Introduction
Yellow passionfruit (Passiflora edulis f. flavicarpa Degener) is one of the most important fruits of Passiflora genus and Passifloraceae family (Isaacs, 2009; Miranda, et al. (2008); Ocampo, 2007; Souza , et al, 2002). Passionfruit is widely used in the food industry due to its edible qualities while passionflower (flowers of P. edulis) is recognized by its effects in the Central Nervous System and its implementation in traditional medicine in South America and India (Patel, 2009; Montanher, et al., 2007; Ocampo, 2007). However, many diseases in the plantations of P. edulis f. flavicarpa decrease yield, reduce productive life of the plants, and in some cases, can completely destroy the crops (Vaca-Vaca, et al., 2016; Ocampo, et al. , 2013; Cavichioli, et al. , 2011; Fischer, et al. , 2010; Fischer, et al. , 2005).
Diseases in passionfruit crops caused by phytopatho-genic species of Fusarium genus produce significant crop losses (Oliveira-Freitas, et al. , 2016; Preisigke, et al. , 2015a; Preisigke, et al. , 2015b; Silva, et al. , 2013; Fischer, et al. , 2010; Bueno, 2009). Although four species of fungi belonging to Fusarium have been described producing symptoms in P. edulis f. flavicarpa in Colombia, Fusarium oxysporum Schltdl. and F. solani f. sp. passiflorae proposed by Bueno, et al . (2014) [teleomorph: Haematonectria haematococca (Berk. & Broome) Samuels & Rossman] repre-sent the most common source of Fusarium wilt (Ortíz, et al. , 2014; Londoño, 2012; Gardner, 1989). Both F. oxysporum and F. solani produce structural damages in xylem, phloem, cambium, and parenchymatous cells (Saniewska, Dyki, & Jarecka, 2004; Bishop & Cooper, 1983). However, F. oxysporum penetrates through the roots and moves into the vascular system and F. solani f. sp. passiflorae concentrates in the collar area causing the collar rot disease of P. edulis (Ortíz, et al. , 2014).
Plants of P. edulis infected by Haematonectria haemato-cocca (Anamorph: F. solani f. sp. passiflorae) present a progressive loss of vigor, wilt, defoliation, roughness in the fruits, chlorosis, vascular necrosis, eventually perithecia formation, collar rot and finally death (Ortíz & Hoyos, 2012). F. solani is favored by high temperature and relative humidity. Irrigation water, rainfalls, and wind are dissemination factors of reproductive structures of the fungus (Nelson, Toussoun, & Marasas, 1983). Chemical and biological control of passionfruit collar rot is ineffective due to the presence of resistance structures of F. solani (chlamydospores) in the soil for long periods of time. This fact forces to eradicate complete crops and performs the cultivation in new areas free from the pathogen (Flores, et al. , 2012; Londoño, 2012; Castaño-Zapata, 2009; Agrios, 2005; Nelson, Dignani, & Anaissie, 1994).
Histopathological features of plants of passionfruit infected by F. solani f. sp. passiflorae and its symptoma-tology associated with collar rot have been studied in detail (Ortíz, et al. , 2014; Ortíz & Hoyos, 2012). However, damages produced by this pathogen in the surface of the plant and its progress over time in P. edulis f. flavicarpa are not well known. This study aims to perform environmental scanning electron microscopy (ESEM) in P. edulis f. flavicarpa seed-lings inoculated with F. solani f. sp. passiflorae observing the progress of the damages over time.
Materials and methods
Seeds of Passiflora edulis f. flavicarpa, obtained from susceptible commercial crops located in the department of Caldas (Colombia), were planted under controlled condi-tions of temperature and light at 29ºC and 12 h photoperiod in autoclaved soil. When seeds germinated and reached two true leaves (three to four weeks after planting), they were carefully removed from the soil and disinfested by immersing each seedling in 2% sodium hypochlorite and 70% ethanol for 2 minutes and 50 seconds, respectively. Posteriorly, seedlings were washed with sterile distillated water and inoculated by placing a segment of potato dextrose agar (PDA) media with the fungus using the modified test-tube screening methodology proposed by Ángel-García, Robledo-Buriticá, & Castaño-Zapata (submitted Boletín Científico Centro de Museos, 2017). Inoculations were carried out in three seedlings every 24 h until the seventh day. From this day to the fifteenth day the interval of inoculation was 72 h. Inoculated seedlings grew up under the same conditions previously mentioned.
