Introduction
Our objective was to use rice husk (RH) as a precursor to produce carbonaceous material and explore its application in different technological fields: The manufacturing of sensors and electronic devices, the optimization of photovoltaic panels to capture solar energy, as an alternative energy source, as porous biochar, and in other fields. As a first step, we developed a pyrolysis system for the thermal decomposition of biomass in a controlled nitrogen atmosphere.
Rice husk is a by-product of the threshing process for obtaining white rice and it represents approximately 23% of the initial weight of paddy rice (Della, et al., 2002). It is generated when the husk of the rice grain is removed, a process that is usually carried out with rubber rollers. Studies have found that approximately one ton of RH results from every five tons of processed paddy rice (Lozano, 2020). On the other hand, when calcinated, RH ash represents between 13 and 29% of the initial weight and it is composed mainly of high-purity silicon (87-97%) (Abril & Abril, 2010) and small amounts of inorganic salts.
Until a few years ago, this waste was burned in open spaces producing air pollution with the subsequent negative impact on the environment and especially in human health. The laws and norms regulating environmental issues in Colombia, as well as the regula tions issued in compliance with the Kyoto Protocol for the reduction of CO2 emissions, prohibit the burning of this type of material in open spaces. However, the compliance with established protocols and procedures has been difficult as the husk must be burned under special conditions, there are effects on the profit margin, and the appropriate technology is lacking (Quiceno & Mosquera, 2010).
According to Quiceno & Mosquera (2010), since the 1980s, several studies have aimed at adding value to this by-product considered as waste. RH has been used as an additive for the manufacturing of cement and rubber, in the extrusion of polymers with natural fibers, in the fabrication of insulating materials, and as fuel to dry paddy rice. In Colombia, however, so far there are no reports on carbonaceous material and silica produced from this important and abundant organic precursor. Other leading rice-producing countries, such as India and China, are taking advantage of the extraordinary properties of this material and its potential applications in science and technology (Prasad & Pandey, 2012). In this context, it is worth highlighting the obtention of silicon from rice husk for the preparation of solar panels (Abril & Abril, 2010) and nanoparticles by pyrolysis (Wang, et al., 2011), with potential application in areas such as biomedicine, biosensors, and solar cells.
Pyrolysis is the chemical decomposition of organic matter and other materials, except metals and glass, by heating them at high temperature in the absence of oxygen (controlled atmosphere). The chemical industry uses this process to produce activated carbon, methanol, and other wood chemicals, as well as for the transformation of medium-weight hydrocarbons into lighter products such as gasoline. These specialized uses of pyrolysis are known by different names, such as dry distillation, destructive distillation, or cracking. Pyrolysis is also used in the generation of nanoparticles (Rockstraw, et al., 2005).
To validate the system, we charred rice husk samples varying the temperature and time of carbonization. We analyzed the synthesized material through scanning electron microscopy (SEM) techniques to determine its structure by means of micrographs and its elementary composition by means of X-ray scattering spectroscopy (EDS). Besides, we performed a Raman spectroscopy analysis to identify the characteristics of the synthesized material associated with the vibrational modes.
Here, we briefly describe the conditions to develop the pyrolysis system: The setting and monitoring of the temperature in the muffle and the outlet duct for pyroligneous gases, as well as of the relative humidity and the air quality in a laboratory environment, all of which are presented in detail in the supplementary material. Finally, we present and discuss the results obtained.
Materials and methods
This section describes the methods and techniques used for the acquisition and conditioning of the signals corresponding to the temperature in both the muffle and the pyroligneous gases pipe. Likewise, it characterizes the relative humidity, air quality, and temperature in the laboratory environment, along with the proportional-integral-derivative (PID) control and the virtual interface.
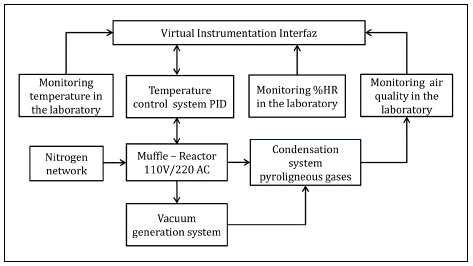
We developed a pyrolysis system similar to that devised by Prías-Barragan, et al . (2011) at the Instituto Interdisciplinario de las Ciencias, Universidad del Quindío, consisting of a muffle as a heating source, a reactor, a nitrogen supply network, a trap to condense the gases produced during the process and avoid environmental pollution, and a pump for vacuum generation. The temperature is the main parameter in pyrolysis processes; in this case, we developed an automatic PID temperature controller to monitor the temperature required for carbonizing the precursor material as a virtual instrumentation software application that tracks air quality, relative humidity, and temperature in the laboratory environment. Figure 1 shows a basic scheme of the developed pyrolysis system and figure 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818, the control and monitoring P&D system diagram.
