Introduction
One of the premises about population genetics predicts that dispersion decreases with an increased geographic distance where gene flow is generally lower among geographically distant populations causing a pattern of isolation by distance (Wright, 1943). It is well known that, besides dispersion, there are vicariant events (river and mountain formation) that explain biologic diversification.
Many hypotheses have been put forward to explain the high levels of diversity in Amazonia (Da Rocha & Kaefer, 2019). One of them, known as the riverine-barrier hypothesis, invokes the barrier effect of the rivers to explain patterns of diversity (Wallace, 1852; Bates, 1874) and proposes that riverine barriers separate once continuous populations leading to differentiation and speciation. However, we know little about the genetic structure of tropical anuran populations, especially those groups with cryptic diversity in tropical regions due to the marked phenotypic conservatism present between species as compared to the marked structuring and high genetic differentiation observed between different populations (Vieites, et al., 2009; Kaefer, et al., 2013; Guayasamin, et al., 2017).
A good example of this effect on anurans is the genus Pristimantis Jiménez De la Espada 1870, the most diverse among all vertebrate groups (Fouquet, et al., 2013). This diversity may be associated with the evolution of the direct development characteristic of Terrarana, which makes them interesting for evolution studies since species with such traits have poor dispersion capacity and philopatry (Beebee & Griffiths, 2005; Fouquet, et al., 2012). As a consequence of their life history, populations tend to be highly genetically structured and retain marked signs of past evolutionary processes making them good models for testing evolutionary hypotheses (Funk, et al., 2012; Fouquet, et al., 2012).
Pristimantis latro Oliveira, Rodrigues, Kaefer, Pinto, and Hernández-Ruz 2017 is found in the eastern Amazon between the Tapajós and the Tocantins- Araguaia Rivers occupying both banks of the Xingu River (Oliveira, et al., 2017). In the region, primary forests have a high degree of fragmentation and degradation with the substitution of the land cover mainly by extensive livestock and the advance of soy crops (Almeida, et al., 2014). Given the increase in environmental threats and habitat reduction on the newly described species and the lack of knowledge about the genetic structure of P. latro, we used fragments of the mitochondrial 16s rRNA and COI genes to: 1) Evaluate patterns of genetic structure; 2) test whether there is a relationship between the genetic distance and the geographic distance of different localities; 3) to reconstruct the phylogenetic relationships among the populations, and 4) to estimate the time of divergence between the sampled localities.
Methodology
Study area and sampling
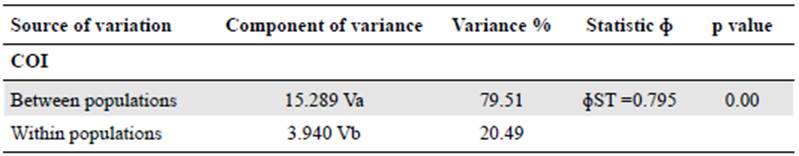
Fifty-two individuals of P. latro were collected in eight municipalities in the state of Pará, Brazil: Santarém, Medicilândia, Brasil Novo, Vitória do Xingu, Altamira, Anapu, Senador José Porfírio, and Marabá (Figure 1). The forest composition in the study area varies from dense ombrophylous forest to lowland ombrophylous forest (Instituto Brasileiro de Geografia e Estatistica - IBGE, 2012) with immense pasture areas.
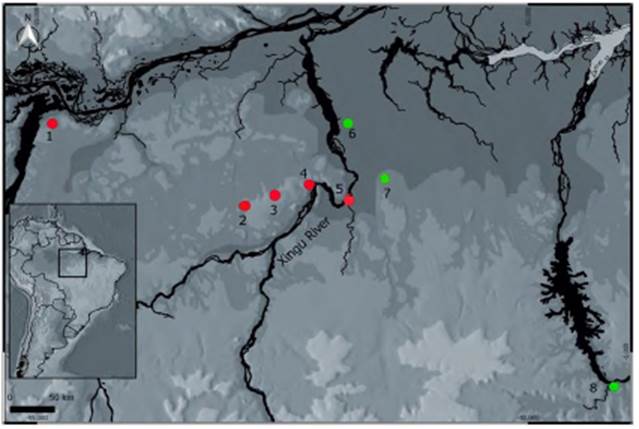
Figure 1 Map of Pristimantis latro capture localities. The red circles represent the localities from the left bank of the Xingu River and the green ones the localities from the right bank. The numbers correspond to the following sampled localities: 1) Santarém; 2) Medicilândia; 3) Brasil Novo; 4) Altamira; 5) Vitória do Xingu; 6) Senador José Porfírio; 7) Anapu (Type locality), and 8) Marabá.
