels, 1934). Pl. 1(D), Fig. 10
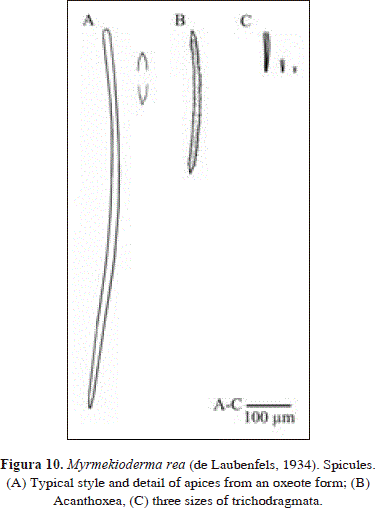
Synonymy in Muricy et al., 2011:67. In addition:
Viles(?) strongyloxea Alcolado & Gotera, 1986: 5, figs. 5b, 6 (synonymy suggested by Díaz et al., 1993).
Myrmekioderma rea; Zea et al., 2009.
Myrmekioderma styx de Laubenfels, 1953: 523, fig. 3; de Rosa-Barbosa, 1995: 120, figs. 1-11; Alcolado, 2002: 63.
[NON Myrmekioderma styx; Díaz et al., 1993: 303 (and many other authors, see also list in Muricy et al., 2011: 96) = Myrmekioderma gyroderma (Alcolado, 1984)] (see table 2 and below)].
Material
INV-POR 0556: Isla Terrón de Azúcar (st.n. 7), pavement, calcareous terrace after coastal cliff, 6-7 m, 30 Sep. 1995, coll. S. Zea.
Description
Massive, approx. 5 cm in diameter. Color orange in vivo (NCG 17-Spectrum Orange), cream in alcohol (NCG 54-Cream color). Consistency firm, somewhat compressible but friable with force. Irregular surface with very low mounds and areas with tiny folds, elongated as protuberances, forming valleys difficult to discern. At the top, there are three oscula, poorly differentiated (possibly damaged by preservation), 1.5 to 3 mm in diameter. Observed filling crevices and densely fouled, with some free areas.
Spicules: styles, acanthoxea, raphides in trichodragmata
Styles, curved, slender, often as oxea or strongyloxea with blunt tips, 735-913(86.4)-1130 x 7.7-12.9(2.6)-19.4 mm; acanthoxea, with fewer spines toward the center, 299-333.5(25.7)-380 x 8.6-11.4(2.4)-14.3 mm; rhaphides in trichodragmata, straight, some sinuous, 38.0-87.9(31.8)-133 x 5.7-9.0(1.9)-11.9 mm, in three size ranges: 92.6-133 x 5.7-11.9 mm (n = 8) vs. 38.0-61.8 x 9.5-11.9 mm (n = 5) vs. 19-28.5 x 4.8-9.5 mm (n = 5).
Distribution
Puerto Rico (de Laubenfels, 1934), Mexico (de Laubenfels, 1953), Venezuela, Bahamas (Diaz et al., 1993; Zea et al., 2009), Barbados (van Soest & Stentoft, 1988, Diaz et al., 1993), Cuba (Alcolado & Gotera, 1986; Alcolado, 1999; 2002), Brazil (de Rosa-Barbosa, 1995; Muricy et al., 2011), Jamaica (Lehnert & van Soest, 1998), Colombia (Urabá).
Comments
Even though the material studied from Urabá bears longer styles/oxea/strongyloxea than other Caribbean areas [735-913-1130 x 7.7-12.9-19.4 mm in Urabá vs. 260-600-800 x 5-11-20 mm in several areas (Diaz et al. 1993)], they are as thin as characteristic for the species. A related species, M. gyroderma, bears characteristic stouter oxeas [570-1125 x 8-45 mm in Diaz et al. (1993) as M. stix, and 180-1000 x 1-31 mm in Alcolado (1984)]. In a similar way, the 2-3 sizes of trichodragmata reported here (2 in van Soest & Stentoft, 1988, but 1 in Diaz et al. 1993) are as thin as characteristic for the species (4.8-11.9 mm in Urabá vs. 3-10 mm in other Caribbean areas, see Diaz et al. 1993). In contrast, they are wider in M. gyroderma (8-32 mm, Diaz et al. 1993 as M. stix).
After revision of holotypes, Myrmekioderma rea (de Laubenfels, 1934) and M. styx de Laubenfels, 1953, were found to be conspecific (K. Ruetzler, USNM, in litt.). On the basis of the descriptions, Alcolado (2002) had previously concluded that they were different. On the other hand, other Caribbean authors used M. styx erroneously for the other species commonly found in Caribbean reefs known now as M. gyroderma (Alcolado, 1984) (Castellanos et al., 2003). Those species can be differentiated by growth form (filling crevices or buried in sand and rubble in M. rea vs. massive and exposed in M. gyroderma), non-fouled surface areas (showing circular grooves that form characteristic warts when contracted in M. rea vs. elongated meandering grooves in M. gyroderma), and principal spicules (thinner, slender styles/oxea/strongyloxea in M. rea vs. wider, stout oxea in M. gyroderma) (Zea et al., 1999).
Family Dictyonellidae van Soest, Díaz & Pomponi, 1990
Genus Svenzea (Alvarez, Erpenbeck & Alvarez, 2002)
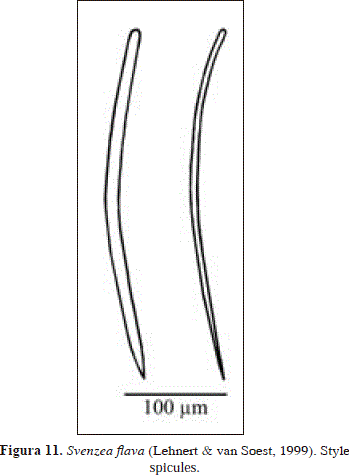
Svenzea flava (Lehnert & van Soest, 1999) PL. 1(E), Fig. 11.