Temperature in the muffle, the gas outlet duct, and the laboratory environment
We monitored the temperature in the muffle, in the gas outlet duct, and in the laboratory environment. We used a type K thermocouple to measure the temperature in the reactor muffle and the gas outlet duct and a PT100 resistance temperature detector (RTD) to check it in the laboratory environment. The temperature in the muffle was the carbonization temperature of the material. This is one of the most important parameters of the process together with the carbonization time and it was conditioned and entered into the virtual instrumentation interface (VI) through the NI-9211 thermocouple input module (National Instrument, NI). The conditioning circuit and the PT100 sensor, as well as the temperature measurement system, are described in detail in the supplementary material. (Figure 6S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818)
Relative humidity monitoring
We monitored the relative humidity in the laboratory environment using an HS1101 capacitive sensor and then sent the signal to the VI using the NI-9201 analog voltage module. The signal conditioning circuit is shown in the supplementary material (Figures 2S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818, 3S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818 and table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818).
Air quality in the laboratory
We monitored air quality in the laboratory environment to control the emission of pyroligneous gases produced during the carbonization process of rice husk. We used the MQ135 resistive type electrochemical sensor (Olimex, 2017) located in the gas outlet duct; this sensor is sensitive to different gases such as NH3, NOx, and CO2. In the supplementary material we describe in detail the conditioning and calibration of the sensor, figures 4S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818 and 5S,https://www.raccefyn.co/index.php/raccefyn/article/view/1109/2818.
Temperature control system
Temperature and carbonization times are the main parameters determining the character istics of the carbonaceous material to be obtained. For controlling the temperature of the process, we developed a PID temperature control system to establish the number of heating ramps and their respective timing, and to raise the temperature inside the muffle to the point selected for the process. This also enabled us to establish the time this temperature level had to be kept to satisfy the carbonization time.
Virtual instrumentation interface
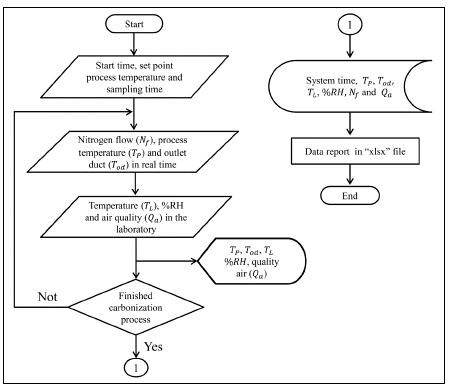
The virtual instrumentation interface monitored and recorded the variables involved in the carbonization process: The temperature in the muffle (process temperature: T P ) and the pyroligneous gases exit pipe (T od ), the flow of nitrogen (N f ) entering the system, the relative humidity (%RH), the temperature (T L ), and the air quality (Q a ) in the laboratory environment. Figure 2 shows the flow chart of the VI developed to implement the virtual instrumentation interface.
Validation of the pyrolysis system
To validate the pyrolysis system, we carried out the rice husk carbonization processes at different temperature levels varying the carbonization time (hours). We analyzed the samples of synthesized material using SEM techniques to determine their structure and topography, EDS to identify their elementary composition, and Raman spectroscopy to determine the characteristics associated with the vibrational modes and crystalline structure.
