Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales
Print version ISSN 0370-3908
Rev. acad. colomb. cienc. exact. fis. nat. vol.39 no.150 Bogotá Jan./Mar. 2015
1 Department of Integrative Biology at Oklahoma State University, Stillwater, Oklahoma, USA
2 Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia. *Corresponding author: José IvánMojica Corzo, jimojicac@unal.edu.co
3 Department of Natural Sciences Mount Marty College, Yankton, Dakota del Sur, USA
Recibido: 20 de octubre de 2014. Aceptado: 19 de enero de 2015
Abstract
Astroblepid species inhabit the Andean Cordilleras in South America. Their habitat has undergone a rapid transformation to the unimaginable levels of degradation since ancient times. State of knowledge on the habitat perturbation and the concomitant extirpation of endemic astroblepid species is scarce. The Andes Cordilleras are characterized by fertile soil and as a result of that, vast regions are transformed for agriculture and pasture for cattle. These processes require the use of water obtained from creeks and springs that causes remarkable changes of the natural configuration of these water systems and the disappearance of the fish fauna together with the zooplankton and aquatic flora. Despite these human actions, some species of Astroblepus may have the ability to respond to the rapid habitat transformation enabling them to survive. In this paper we will discuss the incidence of habitat perturbation in a localized stream segment on the presence of Astroblepus mariae.
Key words: Habitat perturbation, survival, refuge, buffer zone.
Abstract
Astroblepid species inhabit the Andean Cordilleras in South America. Their habitat has undergone a rapid transformation to the unimaginable levels of degradation since ancient times. State of knowledge on the habitat perturbation and the concomitant extirpation of endemic astroblepid species is scarce. The Andes Cordilleras are characterized by fertile soil and as a result of that, vast regions are transformed for agriculture and pasture for cattle. These processes require the use of water obtained from creeks and springs that causes remarkable changes of the natural configuration of these water systems and the disappearance of the fish fauna together with the zooplankton and aquatic flora. Despite these human actions, some species of Astroblepus may have the ability to respond to the rapid habitat transformation enabling them to survive. In this paper we will discuss the incidence of habitat perturbation in a localized stream segment on the presence of Astroblepus mariae.
Key words: Habitat perturbation, survival, refuge, buffer zone.
Introduction
Astroblepus species, also called "climbing" or "suckermouth" catfish, inhabit streams from the piedmont to high altitude of the Andean cordilleras. Their habitat includes small, shallow creeks, and springs characterized by rocks, stones, gravel and mud, and diverse riverine vegetation. Their ability to climb (Johnson, 1912; Arratia, 1990; Howes, 1983; Buitrago-Suárez, 1995; Nelson, 2006; Gerstner, 2007; Schaefer et al., 2011; DeCrop, et al., 2013) enable them to explore unreachable habitats for other species of fish. Some species of this group of catfishes are the only representatives of the fish fauna from the highest Andes.
Little is known about the ecology of astroblepids, but as it has been described in more recent studies, anthropogenic activities cause an increase in habitat alteration with the concomitant extirpation of local cryptic populations (Vélez-Espino, 2003, 2005 and 2006). Habitat degradation in the Andean cordilleras due to deforestation for pasture and agriculture is in fact modifying the population structure of these catfishes. Andean streams are remarkably modified for water supply for humans and cattle (Vélez-Espino, 2006). The Andean landscape is also characterized by the rich and fertile soil for agriculture. Large areas of the Andean slopes are used for industrial agriculture tubercle crops such as potatoes and for leguminous plants as well as for pastures. As a result of these processes, the natural configuration of creeks has undergone a rapid transformation during the last decades. In some cases many of the water bodies are lost forever with their fish species, zooplankton and aquatic flora (Vélez-Espino, 2005).
A habitat suitability model has been established for species such as Astroblepus ubidiai to reflect abundance and make suggestions for the conservation of this species (Vélez-Espino, 2004). Because the Andean cordilleras water bodies share some similarities, these analyses can be applied to most species of Astroblepus. Degrees of habitat fragmentation in the Colombian Cordilleras are coupled with those of the other South American countries such as Ecuador, Bolivia, Chile, Peru and Venezuela. Although there are no studies on habitat fragmentation in the Andean Cordilleras of Colombia, the anthropogenic perturbation may be more devastating as the economy of this country has improved in the last five or so years. We observed and describe here a remarkable ability of these small catfishes to survive extreme habitat alterations.