The specimens collected in the field were euthanized with topical lidocaine ointment (benzotop®). The muscle or liver tissues were removed and stored in 95% ethanol. Specimens were fixed in 10% formalin, stored in 70% alcohol, and deposited in the Zoology Laboratory Collection- Adriano Giorgi of the Faculty of Biological Sciences at the Federal University of Pará - UFPA, Altamira Campus.
DNA extraction, amplification, and sequencing
The total genomic DNA was extracted with the CTAB protocol (Doyle & Doyle, 1987) and resuspended with 30 μL of ultrapure water. DNA quality was verified using 1% agarose gel in electrophoresis. Polymerase chain reaction (PCR) was performed to amplify a 600 bp fragment of the mitochondrial 16S rRNA gene and a 600 bp fragment of the mitochondrial cytochrome oxidase subunit I (COI) gene.
The amplification reaction of the mitochondrial 16S rRNA gene fragment was done under the following conditions: An initial temperature of 92°C (60 sec.), followed by 35 cycles of 92°C (60 sec), 50°C (50 sec) and 72°C (1.5 min) and a final extension at 72°C for 7 min. For the amplification of the mitochondrial COI gene fragment, we used the following conditions: 95°C (3 min), followed by 35 cycles of 95°C (of 30 sec), 53°C (30 sec), and 72°C (40 sec), and a final extension of 72°C (7 min).
The sequencing reactions were performed using the BigDye™ Terminator kit (Life Technology) following the manufacturer's protocol and using the forward primers 16S A (mtDNA) (Palumbi, 1991) and COI LCO 1590 (Folmer, et al., 1989). The products were precipitated with ethanol according to the manufacturer's recommendations (Applied Biosystems™), resuspended in 10 μL of formamide (Thermo Scientific™), and subsequently injected into the automatic ABI sequencer 3500XL (Applied Biosystems™).
Alignment of sequences and molecular dating
We aligned the sequences using the BioEdit software (Hall, 1999) using the ClustalW algorithm (Thompson, et al., 1996) and edited manually (checking the spectrograms for errors). To infer the time of divergence between the P. íatro localities, we built a species tree using the software BEAST v1.8 (Drummond, et al., 2012) using both the 16S and COI genes with the GTR + G evolutionary model chosen in the jModelTest software (Darriba et al., 2012) via corrected Akaike information criterion (AICc) and using a coalescent: constant size as tree prior. The species of Pristimantis used as outgroup were described by Oliveira, et al. (2020). The total number of sequences used for each locality in this study are shown in table 1. Given the absence of a fossil record, we used a general mutation rate for the 16S of 0.0028 per lineages per millions of years (Lymberakis, et al., 2007; Lemmon, et al., 2008) and a fixed substitution rate of 1.0 to COI mtDNA according to Fourdrilis, et al. (2016). The chain (MCMC) had 500 million generations with a tree sampled every one thousand generations. Thus, we recorded 50.000 trees and discarded the first 10.000 considering a 20% burn-in.
Table 1 Sampling localities of Pristimantis latro and respective numbers of mitochondrial genes (16S rRNA and COI) samples
We performed such analyses assuming a lognormal relaxed molecular clock, which presumes independent rates in different branches (Drummond, et al., 2006). The previous trees were modeled according to the Yule speciation process. The burn-in value was selected by viewing the log likelihoods associated with the distribution of the tree with the Tracer v1.5 software (Drummond & Rambaut, 2007), discarding 20% of the trees with the TreeAnnotator v1.6.6 (Drummond & Rambaut, 2007).
We obtained the uncorrected pairwise genetic distances p between localities in the program MEGA 6.0 (Tamura, et al., 2013) using thep distance for the 16S rRNA and the Kimura 2 parameter (K2P) for the COI.
Population structure analysis
We only used the COI gene as it had the largest number of sequenced locations compared to the 16S. We implemented a Bayesian Analysis of Population Structure (BAPS) in the BAPS 5.0 software (Corander, et al., 2008) to find clusters formed with the COI gene sequences (eight localities). The BAPS software uses nucleotide frequencies of the samples to infer the K number of genetically different groups through Bayesian analysis allocating similar sequences in the same group. The maximum number of K chosen for the analysis was eight, which corresponded to each locality. We used the log-likelihood value to choose the most likely grouping configuration. We performed an analysis of molecular variance (AMOVA) to infer the genetic differentiation between and within the P. íatro populations using the Arlequin 3.0 program (Excoffier, et al., 2005).