Synonymy:
Pseudaxinella(?) flava Lehnert & van Soest, 1999: 151, figs. 25-30 (holotype: ZMA POR 13563, type loc.: Discovery Bay, Dairy Bull, Jamaica).
Svenzea flava; Zea et al., 2009.
Material
INV-POR 0537: Cabo Tiburón (st.n. 1), dead coral, calcareous terrace after cliff, 9 m, 28 Sep. 1995, coll. S. Zea.
Description
Massive, cavernous sponge. Color olive yellowish-green externally (NCG 50-Yellowish Olive Green, 49-Greenish Olive), darker on the sides and underneath the green (NCG 31-Marron, possibly due to pigments produced by associated cyanobacteria), cream internally (NCG 54 -Cream Color). Whole specimen turned cream when preserved in alcohol. Consistency soft. Smooth surface, pierced by fields of pores, 0.5-3 mm in diameter, some of them covered by a thin organic veneer.
Spicules: styles
Styles, evenly curved, thick and thin (the latter tend to thin towards the head), some stepped tips, 290-354.4(31.8)-409 x 4.8-10.9(3.3) -14.3 mm; a few styles attain 428 mm in length.
Distribution
Jamaica (Lehnert & van Soest, 1999), Bahamas (Zea et al., 2009), Colombia (Urabá),
Comments
The material examined from Urabá agrees broadly with the original description of Pseudaxinella(?) flava Lehnert & van Soest, 1999. A related species is Svenzea tubulosa (Alcolado & Gotera, 1986). Both species share a similar skeletal organization (isoor anisotropic reticulation, with multispicular ascending tracts) and the same spicules (styles only), but are easily distinguished by habit (massive in S. flava vs. tubular in S. tubulosa), and in spicule shape and robustness (width: 2-14.3 mm in S. flava vs. 12-21.4 mm in S. tubulosa) (see Alcolado & Gotera, 1986, Lehnert & van Soest, 1999).
Pseudaxinella(?) flava is here tentatively assigned to the genus Svenzea Alvarez et al., 2002, especially because of its overall resemblance to S. zeai (Alvarez et al., 1998), with which it shares a similar shape and consistency, a reticulation of styles, and lack of ectosomal specialization. Nevertheless, as stated by Alvarez et al. (2002), a definitive assignment of Pseudaxinella(?) flava to Svenzea is not possible due to its skeletal organization (different to the typical unipaucispicular reticulation of Svenzea) and apparent absence of granular cells and large embryos/larvae.
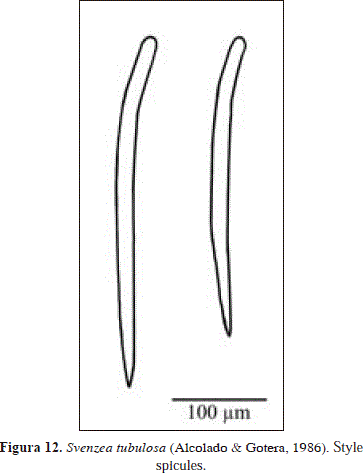
Svenzea tubulosa (Alcolado y Gotera, 1986) PL. 1(F), Fig. 12.
Synonymy:
Scopalina(?) tubulosa Alcolado & Gotera, 1986: 6, figs. 5c, 7 (holotype: IdO 353; type loc.: Playa Baracoa, north-west of Habana province, and Habana city, Cuba).
Pseudaxinella tubulosa; Alcolado, 1999: 121; Alcolado, 2002: 63.
Svenzea tubulosa; Zea et al., 2009.
Material
INV-POR 0550: Sapzurro Cove (st.n. 6), dead coral, slope of reef buttress, 16 m, 29 Sep. 1995, coll. S. Zea.
Description
Tubes arising from cracks and crevices, 10 cm high, 13-15 mm wide, with 2-5 mm-thick walls. Tubes are wider at the apex, having an elongated, bean-shaped apical oscule, up to 17 mm in diameter, sometimes divided. Color brown externally (NCG 31-Maroon), faded to cream at sides, base and internally (NCG 54-Cream Color). Turns cream when preserved in alcohol. Consistency firm, somewhat compressible, easy to tear. Micro-verrucose surface, rough to touch, with scattered smaller openings, 1.3 mm in diameter; only one attained 3.6 mm in diameter and was covered by a skinny organic pinacoderm.
Spicules: styles
Styles, straight, with a basal bend (similar to a rhabdostyle but less pronounced), 323-382.4(29.5)-413 x 13.3-18.1(2.4)-21.4 mm.
Distribution
Cuba (Alcolado & Gotera, 1986; Alcolado, 1999; 2002), Bahamas (Zea et al., 2009), Colombia (Urabá).
Comments
Spicules from the material of Urabá are wider than those from the original description (12-15 mm in Cuba vs. 13.3-21.4 mm in Urabá) (see Alcolado & Gotera, 1986). Those authors did not describe the surface of their material, which is micro-verrucose in Urabá. As stated above, S. flava and S. tubulosa are similar species with dubious generic assignment, being herein tentatively assigned to Svenzea Alvarez et al., 2002. See more comments above under Svenzea flava.
Family Halichondriidae Gray, 1867
Genus Hymeniacidon Bowerbank, 1859
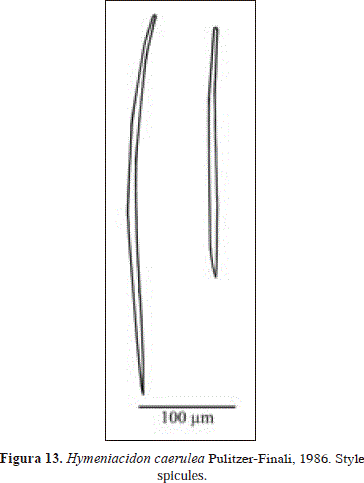
Hymeniacidon caerulea Pulitzer-Finali, 1986 PL. 3(A), Fig. 13.