We describe in this work the habitat conditions and behavior of a population of A. mariae from the Rio Negro drainage in the Departamento de Cundinamarca, in the northeast central part of Colombia. This is a preliminary report on the habitat alteration of this species of Astroblepus inhabiting the Colombian cordilleras and it serves as the baseline for further assessment of the habitat suitability index in the northern ranges of Colombia.
Materials and methods
Study area. Six creeks were sampled for fish and physico-chemical variables. The creeks are located in the watershed of the Rio Negro Basin, Department of Cundinamarca, central part of Colombia (4°39'26"-4°38'43"N and 73°52'5"-73°52'11" W). The area is characterized by extensive human activity such as agriculture, grazing, and human settlements including small villages called Corregimientos and aggregations of houses along the road. Creeks are tributaries of the Río Negro (Figure 1) which in turn is a tributary of the Río Meta, from the Orinoco Basin and are numbered 1 to 6 in the tables. Observations were performed during a 2 weeks period of December 2013 and January 2014. Creek 1 presented extreme anthropogenic perturbation, and it encompasses most of the analyses and discussion in this report.
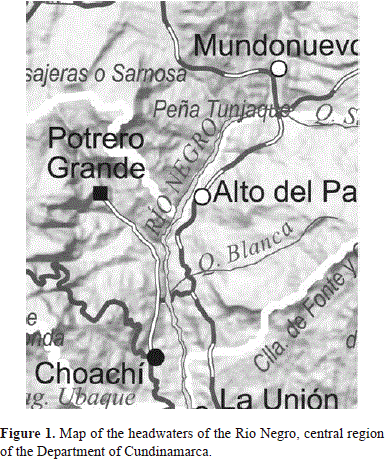
Creek 1, which we refer here as the "Spring", is located about 0.9 miles from the village of "Mundo Nuevo" along the road to Choachi in the province of Cundinamarca (N. 4°39'26" and W. 73°52'5"). The Spring is about 250m (750 feet) long, width=30cms, depth=5cms to 10cms, with an approximate slope of 45°. Two distinctive segments were identified based on the degree of perturbation. The first segment is located on the left side of the road to Choachi and the other on right side of the same road. The segment on the left side of the road is identified as Spring Segment 1 (SprSeg1) and it extended from its origin to the road. With a length of no more than 50ms (150 feet), this segment is located in an area with pasture, and cattle were present. The other segment, Spring Segment 2 (SprSeg2) was also pasture but without cattle. A distinctive third zone was also observed between SprSeg1 and SprSeg2 that is referred to here as the buffer or transition zone. This buffer zone was a short segment of no more than 10m. SprSeg1 and SprSeg2 were sampled for fish and physicochemical values (Table 1).
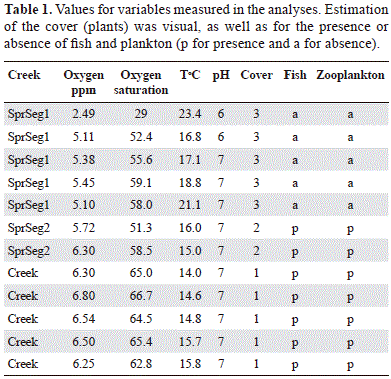
Five sites were selected along SprSeg1 to measure the physicochemical variables (temperature, oxygen saturation (percentage) and concentration (parts per million), pH) were also estimated from its origin to the road. The sites were about 10ms apart from each other. Two sites were selected for SprSeg2 to measure the variables. These variables were also measured along the buffer zone. Substrate composition for both the "Spring" and the other creeks follows the classi- fication of Vadas and Orth (2001). In addition vegetation abundance estimated as: 1 for abundant, 2 not abundant and 3 for absent (Table 1), was also estimated for all sites in the segments and buffer zone. Some plants were collected for further taxonomic determination. These variables were also estimated for Creeks 2 to 6 (referred to here as Creeks 2-6) for further comparison and data analyses.
Sampling for specimens of Astroblepus mariae was performed along the "Spring" and the other adjacent Creeks 2-6. Fish sampling gear included small seine nets and quick-seine nets. Estimated specimens were preserved and fixed in the field, and transported to Instituto de Ciencias Naturales (ICN-MHN Universidad Nacional de Colombia).