To estimate the genealogical relationships among individuals, we produced a network of haplotypes based on the topology of a phylogenetic tree for the COI gene built with the TreeFinder software (Jobb, 2011) with 10,000 non-parametric bootstrap replicas (Felsentein, 1985). We built the haplotypes network using the HaploView software (Salzburger, et al., 2011) to determine the number of haplotypes shared based on likelihood calculations. We did not use 16S gene sequences in the BAPS analysis and haplotypes network.
To calculate the relative importance of isolation by distance, we used the simple Mantel test performed in the Alleles In Space (AIS) program (Miller, 2005) for the COI marker only as it presented more localities. The Mantel test involves estimating the correlation between two matrices through Pearson's correlation r coefficient. The significance of r values was estimated by 1.000 permutations.
Results
Molecular dating and genetic distance
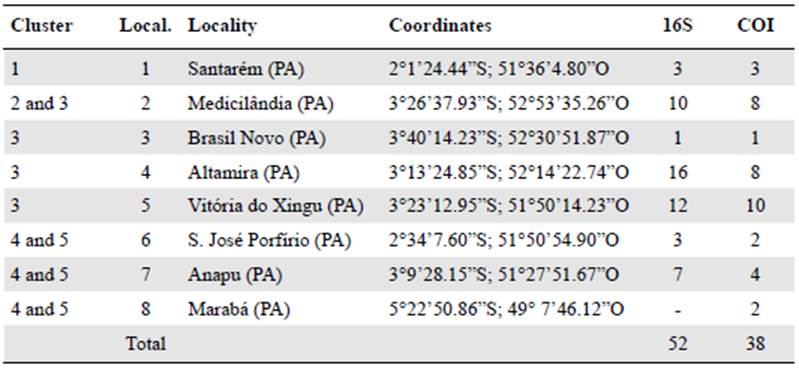
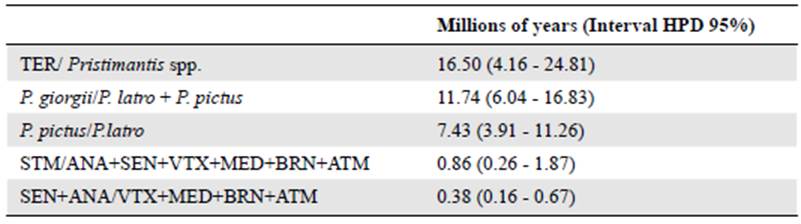
We obtained 52 sequences of the 16S rRNA gene fragment corresponding to eight haplotypes with 521 bp, 507 preserved sites, 14 variable sites, and 11 informative sites, as well as 41 COI DNAmt sequences equivalent to 14 haplotypes with 617 bp, 579 preserved sites, 38 variable sites, and 31 informative sites. Within P latro, the first cladogenesis event occurred around 0.860 Ma (95% HPD: 0.26 - 1.87 Ma), which separated the populations of Santarém (STM) from the others of P. latro. The second cladogenesis event was between the east and west margins around 0.380 Ma (95% HPD: 0.16 - 0.67 Ma) (Figure 2).Table 2 shows all the divergence times and the HPD interval.
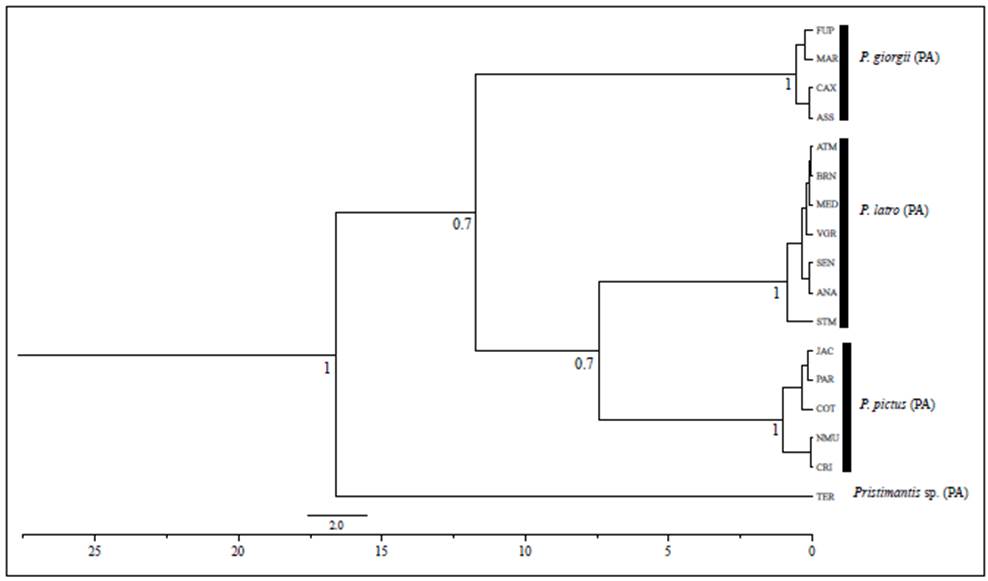
Figure 2 Tree of P. latro species based on the Bayesian analysis for the 16S and COI genes. The values of the branches indicate the posterior probability. The scale bar shows the number of replacements per site. Abbreviations represent the localities where P latro was studied in the Xingu region: Municipality of Santarém (STM), Municipality of Medicilandia (MED), Brazil Novo (BRN), Altamira (ATM), Vitória do Xingu (VTX), Senador José Porfirio (SEN), and Anapu (ANA), those for Pristimantis giorgii: (FUP, Fazenda Uberlândia Portel - PA; MAR, Marabá - PA, and CAX, Caxiuanã - PA), forP. pictus: (JAC, Jacareacanga; PAR, Paranaíta - MT; COT, Cotriguaçu - MT; NMU, New World - MT; CRI, Cristalino - MT), and Pristimantis sp. (TER, Terra do Meio - PA).