Results
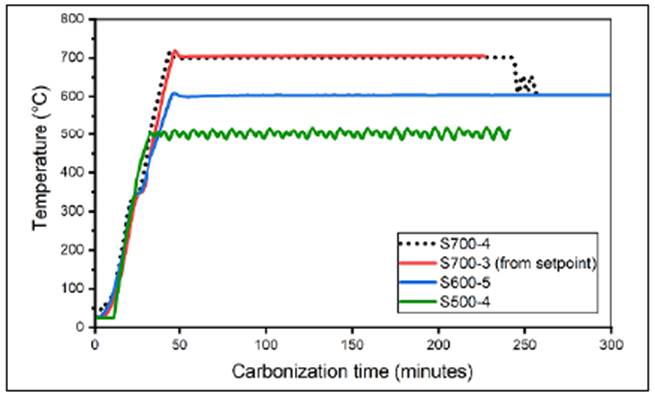
We developed a pyrolysis system with a nitrogen-controlled atmosphere to obtain carbona ceous material from biomass precursors. The temperature of the process varied in a range of up to approximately 1000°C. We controlled the temperature by means of an automatic PID system monitored by a virtual instrument (VI) and we monitored air quality in the gas outlet pipe of the system and the relative humidity and temperature in the laboratory. Figure 3 shows the performance of the system in four carbonization processes in terms of the processing temperature (temperature in the muffle). We adjusted the system to guarantee adequate control of the variables involved in the process.
We synthesized several samples of carbonaceous material from the rice husk during the validation of the pyrolysis system. These samples were analyzed using SEM, EDS, and Raman spectroscopy. No grinding process was performed on the samples after the pyrolysis process, i.e., the charred rice-husk flakes were removed. Our results showed that the thermal decomposition method using a pyrolysis system is a low-cost alternative to obtain graphene oxide from the rice husk (GO-RH) precursor.
Figure 4a shows an RH sample and figure 4b the magnified SEM micrograph of a sample processed at 700°C and carbonized during three hours (S700-3) where the tubular morphology typical of rice husk composed of internal fibers and a highly irregular surface, visible at 500 magnifications, is observed in figure 4c. This morphology is typical of coals obtained from rice husks as described by Ahiduzzaman & Sadrull-Islam (2016). As shown in figure 4d, these fibers are composed of internal hollow structures between 5 and 30 μm in size. This feature is typical of rice husk carbonization from 450° on (Ahmad-Husni, et al., 2014).
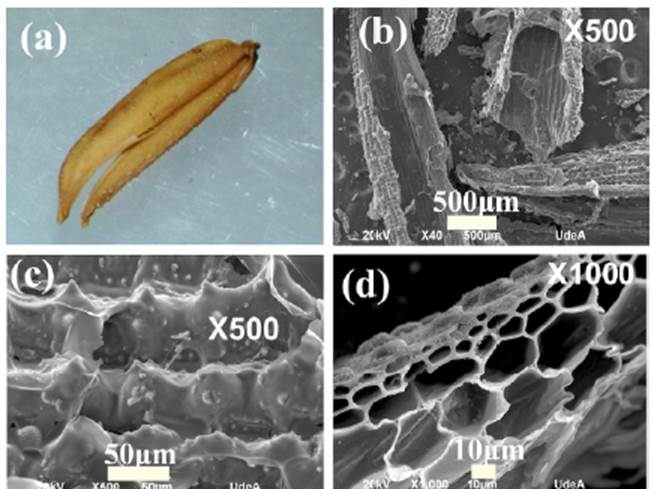
Figure 4 a. Sample rice husk. SEM micrograph of b. S700-3 at 40 x; c. S700-3 at 500 x, and d. S700-3 at 1000 x (CIDEMAT-UDEA)
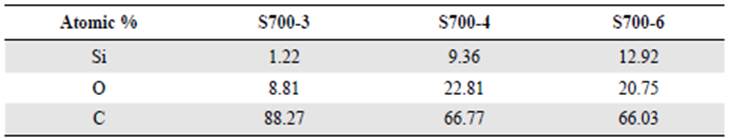
On the other hand, we verified the obtention of carbon and silicon by pyrolysis of the rice husk. The energy-dispersive x-ray spectroscopy (EDS) analyses showed that there the carbon present in the sample decreased as the carbonization time increased. The opposite occurred with the silicon content in the samples as shown in table 1.