Other variables collected but not included in this analysis were; slope, length of the water bodies, width, depth, type of flow, substrate composition and zooplankton. Composition of zooplankton was assessed for the most abundant groups. Habitat perturbation was visually estimated and is described in this paper. Habitat perturbation description follows that in Vélez-Espino (2006).
Statistical analysis. The statistical tests are limited by the small sample sizes of the variables. If provided, levels of confidentiality need to be corroborated with larger sample sizes for the different variables. Some of the tests were adjusted for unbalanced data and included one-way-analysis of variance (ANOVA). Two one-way-analyses of variance were accomplished as follows: the first one was performed on the data collected to assess significant differences between Creeks 2-6 as one single group of data with the SprSeg1 with major human disturbance. Values for the variables from SprSeg2 were included with the group of Creeks 2-6 for these analyses because both groups of water systems shared similar characteristics, such as pH, oxygen concentration and saturation, and more importantly, the presence of fish. The second analysis was applied to assess significant differences within the "Spring" segments 1 and 2 SprSeg1 and SprSeg2 (see spring topography in the results session). The circle intercept test for the means was used to estimate the minimum vital conditions values required by individuals of A. mariae to exist in the sampled water systems. This analysis was performed considering the presence-absence of fish versus those variables with significant mean differences. The statistical analyses were performed with the software package JMP™. Due to the sample size differences between the two segments of the "Spring", a t test with an unbalanced analysis was performed as recommended by the JMP⢠Software Guide. A Tukey-Kramer test for degrees of data overlap was also performed in those cases where the t test did not render higher confidentiality (see pH means). Significant factors determining absence or presence of A. mariae are also estimated and discussed.
Results
The topography and substrate compositions were different for both the Creeks 2-6 and the "Spring". Creeks 2-6 presented waterfalls, bedrock and large rocks. Width, depth and amount of water were substantially larger compared with the "Spring". The Creeks 2-6 watersheds included areas used for agriculture but their shorelines were covered by abundant vegetation characterized by bushes and plants. Both segments of the "Spring", SprSeg1 and SprSeg2 had the same substrate composition including sand, mud, gravel, pebble and no bedrock was observed. Unlike the Creeks 2-6, the "Spring" watershed had no streamside vegetation therefore it had a higher exposure to solar radiation, likely the reason why the temperature values were different (see t test below). The shoreline of SprSeg1 was the most exposed to solar radiation. The margins of this segment of the "Spring" were irregular and somewhat un-identifiable in some fragments. Cattle tracks and cattle feces were observed in the waterbed (Figure 2). The shoreline of this segment had no plants of any kind found along the SprSeg2. Down the slope, when the SprSeg1 reached the road, it changed direction and 10 or so meters it disappeared underneath a bridge. This short fragment of the "Spring" is separated from SprSeg1 by a fence, therefore no cattle were observed and it can be regarded as a buffer zone. Neither fish nor zooplankton were found in SprSeg1.

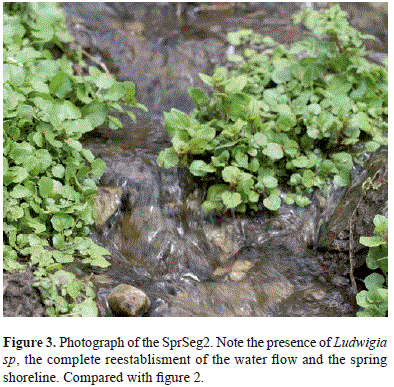
The SprSeg2 watershed consisted of grass and some scattered plants. Unlike the Creeks 2-6 that have bushes and tall plants, SprSeg2 was populated by Ludwigia sp, a small plant (Figure 3). Although this segment did not have cattle, it was used for grazing about a month before the exploration of the area (information taken from interviews in situ). Unlike SprSeg1, the margins of SprSeg2 were defined and had abundant Ludwigia sp on the shorelines and the water bed was composed by small falls, currents and ponds no more than one meter long and 50cms wide. The small falls harbored organic matter represented by leaves, grass, and sticks. Two specimens of A. mariae were collected and there was abundant zooplankton represented by arthropods and annelids. Several specimens of crabs were also observed.