Synonymy:
Laxosuberites coerulea; de Laubenfels, 1936b: 148 [NON Terpios coerulea Carter, 1882 = Terpios fugax Duchassaing & Micheloti, 1864 fide Rützler & Smith, 1993].
Hymeniacidon caerulea Pulitzer-Finali, 1986: 117, fig. 118 (holotype: MSNG 47693; type loc.: La Parguera, Puerto Rico); Díaz et al., 1993: 297, figs. 25, 31; Alcolado, 2002: 63.
Material
INV-POR 0558: Isla Terrón de Azúcar (st.n. 8), under coral and pavement, calcareous terrace, 6-8 m, 30 Sep. 1995, coll. S. Zea.
Description
Massive, growing on coralline algae and rubble. Cavernous interior. Color dark blue (NCG 90-Blue Black, 73-Indigo) in vivo, green-bluish when preserved in alcohol (NCG 63-Paris Green). Consistency fragile, easy to tear. Smooth to the touch. Rare oscula, up to 4 mm in diameter, and smaller pores, up to 1.5 mm in diameter.
Spicules: styles
Styles, slightly but evenly curved that tend to thin towards the head, 232-429.6(122.6)-613 x 4.8-9(3.8)-16.6 mm.
Distribution
Florida (Dry Tortugas, de Laubenfels, 1936b), Puerto Rico (Pulitzer-Finali, 1986), Cuba (Alcolado, 1999; 2002), Colombia (Urabá).
Comments
In agreement with Diaz et al. (1993) this material is assigned to Hymeniacidon caerulea Pulitzer-Finali, 1986, due to its blue color, which distinguishes it from other congeneric species. However, the massive habit of the specimen from Urabá is an innovation for the species, which usually fills cracks and crevices under rocks. Also different is the thinning at the basal end of the styles recorded here (see Pulitzer-Finali, 1986; Diaz et al., 1993).
Order Agelasida Hartman, 1980
Family Agelasidae Verril, 1907
Genus Agelas Duchassaing & Michelotti, 1864
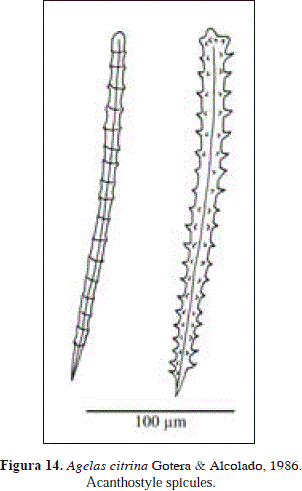
Agelas citrina Gotera & Alcolado, 1987
PL. 3(B), Fig. 14.
Synonymy:
Agelas citrina Gotera & Alcolado, 1987: 1, figs. 1-2 (holotype: IdO 645, type loc.: west margin of the Gulf of Batabanó, Cuba); Alcolado, 2002: 61 (checklist); Zea et al., 2009; Alcolado & Busutil, 2012: 69.
INV-POR 0551: Sapzurro Cove (st.n. 6), under laminar coral, slope of reef buttress, 16 m, 29 Sep. 1995, coll. S. Zea.
Description
Massive, approx. 20 cm in diameter. Orange color (NCG 16-Chrome Orange) in vivo, lighter at the base (NCG 17-pectrum Orange). Color brown when preserved in alcohol (Raw Umber 23-NCG). Consistency rubbery and compressible, difficult to tear. Conulose surface, each conule up to 5 mm high, 3-6 mm apart, covered by a thick, transparent, skinny organic veneer, which looks glossy over valleys between conules. Solitary oscula or in clusters inside depressions. The specimen gives off a sulfur smell.
Spicules: acanthostyles
Acanthostyles, verticillated, 162-263.2(58.4)-363 x 7.1-13.8(3.3)-19 mm, having 13-21.8 (4.2)-26 regular whorls per spicule, and 4-5 spines per whorl; spine development varies between spicules, from well-developed to tiny nubs that are hardly noticeable.
Distribution
Cuba (Gotera & Alcolado, 1987; Alcolado, 2002), Guadalupe (Alcolado & Busutil, 2012), Bahamas (Zea et al., 2009), Colombia (Urabá).
This material is consistent with the original description of Agelas citrina from Cuba (Gotera & Alcolado, 1987), except for the height of the conules (4-5 mm in Urabá vs. 2-3 mm in Cuba). Further differences in spicule size (102-218 x 10-13 mm in Cuba vs. 162-364 x 7.1-19 mm in Urabá), being much larger in Urabá than in Cuba, in correspondence with the conditions in Urabá that promote hypersilicification. At any rate, it is possible to assign the material examined here to Agelas citrina from features such as a conulose surface, a rotten smell, and the presence of long spicules, all of which distinguish it from other Agelas species.
Order Haplosclerida Topsent, 1928
Suborder Haplosclerina Topsent, 1928
Family Chalinidae Gray, 1867
Genus Haliclona Grant, 1835
Subgenus Halichoclonade Laubenfels, 1932
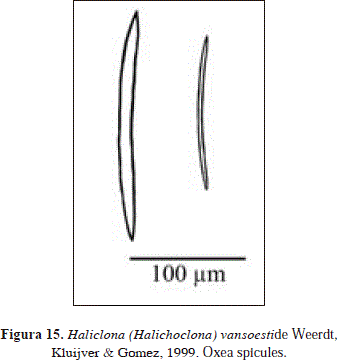
Haliclona (Halichoclona) vansoesti de Weerdt, Kluijver & Gomez, 1999
PL. 3(C), Fig. 15.