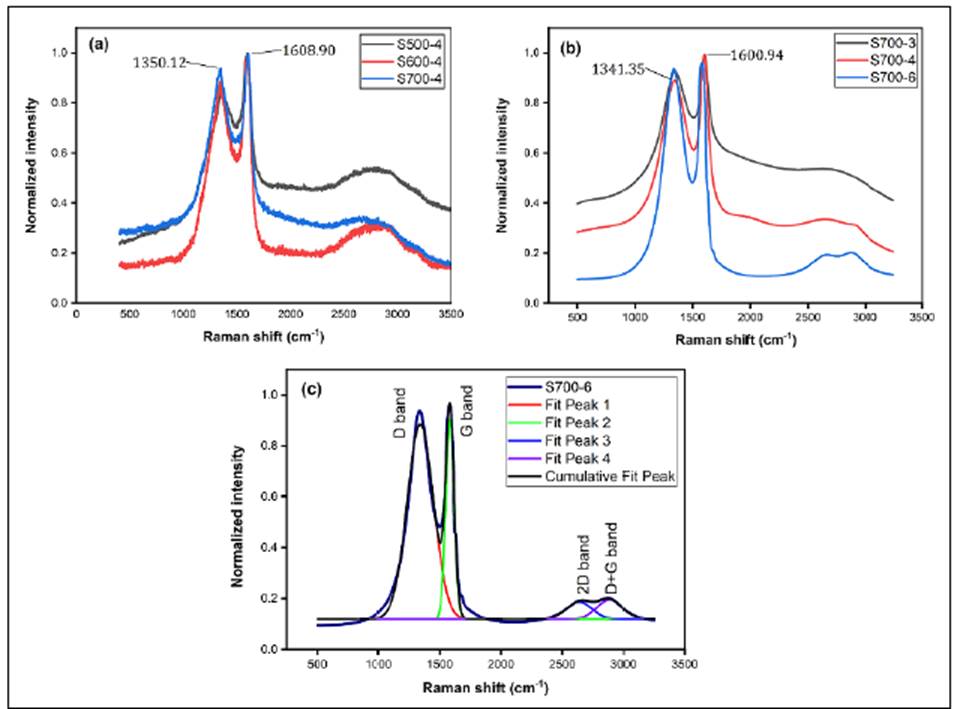
Raman spectroscopy is a well-established and suitable characterization tool to determine the quality of graphene and graphene oxide (GO) (Eigler & Dimiev, 2017). The Raman spectra of the carbonaceous material samples (Figures 5a and5b) corresponded to a typical Raman GO spectrum characterized by the G band (~1600 cm-1) (Johra, et al., 2014) showing the degree of charge transfer and the sensitivity to external electromagnetic perturbations (Souza, et al., 2019) while the D band (~1350 cm-1) indicated the amorphous carbon species (Johra, et al., 2014), as well as the formation of a graphitized structure (Prías, et al., 2016). The ratio between the band intensities (I D /I G ) increased as the pyrolysis temperature increases, which represents the transformation of a disordered structure into an ordered one (Rybarczyk, et al., 2019). Figure 5c shows how the spectrum of sample S700-6 was deconvolved in four peaks and the wide 2D and D+G bands around the 2800 cm-1 suggesting the presence of many graphene layers with edges, defects, and sp2 regions (Prías, et al., 2016, Johra, et al., 2014; Bharathidasan, et al., 2018).
Discussion
The pyrolysis system developed allowed for the generation of new materials using agro-industrial waste. In this particular case, we used rice husk, which can negatively affect the environment, to generate a carbonaceous material useful in science and technology research. The system included the monitoring of variables in the laboratory environment: Temperature, relative humidity, and air quality, important parameters for sample processing.
In the semi-quantitative analysis of the carbonaceous material obtained, the EDS analysis with an electronic scanning microscope showed that there were two main elements, carbon and silicon (Table 1). However, there is a high level of uncertainty given that we performed the analysis on the material as it came out of the carbonization process, i.e., without prior grinding. This analysis also showed a decrease in the carbon present in the samples as carbonization time increased.
The Raman spectra in the GO-RH samples (Figure 5) showed a multilayer oxidized graphene-type vibrational response with overtones strongly influenced by carbonization time and temperature, which could be attributed to the increase in the desorption of multifunctional oxides and organic compounds.
The slight modification of the G band can be attributed to voltage and temperature modifications (Figures 5a and5b) associated with the degree of charge transfer and the sensitivity to external electromagnetic disturbances (Souza, et al., 2019). Additionally, band D can be associated with defects such as vacancies, grain boundaries, and amorphous carbon species (Johra, et al., 2014).
The two characteristic bands obtained confirmed the presence of conjugated bonds and carbon-carbon double bonds typical of oxidized graphene showing that in the 500 to 700 ° C temperature range, the graphene oxide-type structure is very stable and structurally conformable. These results suggest that the GO derived from rice husk (GO-RH) is a potential candidate for the development of electronic devices and sensors.