The buffer zone in between the two segments of the "Spring" can be considered as a transition zone between the highly perturbed SprSeg1 and SprSeg2. The zone has distinctive features compared with both, SprSeg1 and SprSeg2. Unlike the two segments of the "Spring", the buffer zone remains untouched and may have more stable conditions year round. It had abundant Ludwigia sp and may also serve as a refuge for A. mariae when cattle are switched to the SprSeg2.
In general, the group of Creeks 2-6 presented fairly stable conditions. The variables were constant along the length of their shoreline. This homogeneity represented more habitat stability and therefore more fish were collected in these creeks. Fish were collected in different parts of the creeks which is an indication of the favorable conditions for A. mariae in these small water systems. However, none of the creeks presented primary vegetation and plants on the shoreline of were exotic species. Most of the specific taxa are introduced and include representative species of Melastomataceae, Rosaceae, Solanaceae, Platanaceae and Scrofulariaceae. As expected, plants provide shade and are crucial suppliers of organic matter to sustain the creek habitat.
Zooplankton in these creeks was present and abundant. Composition of the zooplankton was mostly arthropods followed by annelids. Variables such as oxygen concentration, oxygen saturation, pH and temperature had no significant variation along the creek's length and within the creeks with more stable conditions.
When the means of the variables from the "Creeks 2-6" and SprSeg2 were compared with those of the SprSeg1 (Table 1), significant differences were found from the analyses except for pH (p > t =0.1). To reinforce this result a Tukey-Kramer test to assess closeness of the means for pH was also applied and the results concurred with the t test. ANOVA tests for the other mean variables (oxygen concentration, oxygen saturation and temperature) rendered significant differences with p values 0.01, 0.02 and 0.03 respectively.
Significant differences were also recovered from the second analysis for oxygen concentration, and pH when SprSeg1 (Table 1) was compared with SprSeg2, (p>t=0.05 and p>t=0.08 respectively). Probabilities of getting greater t values were observed for oxygen saturation and temperature. The means for these two variables were not different.
The third statistical analysis (the circle intercept test for the recovered means) rendered the following conclusions below when both presence and absence of fish (see also Table 1) were plotted versus the significant mean values. Individuals of A. mariae live in the Creeks 2-6 and SprSeg1 where the oxygen concentration had a minimum concentration equal or above 5.5ppm and temperatures of 16°C or lower. Borderline conditions were somewhat difficult to observe for oxygen saturation and pH. However these catfishes may prefer oxygen saturations of 55% or above and a pH value of 7.
Discussion
With the two recognized segments presenting different degrees of perturbation, creek 1 (or the "Spring", Figs. 2 and 3) is regarded here as a natural laboratory to study not only the ecology but also behavioral responses of A. mariae to drastic environmental changes. The fact that fishes were found in this spring requires more attention and demands long-term studies to assess the habitat perturbation index, suitability index, management recommendations and most importantly, to estimate the evolutionary strategies of the individuals of A. mariae to habitat contingencies.
As described above, the characteristics for the "Spring" or Creek 1 are remarkably different from the other creeks (Creeks 2-6). It did not have cover provided by trees, and the size and width were comparatively smaller than those of Creeks 2-6. Added to these conditions is the fact that farmers switch their cattle constantly from SprSeg1 to SprSeg2 and vice versa when the pasture is depleted on the other segment. According with an interview, the farmers switch their cattle every month. Recovery of the suitable living conditions for A. mariae after grazing in either SprSeg1 or SprSeg2 may require longer time.
The presence of A. mariae individuals in this spring with such different conditions elicits different questions. For instance, what are the required factors of these catfishes for survival? Do they migrate long distances when the cattle are switched to SprSeg2 or do they stay in the refuge along that segment waiting for suitable conditions in the other side? How do these fishes respond to the increased temperature and other conditions related to the lack of shade provided by trees? All of these questions need to be answered with long term studies designed to provide us with more data.