The monosporic isolate of F. solani f. sp. passiflorae MViRi01 was used as inoculum. This isolate was tested for its pathogenicity following the Koch´s postulates. Morphological characterization was performed through optical and elec-tronic microscopy on carnation leaf agar (CLA) and PDA media (Figure 1) following the recommendations of Leslie & Summerell (2006). For the inoculation, F. solani f. sp. passiflorae MViRi01 was cultivated in PDA media until reproductive structures (microconidia) were observed.
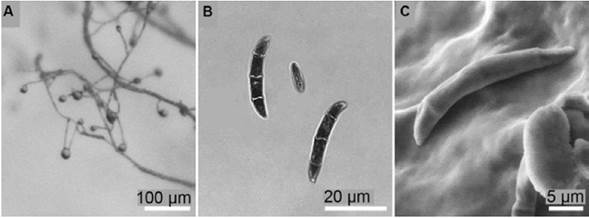
Figure 1 Morphological features of F. solani MViRi01 isolate on CLA. (A) Monophialides with microconidia in situ. (B) Macroconidia and microconidium. (C) Scanning electron microscopy of a macroconidium.
Samples of 5 mm length of the external surface (Figure 2A) and longitudinal section (Figure 2B) of tissues from collar, stem and leaves of each inoculated plant were observed. Sections of the tissues were operated manually after treating them at temperature of 4ºC for two minutes. Every tissue was summited to an amplification of 200x, 500x, 1000x, 2000x, 5000x, and a maximum of 8000x. The total number of observations was 30 plants.
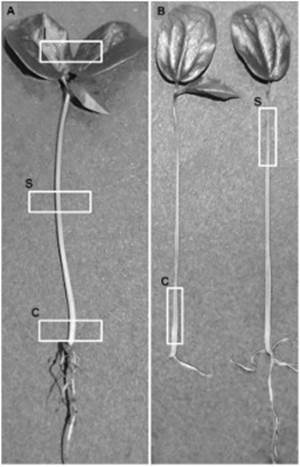
Figure 2 Observed areas in P. edulis f. flavicarpa seedlings. (A) Examination sites on the surface of the seedling: c, collar; s, stem; l, leaves. (B) Examination sites of longitudinal sections of the seedling: c, collar; s, stem.
Observation was carried out by using a scanning electron microscope FEI-QUANTA FEG 250 in environ-mental scanning electron microscopy (ESEM™) mode at low vacuum (2.600 Pa) and at an acceleration voltage of 10 kV. Images were analyzed by using the software ImageJ (Schneider, Rasband, & Eliceiri, 2012).
Results
Surface and longitudinal section of the collar. Epidermal cells of the collar of non-inoculated seedlings of P. edulis f. flavicarpa presented a rectangular, semi-square, and polygonal shape with an average surface area of 536.2 μm2, well-defined cell walls, and complete absence of hyphae and reproductive structures of the fungus (Figure 3A, B). Inoculated plants 24 h after inoculation were deeply overgrown with septate hyphae causing moderate rugosity over the collar epidermis (Figure 3C). Epidermal cells of the collar four days after inoculation showed severe rugosity, 25% reduction of the cell wall thickness, and cell wall collapse (Figure 3D). Collar cells of the seedlings ten days after inoculation were unrecognizable, cell walls were not distinguished, the collar total width was reduced from 539.7 μm to 258.3 μm, and the tissue presented a heterogeneous texture indicating rot (Figure 3E). Although reproductive structures were not observed massively in the collar surface, some areas of the collar presented disperse macroconidium (Figure 3F).
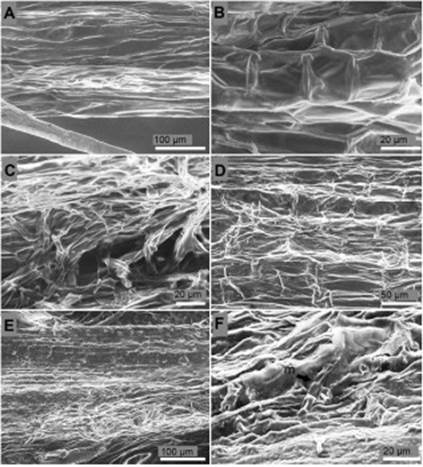
Figure 3 Surface of the collar of P. edulis f. flavicarpa seedlings. (A, B) Surface of the collar of non-inoculated seedling. (C) Septate hyphae colonization over the surface of the collar 24 h after inoculation. (D) Cell wall degradation and loss of turgor pressure of the epidermal cells of the collar four days after inoculation. (E) Degradation of the epidermis of the collar ten days after inoculation. (F) Single macrocodium (m) on rot tissues over the epidermis of the collar ten days after inoculation.