Synonymy:
Haliclona (Halichoclona) vansoesti de Weerdt et al., 1999: 47, figs. 1-3 (holotype: ZMA POR 13391, type loc.: Piscadera Baai, Curaçao).
Material
INV-POR 0540: Cabo Tiburón (st.n. 1), dead coral, calcareous terrace after cliff, 9 m, 28 Sep. 1995, coll. S. Zea.
Description
Lobated, massively encrusting. Color light blue in vivo, almost white (NCG 74-Cyanine Blue) to cream-transparent when preserved in alcohol. Consistency firm, but brittle. Slightly raised oscula, approx. 5 mm in diameter, scattered over the surface.
Spicules: Oxea
Oxea, hastate, 166-200(12.8)-214 x 4.8-9(1.4)-11.9 mm.
Distribution
Curaçao, Jamaica, St. Vincent, Martinica (de Weerdt et al., 1999), Colombia (Urabá).
Comments
The material studied here is consistent with the original description of Haliclona (Halichoclona) vansoesti de Weerdt et al., 1999. This species was originally described as having the choanosome light purple and the ectosome white semi-transparent. Although in Urabá the specimen had a bluish tone, its color was very similar to that originally reported [see Pl.3(C)]. For a better understanding of the species refer to the original description.
Order Dictyoceratida Minchin, 1900
Family Thorectidae Bergquist, 1978
Subfamily Thorectinae Bergquist, 1978
Genus Smenospongia Wiedenmayer, 1977
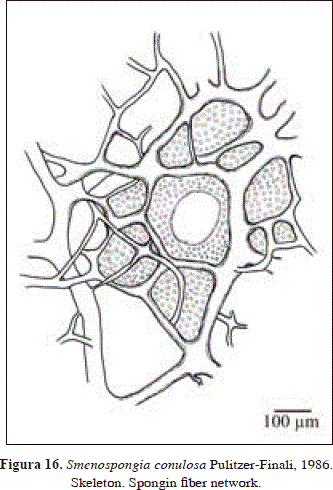
Smenospongia conulosa Pulitzer-Finali, 1986
PL. 3(D), Fig. 16.
Synonymy:
Smenospongia conulosa Pulitzer-Finali, 1986: 179, fig. 86 (holotype: MSNG 47711, type loc.: La Parguera, Puerto Rico); Lehnert & van Soest, 1998: 94, Alcolado, 1999: 123; 2002: 70; Zea et al., 2009.
Material
INV-POR 0544: Sapzurro Cove (est.n. 2), dead coral, reef base, 16-18 m, 28 Sep. 1995, coll. S. Zea.
Description
Massive, flabellate. The color of the specimen collected was bright light green in vivo (NCG 161-Pistachio), with lighter shades of brown internally (NCG 22-Burnt Umber) becoming dark brown, almost black, when preserved in alcohol (NCG 19-Dusky Brown). Other individuals seen and photographed [Pl 3(D)] were dark, brownish green. Consistency compressible, elastic, easy to tear. Oscules are slightly elevated and aligned along ridges, approx. 13 mm in diameter, surrounded by a smooth membranous rim. Several exhalant canals converge inside atria. The surface is abundantly covered by blunt conules, up to 3 mm in height, spaced approx. 2.5 mm, not connected by ridges. Between conules there are openings flush with the surface, 1-2 and 4-7 mm in diameter. The specimen gives off a sulfur smell and releases mucus.
Skeleton:
Regular reticulation of spongin fibers, orange in color, which lack a pith or any foreign material inside. Although thick and thin fibers are distinguishable, they are interconnected, being not differentiated as primary or secondary. Fibers 12.9-64.5 mm in diameter. Reticulation with meshes 86-518 mm in diameter, which are obscured by pigment granules(?). Some meshes are partially obscured, forming circular shapes with the appearance of ascending fascicles, 60-250 mm in diameter.
Distribution
Puerto Rico and Dominican Republic (Pulitzer-Finali, 1986), Jamaica (Lehnert & van Soest, 1998), Cuba (Alcolado, 1999: 2002), Bahamas (Zea et al., 2009), Colombia (Urabá).
Comments
The description of this material is broadly consistent with the original description of Smenospongia conulosa Pulitzer-Finali, 1986. However, the bright light green color of some specimen from Urabá contrasts with the darker tones of brown and olive green seen for the species here and in other Caribbean areas (cf. Pulitzer-Finali, 1986, Lehnert & van Soest, 1998). Whether these color morphotypes are different species remains to be determined. Zea et al. (2009) have tentatively separated the two color morphotypes as S. conulosa (dark green) and Smenospongia sp.-parrot green (light green).
Family Dysideidae Gray, 1867
Genus Pleraplysilla Topsent, 1905
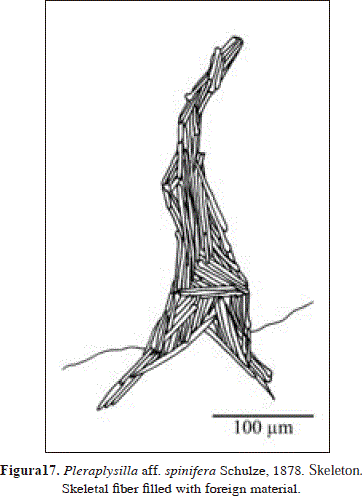
Pleraplysilla aff. spinifera Schulze, 1878
Fig. 17.
Synonymy in Cook & Bergquist, 2002: 1063 (as Pleraplysilla spinifera).
Material
INV-POR 0547: Sapzurro Cove (st.n. 4), dead coral, reef flat of Siderastrea siderea, 2-3 m, 29 Sep. 1995, coll. S. Zea.