When the cattle are switched to the SprSeg1, the individuals of A. mariae may have one of the following two responses or both. First, they may migrate in the search for better conditions, mainly food. This response implies migration up or down the stream of the main river channel. This response presents difficult barriers for individuals of A. mariae. Although no direct observations were made and were based on information gathered from local people, the main channel is the preferred habitat for exotic species such rainbow trout, cited as one of the most predaceous fish on native species in North America (Miller et al., 1989). Despite the fact that no studies have been done addressing local extinction of astroblepids in Colombia, we predict that this exotic fish may be one of the main causes of decline of A. mariae, combined with habitat perturbation by humans (see also Vélez-Espino, 2003). Other barriers are the water conditions on the main channel that may be unsuitable for long distance migrations. Oxygen concentration and pH may be too low in combination with the high turbidity of the Rio Negro that might prevent these catfishes from migrating along that river.
The other answer to the habitat alteration in the spring is a short migration to refuges along the "Spring" itself. These refuges are buffer zones or transition zones between high and medium perturbed habitats for both, SprSeg1 and SprSg2. As observed in the field, the "Spring" presents two recognizable zones or segments and an untouched zone in between. The third zone may have stable conditions year round and serve as a micro-refuge for individuals of A. mariae during sudden and unexpected habitat alterations. We proposed the strategy of micro-refuge exploitation by these catfishes of the transition zone as the possible response when the conditions change dramatically after the introduction of cattle in the SprSeg2. Although in a small scale, our proposed refuge can be a homologue of the buffer zones and ecological corridors from current literature. The theory on ecological corridors and buffer zones is rather old and abundant (Simpson, 1936; Preston, 1960; Forman, 1983 and Simberloff & Cox, 1987). Both, buffer zones and ecological corridors are used by animals during a certain period of their life cycle and regardless of their size, these areas increase the chances of survival (Preston, 1960), conservation and sustainability of animal diversity (sensu Bennett, 1991 and Shafer, 1990). Buffer zones and ecological corridors permeate physical conditions and in general present gradients of the environmental factors between disparate habitats (Simberloff and Cox, 1987). Our proposed micro-refuge may present suitable conditions for the individuals of A. mariae until the SprSeg1 recovers.
Recovering of SprSeg1 includes the reestablishment of the conditions for these catfishes to survive. The process has to be fast and it starts with and is determined by the blooming of the non-perennial plant Ludwigia sp. This plant blooms in the margins of highly perturbed springs and creeks to the extent that it can take over the waterbed of little water systems. This characteristic is advantageous not only for the plant itself but also for the zooplankton and astroblepids. The process starts with the establishment of the non-perennial plant followed by the surge of zooplankton (mainly arthropods and annelids) and later the invasion of A. mariae. Simultaneously with the reestablishment of the plant, the abiotic conditions, i.e., oxygen concentration, pH, temperature reach the suitable levels for animals to populate the recovering spring.
The presence of the plant in the transition zone is fundamental because it provides shelter to the fish and prevents undesirable matter from cattle, i.e., feces and urine from reaching the SprSeg2. The plant may also reestablish the normal oxygen levels, pH, and temperature before the water reaches the SprSeg2. These predictions need to be corroborated with more data and statistical analysis.
Assessing the existing habitat conditions for A. mariae is a daunting task mostly because of the lack of information on its taxonomic and systematic status. The distribution of A. mariae is unknown as it is for most of the 54 recognized species (Schaefer, 2003, and 2011). This situation makes the application of models assessing the ecological status of the species even more difficult because each species presents different responses to habitat disturbances. Studies on the taxonomy and distribution are fundamental before estimating habitat alteration and the species response to ecological changes. Systematics studies based on morphology and genetics using DNA currently underway by the main author will shed some light on the distribution and population structure of this species. Our aim in this report was not to present a complete analysis of the ecological status of A. mariae, but to present baseline conclusions for further and more comprehensive studies, i.e., habitat suitability index, human perturbation index and others.
Acknowledgements
We thank Miguel Leonardo Martínez for his help during the fieldwork. We also thank Miguel Leonardo MartínezSr for driving the main author to the areas of study. We extend our thanks to José Espitia, Chief of the Museum of Natural History (Universidad de La Salle) and Marta García, Chief of the Zoology Collection (Departamento de Biología, Universidad PedagógicaNacional at Bogotá). Julio Betancur (MHN-ICN) determined the specific name of the small plant found along the SprSeg2. We extend our special thanks to Francisco Medellín (Departamento de Biología, Universidad Pedagógica Nacional). We thank the Biochemistry Lab personnel, Universidad de La Salle (Bogotá) for providing the main author with glassware and pH-meter for fieldwork. Donald Taphorn and an anonymous reviewer provided insightful comments and corrections to improve this manuscript.