Table 2 Divergence time between populations of Pristimantis latro. Terra do Meio (TER); Santarém (STM); Medicilândia (MED); Brasil Novo (BRN); Altamira (ATM); Vitória do Xingu (VTX); Senador José Porfirio (SEN), and Anapu (ANA)
The genetic distance within P. latro presented a variation of 0.1 - 8.3% for the COI gene (Supplementary material 1,https://www.raccefyn.co/index.php/raccefyn/article/view/956/2807) and 0 - 1.7% for the 16S rRNA gene (Supplementary material 2,https://www.raccefyn.co/index.php/raccefyn/article/view/956/2808). Between the left and right banks, the distance was 2.8 - 4.6% for COI and 0 - 1.5% for 16S. The greatest genetic distance (8.3% for COI) was between P. latro from the Marabá and Senador José Porfirio municipalities with a geographic distance of 443 Km, whereas for 16S (1.7%), it was between P. latro from the Brasil Novo + Medicilândia and Santarém municipalities with a geographic distance of 240 Km. The most geographically distant localities (Santarém and Marabá, located on the opposite banks of the Xingu River, 701 Km), showed a genetic distance of 5.1% for the COI. Due to the absence of sequences for the 16S gene from Marabá, this comparison could not be made, therefore, we compared the Santarém and Anapu municipalities (opposite banks of the Xingu River) ~377 Km apart, which presented a 1% genetic distance between one another.
Population structure
Pristimantis latro presented a marked genetic structure for the COI gene (logML = -382.8427; probability = 0.99) forming five clusters (Figure 3): Cluster 1 (Santarém); Cluster 2 (Medicilândia - A); Cluster 3 (Medicilândia-B, Brasil Novo, Altamira, and Vitória do Xingu); Cluster 4 (Senador José Porfírio-A and Anapu - A); and Cluster 5 (Anapu-B, Senador José Porfírio-B, and Marabá). In the haplotypes network (Figure 4), we observed no shared of haplotype between the east and west banks of the Xingu River corroborating the result of the BAPS.

Figure 3 Population structure of P. latro from the COI mtDNA data. The vertically arranged colors represent each of the genetic groups (clusters). The numbers correspond to the sampled localities: 1) Santarém; 2) Medicilândia; 3) Brasil Novo; 4) Altamira; 5) Vitória do Xingu; 6) Senador José Porfírio; 7) Anapu, and 8) Marabá.