Description
Thinly encrusting. Color black in vivo, dark brown when preserved in alcohol (NCG 28-Olive Brown). Consistency soft, easy to tear. Conulose surface.
Skeleton:
Organic, notably pigmented. Erect fibers 52-100 mm in diameter, completely filled with spicule fragments (foreign material), as no free sponging was evident around them. Some ramifications were observed, but not anastomosing.
Distribution
According to Cook & Bergquist (2002): English Channel, Portugal, Western Mediterranean and Adriatic (Lesina, the type locality). In Addition: Colombia (Urabá).
Comments
According to George & Wilson (1919) and van Soest (1978), Pleraplysilla minchini Topsent, 1905, was originally described as a 2 mm-thick encrustation, chocolate color, conulose surface, conules 2 mm apart, dendritic skeleton, fibers 100-110 µm in diameter, and few ramifications. This description is consistent with the specimen studied from Urabá, although it bears thinner fibers (52-100 µm).
Pleraplysilla stocki van Soest, 1978, the only other Pleraplysilla species described so far from the Caribbean (Puerto Rico), is clearly distinguished by habit (massive vs. thin encrusting in Urabá), color (alive reddish violet vs. black in Urabá), oscules (conspicuous vs. unconspicuous in Urabá) and fiber diameter (80-300 µm vs. 52-100 µm in Urabá) (see van Soest, 1978).
It is possible, however, that a further record of Pleraplysilla stocki, also from Puerto Rico, by Pulitzer-Finali (1986, irregularly massive 7x5x4 cm, black in vivo, shades of brown in spirit, conulose surface, dendritic fibers 40-120 µm in diameter, abundantly cored by foreign material and protruding conspicuously from the conules, branching and anastomosing rather frequently, forming few meshes) corresponds to the variation found in Urabá. Interestingly, Pulitzer-Finali (1986) notes that the pale yellow color of his specimen's fibers, is the same color which is observed in the fibers of Pleraplysilla minchini and P. spinifera, but different to that recorded originally for P. stocki (dark purple).
As Pleraplysilla minchini Topsent, 1905 is currently recognized as a junior synonym of P. spinifera Schulze, 1879 (Cook and & Bergquist, 2002), the material examined here is tentatively assigned to P. spinifera, until its conspecificity with eastern Atlantic populations, the area in which the species was originally described, is proven. If the specimens are not conspecific, then P. minchini could be the valid name.



Acknowledgments
The authors wish to thank especially the Instituto de Investigaciones Marinas y Costeras-INVEMAR for its help through the project Bioecologic and Environmental Assessment of Colombian Caribbean Reef Areas, Phase I (Sponsored by COLCIENCIAS, grant CO-2105-09-023-93) and the Inventories Line of the Marine Biodiversity and Ecosystems Program-BEM. The maps (Figures 1-2) were made by Venus Rocha at INVEMAR's Information Systems Lab (LabSIS), 2012. This paper is a partial result of the graduating research work of Diego Valderrama to obtain the degree of Marine Biologist (Universidad de Bogotá Jorge Tadeo Lozano), under the guidance of Sven Zea and the advice of Arturo Acero (Universidad Nacional de Colombia). This is contribution 1135 of INVEMAR and 386 of Centro de Estudios en Ciencias del Mar - CECIMAR, Universidad Nacional de Colombia, Caribbean campus.
Rerefences
Alcolado, P.M. 1976. Lista de nuevos registros de poríferos para Cuba. Série Oceanología, Academia de Ciencias de Cuba, 36: 1-11. [ Links ]
Alcolado, P. M. 1999. Comunidades de esponjas de los arrecifes del archipiélago Sabana-Camagüey, Cuba. Bol. Invest. Mar Cost., 28: 95-124. [ Links ]
Alcolado, P. M. 2002. Catálogo de esponjas de Cuba. Avicennia, 15: 53-72. [ Links ]
Alcolado, P. M. & L. Busutil. 2012. Inventaire des spongiaires néritiques du Parc National de la Guadeloupe. Serie Oceanológica, 10: 62-76. [ Links ]
Alcolado, P. M. & G. G. Gotera. 1986. Nuevas adiciones a la fauna de Poríferos de Cuba. Poeyana, 331: 1-19. [ Links ]
Alvarado, E. M. (Ed.). 1992. Sistemas arrecifales en Colombia: investigación y manejo. Bol. Ecotrópica. Suppl. 1: 1-85 [ Links ]
Alvarez, B., Soest, R. W. M. Van & K. Rützler. 2002. Svenzea, a new genus of Dictyonellidae (Porifera: Demospongiae) fromtropical reef environments, with description of two new species. Contrib. Zool., 71(4): 171-176. [ Links ]
Alvarez, B., Soest, R. W. M. Van & K. Rützler. 1998. A revision of Axinellidae (Porifera: Demospongiae) of the central West Atlantic region. Smithsonian Contr. Zool., 598: 1-47. [ Links ]
Boury-Esnault, N., Klautau, M., Béza C, C., Wulff, J. & A. M. Solé-Cava. 1999. Comparative study of putative conspecific sponge populations from both sides of the Isthmus of Panama. J. Mar. Biol. Ass. U. K., 79: 39-50. [ Links ]