Conflicts of interest
The authors declare no conflict of interest of any kind
Bibliography
Arratia, G. 1990. Development and diversity of the suspensorium of trichomycterids and comparison with loricarioids (Teleostei: Siluriformes). Journal of Morphology 205: 193-218. [ Links ]
Bennett, G. (Ed). 1991. Towards a European Ecological Network. IEEP. Arnhem. The Netherlands. [ Links ]
Buitrago-Suaréz, U.A. 1995. Sistemática de las especies colombianas del género Astroblepus Humboldt 1805 (Pisces: Siluroidei: Astroblepidae). MSc. Dissertation ICN-UNal Colombia. [ Links ]
DeCropp, W., E. Pauwels, L. Van Hoorebeke, T. Geerinckx. 2013. Functional morphology of the Andean climbing catfish (Astroblepidae: Siluriformes): Alternative ways of respiration, adhesion, and locomotion using the mouth. Journal of Morphology 274: 1164-1179. [ Links ]
Forman, R.T. 1983. Corridors in a landscape: their ecological structure and function. Ekologia 2: 375-387. [ Links ]
Gerstner, C.L. 2007. Effects of oral suction and other friction- enhancing behaviors on the station-holding performance of suckermouth catfish (Hypostomus spp.). Canadian Journal of Zoology 85: 133-140. [ Links ]
Howes, G.J. 1983. The cranial muscles of loricarioid catfishes, their homologies and values as taxonomic characters (Teleostei: Siluroidei). Bulletin of the British Museum of Natural History (Zoology) 45: 309-345. [ Links ]
Nelson, J.S. 2006. Fish of the World. New Jersey: Wiley. 601p. [ Links ]
Johnson, R.D. 1912. Notes on the habits of a climbing catfish (Arges marmoratus) from the republic of Colombia. Annals of the New York Academy of Sciences 22: 327-333. [ Links ]
Miller, R.R., J.D. Williams, J.E. Williams. 1989. Extinctions of North American Fishes during the last century. Fisheries 14 (6): 22-38. [ Links ]
Preston, F.W. 1960. Time and space and the variation of species. Ecology 29: 254-283. [ Links ]
Schaefer, S.A. 2003. Family Astroblepidae. In Reis RE, Kullander SO, Ferraris CJ Jr, eds. Check List of the Freshwater Fishes of South and Central America. Porto Alegre: Edipucrs, 312-317 [ Links ]
Schaefer, S.A., P. Chakrabarty, A. Geneva, M. Sabaj-Pérez. 2011. Nucleotide sequence data confirm diagnosis and local endemism of variable morphospecies of Andean astroblepids catfishes (Siluriformes: Astroblepidae). Zoological Journal of the Linnean Society 2011: 1-13. [ Links ]
Shafer, C.L. 1990. Nature reserves. Smithsonian Institution Press. [ Links ]
Simberloff, D., J. Cox. 1987. Consequences and costs of conservation corridors. Conservation Biology 1: 63-71. [ Links ]
Simpson, G.G. 1936. Data on the relationships of local and continental mammalian faunas. Journal of Paleontology 10: 410-414. [ Links ]
Vadas, R.L., D.J. Orth. 2001. Formulation of habitat suitability models for stream fish guilds: do the standard methods work? Transactions of the American Fish Society 130: 217-235. [ Links ]
Vélez-Espino, L.A. 2003. Taxonomic revision, ecology and endangerment categorization of the Andean catfish Astroblepus ubidiai (Teleostei: Astroblepidae). Review in Fish Biology and Fisheries 13: 367-378. [ Links ]
Vélez-Espino, L.A, M.G. Fox. 2005. Demography and environmental influences on life-history traits of isolated populations of the Andean catfish Astroblepus ubidiai. Environmental Biology of Fishes. 72: 189-204. [ Links ]
Vélez-Espino, L.A. 2006. Distribution and habitat index model for the Andean catfish Astroblepus ubidiai (Pisces: Siluriformes) in Ecuador. Revista Biologia Tropical 54 (2): 623-638. [ Links ]