Longitudinal section of the collar of non-inoculated seed-lings presented parenchymatous elongated cells in the center (pith or medulla) separated by an average length of 126.9 μm. Xylem cells surrounded pith cells and subsequently phloem cells encircled xylem cells. No hyphae or conidia were found in the collar of non-inoculated seedlings (Figure 4A, B). Pith cells ten days after inoculation suggested the presence of inclusions (Figure 4C) and hyphae colonization (Figure 4C, D). Phloem and xylem cells developed hypertrophy, degradation of the cell wall, and rot (Figure 4E). No conidia were found inside the tissue of the collar.
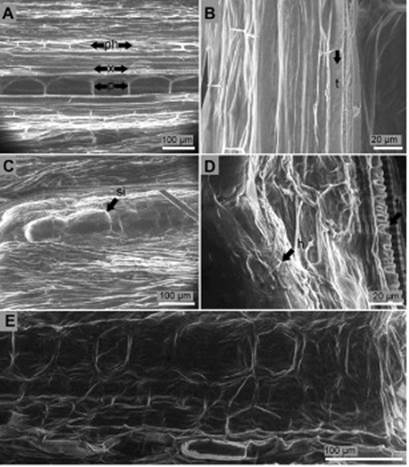
Figure 4 Longitudinal section of the collar of P. edulis f. flavicarpa seedlings. (A, B) Non-inoculated seedling: p, pith; ph, phloem; x, xylem; t, tracheid. (C) Suggested inclusion (si) inside pith cells of the collar ten days after inoculation. (D) Hyphae (h) colonization of the inner tissues of the collar ten days after inoculation: t, tracheid. (E) Degraded cells and cell walls of the inner tissues of the collar ten days after inoculation.
Surface and longitudinal section of the stem. Stem surface of non-inoculated seedlings of P. edulis f. flavicarpa were completely free from mycelia and conidia (Figure 5A). On the surface of the seedlings 24 hours after inoculation straight and slightly curved macroconidia of 36 μm length, blunt apical cell, and barely notched basal cell were observed. Microconidia of 8.3 μm length, ellipsoid, reniform and fusi-form shape were also detected (Figure 5B). Five days after inoculation formation of macroconidia in a dense non mature sporodochium and aerial mycelium in short monophialides were distinguished (Figure 5C, D). During this period abun-dant mycelium was observed around the stem (Figure 5E). Ten days after inoculation microconidia were developed in relatively long and abundant monophialides (Figure 5F). Thirteen days after inoculation sporodochia were fully developed (Figure 5G) and monophialides with microconidia in situ reached a spherical shape with equal longitudinal and transversal radio of 9.9 μm length (Figure 5H).
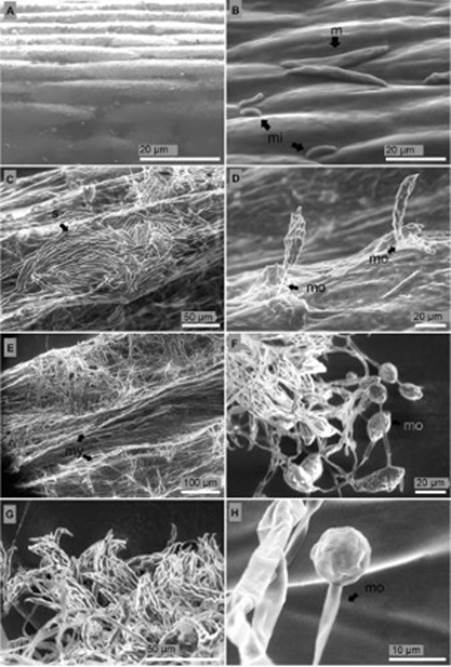
Figure 5 Longitudinal section of the stem of P. edulis f. flavicarpa seedlings. (A) Non-inoculated seedling. (B) Degraded vascular vessels 24 hours after inoculation: t, tracheid; ph, phloem. (C) Tracheid (t) with no signs of side-to-side hyphae colonization 24 h after inoculation. (D) Losses of turgor pressure of parenchymatous cells of the cortex four days after inoculation: c, cortex; ph, phloem; x, xylem. (E) Rot tissues five days after inoculation. (F) Rot tissues seven days after inoculation.