Bula-Meyer, G. & R. Schnetter, 1988. Las macroalgas recolectadas durante la expedición Urabá II, costa Caribe del noroeste chocoano, Colombia. Bol. Ecotrópica, 18: 19-32. [ Links ]
Castellanos, L., Zea, S., Osorno O. & C. Duque. 2003. Phylogenetic analysis of the order Halichondrida (Porifera, Demospongiae), using 3b-hydroxysterols as chemical characters. Biochem. Syst. Ecol. 31: 1163-1183. [ Links ]
Carter, H. J. 1876. Description and figures of deep-sea sponges and their spicules, from the Atlantic Ocean, dredged up on board H. M. S. "Porcupine",chiefly in 1869. Ann. Mag. Nat. Hist., (4) 18(105): 226-240; (106): 307-324; (107): 388-410; (108): 458-479. [ Links ]
Chevillot, P., Molina, A., Giraldo, L. & C. Molina. 1993. Estudio geológico e hidrológico del Golfo de Urabá. Bol. Cient. CIOH. (14): 79-89. [ Links ]
Coelho, E. P. & A. de Mello-Leitão. 1978. Placospongia carinata e sua ocorrencia em costas brasileiras. Departamento de Zoologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Avulso 29: 1-5. [ Links ]
Cook S de C. & P. R. Bergquist. 2002. Family Dysideidae Gray, 1867 In: HOOPER, J. N. & R. W. M. VAN SOEST (Ed.). 2002. Systema Porifera: a guide to the classification of sponges. Kluwer Academic/ Plenum Publishers. New York: 835-851. [ Links ]
Díaz, J. M, Díaz-Pulido, G. & J. A. Sánches. 2000. Distribution and structure of the southermost Caribbean coral reefs: Golfo de Urabá, Colombia. Sci. Mar. 64 (3): 327-336. [ Links ]
Díaz, M. C., Pomponi, S. A. & R. W. N. Van Soest. 1993. A systematic revision of the central West Atlantic Halichondrida (Demospongiae, Porifera). Part III: description of valid species. Sci. Mar., 57 (4): 283-306. [ Links ]
Dickinson, M. G. 1945. Sponge of the Gulf of California. Allan Handcock Pacific Expeditions, 11: 1-251. [ Links ]
Duchassaing de Fonbressin, P. & G. Michelotti. 1864. Spongíaires de la mer Caraïbe. Natkd. Verh. holl. Maatsch. Wetensch. Haarlem. (2) 21(3): 1-124. [ Links ]
Duque-Caro, H. 1990. Neogene stratigraphy, paleoceanography, and paleobiology in northwest South America and the evolution of the Panama Seaway. Paleogeogr. Paleoclimatol. Paleoecol.,77: 203-234. [ Links ]
Galeano, E. & A. Martinez. 2007. Antimicrobial Activity of Marine Sponges from Urabá Gulf, Colombian Caribbean region. J. Mycol. Med., 17(1): 21-24. [ Links ]
George, W. C. & H. V. Wilson, 1919. Sponges of Beaufort (N. C.) Harbor and vicinity. Bull. U. S. Bur. Fish., 36 (876): 130-179. [ Links ]
Gotera, G. G. & P. M. Alcolado. 1987. Nueva especie del genero Agelas (Porifera) colectada en Cuba. Poeyana. (342): 1-4. [ Links ]
Hajdu, E., Peixinho, S., Fernandez, J.C.C. 2011. Esponjas marinhas da Bahia. Guia de campo e laboratório. Museu Nacional, Serie livros, Rio de Janeiro: 1-276. [ Links ]
Hajdu, E., Zea, S., Kielman, M. & S. Peixinho. 1995. Mycale escarlatei n.sp and Mycale unguifera n.sp. (Mycalidae, Poecilosclerida, Desmospongiae) from the tropical western Atlantic. Beufortia, 45: 1-16. [ Links ]
Hechtel, G. J. 1965. A systematic study of the Demospongiae of Port Royal, Jamaica. Bull. Peabody Mus. Nat. Hist. 20: 1-103. [ Links ]
Hofman C.C. & M. Kielman. 1992. The excavating sponges of the Santa Marta area, Colombia, with description of a new species. Bijdr. Dierkd.,61 (4): 205-217. [ Links ]
Hooper, J. N. 1996. Revision of microcionidae (Porifera: Poecilosclerida: Demospongiae), with description of Australian species. Mem. Queensl. Mus.,40: 1-626. [ Links ]
Hooper, J. N. & R. W. M. Van Soest (Ed.). 2002. Systema Porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers. New York: 1-1101, 1103-1706 (2 volumes). [ Links ]
Jones, W. C. 1979. The microstructure and genesis of sponge biominerals En: Lévi, C. & N. Boury-Esnault (eds.). Biologie des Spongiaires. Colloques Internationaux du C. N. R. S., 291: 425-447. [ Links ]
Kelly-Borges, M. & C. Valentine. 1995. The sponges of the tropical island region of Oceania: a taxonomic status review. In: J. E. Maragos, M. N. A. Peterson, L. G. Eldredge, J. E. Bardach & H. F. Takeuchi (eds.). Marine and coastal biodiversity in the tropical island pacific region. Volumen 1. Species systematics and information management priorities. Hawaii: 83-120. [ Links ]
Kobluk D.R. & R.W.M. Van Soest. 1989. Cavity-dwelling sponges in a southern caribbean coral reef and their paleontological implications. Bull. Mar. Sci., 44 (3): 1207-1235. [ Links ]
Laubenfels, M. W. DE. 1934. New sponges from the Puerto Rican deep. Smithson. misc. Collect., 91 (17): 1-28. [ Links ]
Laubenfels, M. W. DE. 1936a. A comparison of the shallow-water sponges near the pacific end of the Panama canal with those at the Caribbean end. Poc. U. S. Nat. Mus., 83 (2993): 441-466. [ Links ]