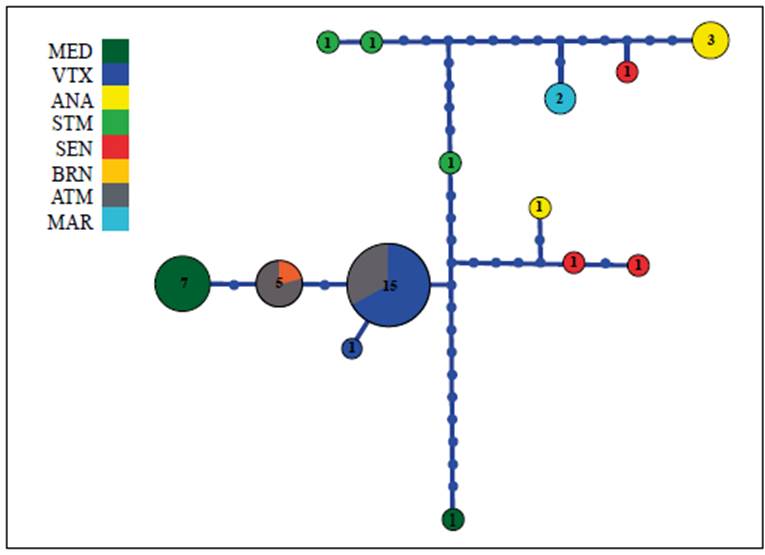
Figure 4. Median-joining haplotypes network based on DNAmt COI between populations of Pristimantis latro. Each haplotype is represented by a circle where the area is proportional to its frequency. The traits indicate additional mutational steps of branches with more than one mutation. The colors indicate each location. Blue dots represent lost or unsampled haplotypes. The abbreviations correspond to the sampled localities: Santarém (STM); Medicilândia (MED); Brasil Novo (BRN); Altamira (ATM); Vitória do Xingu (VTX); Senador José Porfírio (SEN); Anapu (ANA), and Marabá (MAR).
The result of the AMOVA for the COI gene revealed that most of the genetic differentiation is distributed among the five clusters resulting from the haplotypes network and the BAPS (79.51%) while a smaller part within them (20.49%) (ΦST = 0.79; p=0.00) (Table 3) showed a marked population structure in P. latro. The Mantel test revealed a positive and significant correlation between geographic and genetic distances (P<0.001; r=0.558) indicating a marked role of random events in the reproductive isolation between populations and genetic drifting in the process of evolutionary diversification of the studied marker.
Discussion
Pristimantis latro showed a strong genetic structure between the populations of the left and right bank of the Xingu River, as well as in the same bank. Anurans with direct development have life strategies that promote high population structure with species dispersing only by land where habitat heterogeneity inhibits dispersal across the landscape (Voss, et al, 2001).
The genetic structure pattern found in the present study is similar to those described in other Amazon frogs by Fouquet, et al. (2015) where species without tadpoles exhibited a genetic structure associated with rivers as barriers. Also consistent with our results are those of the report by Guayasamin, et al. (2017) showing that color patterns in Pristimantis ornatissimus can be delimited by geographical barriers (elevation gradients, rivers). However, we could not make a thorough comparison with other species of the genus Pristimantis since most of the published works refer to Andean species while P. latro is found in lowlands.
We found a spatial pattern of distribution of genetic variability similar to that proposed in the rivers-as-barrier hypothesis by Wallace (1852). Such a pattern has been found in several studies of different terrestrial vertebrates (Antonelli, et al., 2010). Finally, the sample design used in the present study did not evaluate if there was "permeability" of the rivers to gene flow in the respective extensions. Thus, our results add to a growing body of knowledge indicating that genetic variability at intra and interspecific levels in amphibians is related to the transposition of large Amazonian rivers (Funk, et al., 2007; Fouquet, et al., 2012; Kaefer, et al., 2012; 2013; Simões, et al., 2014).
The municipalities of Medicilândia (left bank of the Xingu River) and Senador José Porfírio and Anapu (right bank) presented more than one cluster per locality supporting local genetic structuring. Genetic structuring in amphibians at distances less than 5 km has already been documented (Lampert, et al., 2003; Andersen, et al., 2004; Burns, et al., 2004) showing that limited gene flow can promote differentiation in local populations mainly due to habitat fragmentation. The Mantel test explains that 31% (r2) of the variation is caused by geographic distance, but is not responsible for all structuring, which indicates that other factors are associated with this pronounced structuring (rivers).
The separation between the left and right banks of the Xingu River (middle of the Pleistocene) is relatively recent, however, it played an important role in population structuring (BAPS in figure 3) and the absence of haplotypes shared between the banks (haplotypes network in figure 4).Ribas, et al. (2012) suggested that the Xingu River acted a geographic barrier separating Psophia interjecta and P. dextralis around 0.8 -0.3 Ma and was responsible for the separation of younger lineages. Recent rivers may represent recent or semipermeable barriers, as found for Amphibia and Squamata in the Tapajós River (Moraes, et al., 2016).
Conclusions
Pristimantis latro presents genetic structure between the left and right banks of the Xingu River, as well as within the Tapajós-Xingu and Xingu-Tocantins interfluvial regions. The pattern of genetic structure found here corresponds to that described in recent articles for the genus Pristimantis, which reveals that species without tadpoles exhibit a genetic structure explained by the hypothesis of rivers as barriers.