Rectangular elongated cells with cell walls well defined composed the healthy stem from non-inoculated seedlings (Figure 6A). Phloem cells from 24 hours after inoculation seedlings presented degradation. However, xylem cells did not acquire any damage (Figure 6B) and tracheid side-to-side hyphae colonization was not seen (Figure 6C). Four days after inoculation both phloem and cortex cells presented rugosity indicating water deficit (Figure 6D). Five days after inoculation structure of the cells of stem of inoculated seedlings was destroyed, no cell walls were distinguished, and cell structure was non-functional (Figure 6E). Similar results were obtained seven days after inoculation (Figure 6F).
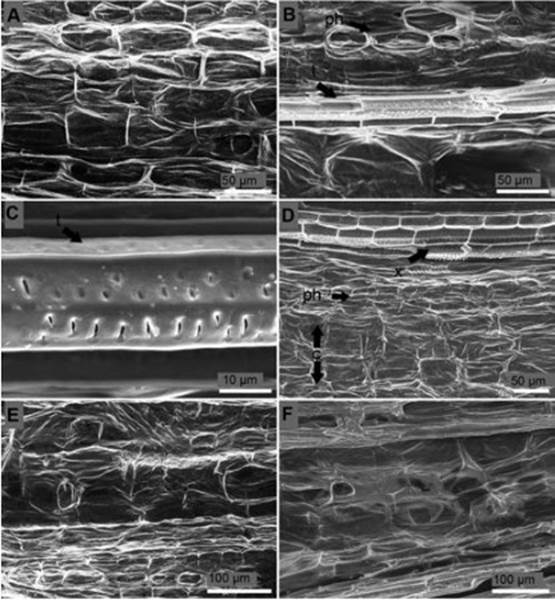
Figure 6 Longitudinal section of the stem of P. edulis f. flavicarpa seedlings. (A) Non-inoculated seedling. (B) Degraded vascular vessels 24 hours after inoculation: t, tracheid; ph, phloem. (C) Tracheid (t) with no signs of side-to-side hyphae colonization 24 h after inoculation. (D) Losses of turgor pressure of parenchymatous cells of the cortex four days after inoculation: c, cortex; ph, phloem; x, xylem. (E) Rot tissues five days after inoculation. (F) Rot tissues seven days after inoculation.
Surface of the leaves. Fusarium structures over the leaf surface came out from stomata six days after inoculation (Figure 7B). Leaf surface of non-inoculated seedlings were not overgrowth with mycelia of the fungus and stomata were free from any sign of the pathogen (Figure 7A, D). Thirteen days after inoculation monophialides appeared. Apical cell (false heads) of monophialides was joined to a relatively long conidiophore (74 μm length). Microconidia in situ were observed thirteen days after inoculation in leaf surface (Figure 7C) while macroconidia were not observed in these tissues. Deep colonization of leaf surface was carried out thirteen days after inoculation (Figure 7E).
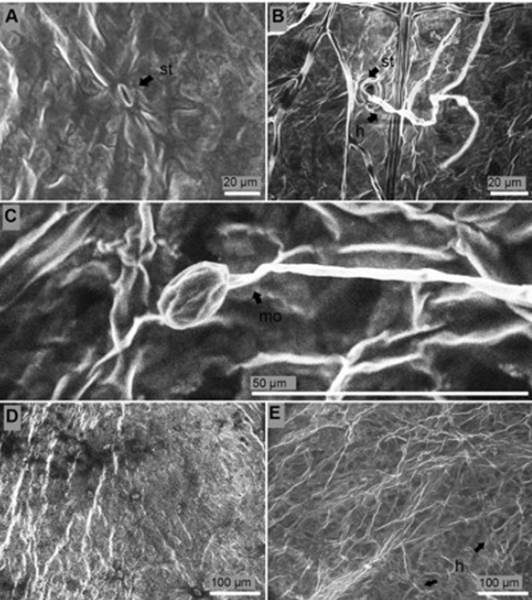
Figure 7 Surface of the leaves of P. edulis f. flavicarpa seedlings. (A) Stoma (st) of non-inoculated seedling. (B) Hyphae (h) colonized stomata (st) six days after inoculation. (C) Monophialide (mo) with micoconiadia in situ thirteen days after inoculation. (D) Healthy leaves. (E) Presence of hyphae (h) thirteen days after inoculation.