Laubenfels, M. W. DE. 1936b. A discussion of the sponge fauna of the Dry Tortugas in particular, and the West Indies in general, with material for a revision of the families and orders of the Porifera. Papers Tortugas Lab., 30: 1-225. [ Links ]
Laubenfels, M. W. DE, 1949. Sponges of the western Bahamas. Amer. Mus. Novitates, 1431: 1-25. [ Links ]
Laubenfels, M. W. DE, 1953.Sponges from the Gulf of Mexico. Bull. Mar. Sci. Gulf Caribbean, 2 (3): 511-557. [ Links ]
Lehnert, H. & R. W. M. Van Soest. 1998. Shallow water sponges of Jamaica. Beufortia, 48 (5): 71-103. [ Links ]
Lehnert H. & R. W. M. Van Soest. 1999. More north Jamaican deep forereef sponges. Beufortia, 49 (12): 141-169. [ Links ]
Little, F. J. 1963. The sponge fauna of the St. George's Sound, Apalachee Bay, and Panama City regions of the Florida Gulf coast. Tulanne Stud. Zool. Bot.,11 (2): 31-71. [ Links ]
Maldonado, M., Carmen-Carmona, M., Uriz M. J. & A. Cruzado. 1999. Decline in Mesozoic reef-building sponges explained by silicon limitation. Nature, 401 (21): 785-788. [ Links ]
Martínez, A., Galeano E., Cadavid, J., Miranda Y., Llano J. & K. Montalvo. 2007a. Acción insecticida de extractos etanólicos de esponjas del Golfo de Urabá sobre larvas de Aedes aegypti y Culex quinquefasciatus. Vitae, 14(2): 90-94. [ Links ]
Martínez, A., Galeano, E. & D. Valderrama. 2007b. Antimicrobial activity of Caribbean Reef sponges (north-west Gulf of Urabá, Colombia). In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G (eds). Biodiversity, innovation and sustainability: Book of abstracts. VII International Sponge Symposium, Armaçao dos Búzios, Rio de Janeiro, Brazil: 27. [ Links ]
Moraes, F. Coxeiras de. 2011. Esponjas das ilhas oceánicas brasileiras. Museu Nacional, Rio de Janeiro, 1-252. [ Links ]
Mothes B. & M. C. K. de A. Bastian. 1993. Esponjas do arquipélago de Fernando de Noronha, Brasil (Porifera, Demospongiae). Iheringia, Sér. Zool., (75): 15-31. [ Links ]
Muricy, G., Esteves, E.L., Moraes, F., Santos, J.P., Da Silva, S., Klautau, M. & E. Lanna. 2008. Biodiversidade marinha da Bacia Potiguar. Porifera. Museu Nacional, Rio de Janeiro, 1-156. [ Links ]
Muricy, G., Lopes, D.A., Hajdu, E., Carvalho, M. De S., Moraes, F.C., Klautau, M., Menegola, C. & U. Pinheiro. 2011. Catalogue of Brazilian Porifera. Museu Nacional, Rio de Janeiro, 1-299. [ Links ]
Pulitzer-Finali, G. 1986. A Collection of West Indian Demospongiae (Porifera). In appendix a list of Demospongiae hitherto recorded from the West Indies. Ann. Mus. Civico Storia Nat. Genova, 86: 65-216. [ Links ]
Rathe Peralta, L. 1981. Estudio sistemático de las esponjas (Porifera) del litoral de República Dominicana. B.Sc. Thesis, Biology. Universidad Autónoma de Santo Domingo, Dominican Republic, Santo Domingo: 1-18. [ Links ]
Rosa-Barbosa, R. de. 1995. Primeiro registro de Myrmekioderma styx Laubenfels, 1953 (Porifera-Demospongiae) no Atlântico Sudoeste com novos aportes para a caracterização da espécie. Biociências, Porto Alegre, 3 (2): 119-128. [ Links ]
Rua, C. P. J., Mattos, A. & M. Solé-Cava. 2006. Cryptic speciation and correspondence between spiculation and molecular markers in Placospongia. In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G (eds). Biodiversity, innovation and sustainability: Book of abstracts. VII International Sponge Symposium, Armaçao dos Búzios, Rio de Janeiro, Brazil: 197. [ Links ]
Rützler, K. 1978. Sponges in Coral reefs. In: Stoddard, D.R. y R.E. Johannes (Eds.). Coral Reefs: research methods. Monogr. Oceanogr. Meth. 5, UNESCO, Paris, 21: 299-313. [ Links ]
Rützler, K. 1986. Phylum Porifera (sponges) In: W. Sterrer (Ed.). Marine fauna and flora of Bermuda. A systematic guide to the identification of marine organisms. John Wiley & sons, Inc. New York: 111-128. [ Links ]
Rützler, K. & K. P. Smith. 1992. Guide to Western Atlantic species of Cinachyrella (Porifera: Tetillidae). Proc. Biol. Soc. Wash., 105 (1): 148-164. [ Links ]
Rützler, K. & K. P. Smith. 1993. The genus Terpios (Suberitidae) and new species in the "Lobiceps" complex. Pp. 381-393. In: Uriz, M.-J.&Rützler, K. (Eds), Recent Advances in Ecology and Systematics of Sponges. Sci. Mar., 57(4): 273-432. [ Links ]
Simpson, T. L. 1978. The biology of the marine sponge Microciona prolifera (Ellis and Solander). III. Spicule secretion and the effect of temperature on spicule size. J. Exp. Mar. Biol. Ecol., 35: 31-42. [ Links ]
Simpson, T. L., Gil, M., Connes, R., Díaz, J-P. & J. Paris. 1985. Effects of germanium (Ge) on the silica spicules of the marine sponge Suberites domucula: transformation of spicule type. J. Morphol., 183 (1): 117-128. [ Links ]