Discussion
Environmental scanning electron microscopy (ESEM) per-formed in this study allowed to reveal the presence, progress, distribution and localization of F. solani f. sp. passiflorae hyphae and reproductive structures (macro and microconidia) on the surface of collar, stem, and the leaves of infected P. edulis f. flavicarpa seedlings. The results suggest that, even though mycelium was detected in the external of both leaves and stem, faster and larger amount of hyphae colonization appear over the collar surface and concentrate on the collar internal tissues after hyphae penetrate from the epidermis to the xylem and pith. These observations coincide with studies developed on seedlings of pea inoculated with Fusarium solani f. sp. pisi that claim that infection of this pathogen can start at the base of the stem or stomata through directly cuticle degradation via cutinase enzymes (Pietro, et al ., 2003; Rogers, Flaishman, & Kolattukudy, 1994; Köller, Allan, & Kolattukudy, 1982; Shaykh, Soliday, & Kolattukudy, 1977). Natural infection of Fusarium solani in P. edulis f. flavicarpa occurs from the periderm to the pith of the collar tissues directly associated with symptoms observed in the field (Ortíz, et al. , 2014; Ortíz & Hoyos, 2012).
The surface of the leaves of inoculated seedlings suggested an infection caused by hyphae. However, hyphae colonization in these tissues was significantly lower compared to those obtained in collar sections. Images from internal tissues of the stem did not reveal presence of hyphae. Nevertheless, hypertrophy, cell wall degradation, losses of turgor pressure in the cells, and rot was observed in stem internal tissues. These symptoms are likely a sign of enzymatic activity produced by the fungus (Ortíz, et al. , 2014; Köller, et al. , 1982; Bateman & Basham, 1976). According to Bueno, et al. (2009) different isolates of F. solani f. sp. passiflorae from collar rot tissues of P. edulis f. flavicarpa produce extracellular enzymes such as amylase, lipase, cellulase, protease, and lacase. Some similar symptoms of cell degradation have been reported in wheat infected by F. culmorum and F. graminearum (Jackowiak, et al. , 2005; Kang & Buchenauer, 2002; Schwarz, et al. , 1997; Meyer, Weipert, & Mielke, 1986) banana infected by F. oxysporum f. sp. cubense (Meiting & Shaosheng, 2010), potato infected by F. roseum (Mullen & Bateman, 1975), and maize infected by F. graminearum (Gao, et al. , 2004).
According to Ángel-García, Robledo-Buriticá, Castaño- Zapata (submitted Boletín Cinetífico Centro de Museos, 2017) the first symptoms of collar rot of P. edulis f. flavicarpa seedlings in the modified test-tube screening methodology appear 2.4 days after inoculation. Results obtained in this study show that infection starts within 24 h after inoculation and 5 days after inoculation reproductive structures of F. solani f. sp. passiflorae were observed. These values suggest an approximate incubation period and latent period (respectively defined by Van der Plank (1963) as the time needed for infection to become visible and the time infected tissues take to become infectious) of 1.4 and 4 days. Similar results have been obtained in F. solani f. sp. pisi by Short & Lacy (1974) who determine that chlamydospores of the pathogen germinate after 24 h when a germinating seed is placed 7 mm away. Shaykh, et al. (1977) also claim that this pathogen began to penetrate the host within 10 h after inoculation and after 18 h the hyphae penetrates the tissue.
Although reproductive structures of Fusarium solani f. sp. passiflorae were mainly observed over the surface of the stem and leaves, no conidia were detected into internal tissues, and side-to-side vessel colonization was not confirmed. In addition, images that suggest inclusions inside pith cells of the collar are not conclusive and new studies must be proposed. However, Ortíz, et al. (2014) report that F. solani f. sp. passiflorae possibly colonizes from the collar to the stem through the xylem, microconidia are present among vessel lumen in P. edulis tissues, and gels are the most common type of inclusion. Morphology of macroconidia, microconidia and monophialides presented on the stem and leaves surface always coincide with the description of F. solani reported by Leslie & Summerel (2006).
Conclusions
Hyphae of F. solani f. sp. passiflorae colonize collar, stem, and leaves surface of P. edulis f. flavicarpa seedlings. However, hyphae colonization of internal structures concentrates on the collar while macroconidia, microconidia, and monophialides concentrate mainly on the stem epidermis and at lower levels on the leaves.
Damages caused in the cell wall of pith, xylem, phloem, and parenchymatous tissues of P. edulis f. flavicarpa seedlings, even when hyphae colonization is not present; indicate an extracellular enzymatic activity produced by F. solani f. sp. passiflorae.
Based on this study and additional studies, the incubation and latent periods of F. solani f. sp. passiflorae inoculated through the modified test-screening methodology in P. edulis f. flavicarpa seedlings are 1.4 days and 4 days, respectively
Although inclusions in pith cells is suggested in this study and related studies, new research is needed in order to verify this hypothesis.