Smithe, F. B. 1975. Naturalist's Color Guide. The American Museum of Natural History, New York. Part I. Color Guide, 86 + 96 colores, Part II (1974). Color Guide Supplement: 1-299. [ Links ]
Soest, R. W. M. Van. 1978. Marine sponges from Curaçao and other Caribbean localities. Part I. Keratosa. Stud. Fauna Curaçao Caribb. Isl., 56 (179): 1-94. [ Links ]
Soest, R. W. M. Van. 1984. Marine sponges from Curaçao and other Caribbean localities. Part III. Poecilosclerida. Stud. Fauna CuraçaoCaribb. isl., 66 (199): 1-177. [ Links ]
Soest, R. W. M. Van. 1993. Affinities of the marine Demospongiae fauna of the Cape Verde Islands and Tropical West Africa. Cour. Forsch. Inst. Senck.,159: 205-219. [ Links ]
Soest, R. W. M. Van. 2002. Family Coelosphaeridae Dendy, 1922 In: HOOPER, J. N. & R. W. M. VAN SOEST (Ed.). 2002. Systema Porifera: a guide to the classification of sponges. Kluwer Academic/ Plenum Publishers. New York: 528-546. [ Links ]
Soest, R. W. Van. 2009. New sciophilous sponges from the Caribbean (Porifera: Demospongiae). Zootaxa 2107: 1-40. [ Links ]
Soest, R. W. M. Van & N. Stentoft, 1988. Barbados deep-water sponges. Stud. fauna Curaçao Caribb. Isl., 70 (215): 1-175. [ Links ]
Sollas, W. J. 1888. Report on the Tetractinellida collected by H. M. S. Challenger, during the years 1873-1876. In: Report on the scientific results of the voyage of H. M. S. Challenger during the years 1873-1876, Zoology 25 (63) CLXVI: 1-458. [ Links ]
Valderrama, D. 2001. Taxonomía y distribución de esponjas arrecifales (Porifera) del noroccidente del Golfo de Urabá, Caribe colombiano. B.Sc. Thesis, Marine Biology. FundaciónUniversidad Jorge Tadeo Lozano, Santa Marta, Colombia: 1-187. [ Links ]
Valderrama, D. & S. Zea. 2003. Esquemas de distribución de esponjas arrecifales (porifera) del noroccidente del Golfo de Urabá, Caribe colombiano. Bol. Invest. Mar. Cost.32: 37-56. [ Links ]
Valderrama, D., Rossi A. L., Solé-Cava A. M., Rapp H.T. & M. Klautau. 2009. Revalidation of Leucetta floridana (Haeckel, 1872) (Calcarea, Clathrinida, Leucettidae): a wide spread species in the tropical western Atlantic. Zoological Journal of the Linnean Society, 157, 1-16. [ Links ]
Weerdt, W. H. DE. 2000. A monograph of the shallow-water Chalinidae (Porifera, Haplosclerida) of the Caribbean. Beaufortia, 50 (1): 1-67. [ Links ]
Weerdt, W. H. De, Kluijver, M. J. DE & R. Gomez. 1999. Haliclona (Halichoclona) vansoesti n. sp., a new chalinid sponge species (Porifera, Demospongiae, Haplosclerida) from the Caribbean. Beufortia, 49 (6): 47-54. [ Links ] [ Links ]
Wells, H. W., Wells, M. J. & I. E. Gray. 1960. Marine sponges of North Carolina. J. Elisha Mitchel Sci. Soc. 76: 200-245. [ Links ]
Wiedenmayer, F. 1977. Shallow-water sponges of the western Bahamas. Exp. Supl. 28, Birkhauser Verlag, Basel & Suttgart: 1-278. [ Links ]
Wintermann-Kilian, G. & E. F. Kilian. 1983. Marine sponges of the region of Santa Marta (Colombia) Part I. Dictyoceratida and Verongida. Stud. Neotrop. Fauna Environ. 18: 1-17. [ Links ]
Wintermann-Kilian, G. & E. F. Kilian. 1984. Marine sponges of the region of Santa Marta (Colombia) Part II. Homosclerophorida, Choristida, Spirophorida, Hadromerida, Axinellida, Halichondrida, Poecilosclerida. Stud. Neotrop. Fauna Environ, 19 (3): 121-135. [ Links ]
Zabala, D. A., Echavarría, B. & A. Martínez. 2008. Actividad inhibitoria sobre la enzima dihidrofolato reductasa de extractos de esponjas marinas del Golfo de Urabá. Vitae, 15(2): 285-289. [ Links ]
Zea, S. 1985. Demosponges of the Colombian Caribbean: report on geographic variation in spicule size. Third International Conference on the Biology of Sponges, Woods Hole (Poster). [ Links ]
Zea, S. 1987. Esponjas del Caribe Colombiano. Dictyoceratida, Dendroceratida, Verongida, Haplosclerida, Poecilosclerida, Halichondrida, Axinellida, Desmophorida y Homosclerophorida. Catálogo Científico, Bogotá: 1-286. [ Links ]
Zea, S. 1998. Estado actual del conocimiento en sistemática de esponjas marinas (Porifera) del Caribe Colombiano. Bol. Ecotrópica, (33): 45-59. [ Links ]
Zea, S. & R. M. W. Van Soest. 1986. Three new species of sponges from the Colombian Caribbean. Bull. Mar. Sci. 38 (2): 355-365. [ Links ]
Zea, S. & E. Weil. 2003.Taxonomy of the Caribbean excavating sponge species complex Cliona caribbaea - C. aprica - C. langae (Porifera, Hadromerida, Clionaidae). Caribb. J. Sci., 39(3): 348-370. [ Links ]
Zea, S., Henkel, T. P. & J. R. Pawlik, J.R. 2009. The Sponge Guide: a picture guide to Caribbean sponges. Available: www.spongeguide. org. Accessed June 27, 2012. [ Links ]
Recibido: 10 de febrero de 2013
Aceptado para su publicación: 6 de septiembre de 2013













