Introduction
Reproductive patterns in anurans are related to environmental variables (Moreira & Lima, 1991; Donnelly & Guyer, 1994; Bertoluci, 1998), and they differ within tropical and subtropical frog communities. The anuran fauna of 'non-seasonal' tropical environments is composed of a large number of species that reproduce year-round (Crump, 1974; Gascon, 1991; Donnelly, 1999) while the breeding season of most species in seasonal tropical environments is typically associated with the rainy season (Aichinger, 1987; Bertoluci, 1998; Bertoluci & Rodrigues, 2002). However, both reproductive patterns, year-round reproduction and reproductive activity concentrated in the rainy seasons, can be found simultaneously in many tropical frog communities (Rossa-Feres & Jim, 1994; Moreira & Barreto, 1997; Prado, et al., 2000). This strategy might serve to avoid overlapping in time and space and minimize competition among sympatric species (Moreira & Barreto, 1997; Bertoluci & Rodrigues, 2002).
Differences among reproductive patterns can play a significant role in the composition of an assemblage over relatively short time scales. This happens through temporal fluctuations in the structure of populations (Fitzgerald, et al., 1999), or by mechanisms of reproductive isolation that maintain the entity of the species developing variants in their reproductive modes (Duellman, 1967; Crump, 1982). Crump (1982) suggested that the differences in the population structures related to the reproductive season have direct effects on the structure of the community because the duration of the reproductive activity of a species is influenced by the reproductive patterns of other species. Moreover, reproductive phenology is known to be an important factor for the coexistence of sympatric species of Neotropical anuran communities (Bertolucci, 1998; Donnelly & Guyer, 1994; Bertolucci & Rodrigues, 2002).
With more than 500 species, the genus Pristimantis is the most diverse amphibian genus with the largest number of endemic species (Frost, 2020). Its great diversity, especially in the northern Andes (Lynch & Duellman, 1997; Hedges, et al., 2008, Meza-Joya & Torres, 2016), is attributed to the use of terrestrial sites for reproduction and the direct development of eggs (Terrarana clade sensu Hedges, et al., 2008). Although continuous reproductive cycles are to be expected in these species considering their relative independence of water bodies (Summers, et al., 2007; Pacheco-Florez & Ramírez-Pinilla, 2014), there are no studies on the reproductive phenology of Terrarana congeneric assemblages, which makes Pristimantis species assemblages a very interesting study subject.
We found assemblages of up to ten Pristimantis species in the same area within two Andean cloud forests in the Cordillera Oriental of Santander, Colombia, (Arroyo, et al., 2003; Gutiérrez-Lamus, et al., 2004), most of them with similar body sizes and morphology (unistrigatus phenetic group) (Arroyo, et al., 2005). Each assemblage included three abundant species, three common species, and few rare species (Arroyo, et al., 2003; Gutiérrez-Lamus, et al., 2004).
Morphological similarity among coexisting animal species may cause potential interactions and lead to competition and niche segregation (Huey & Pianka, 1977). However, pseudo community analyses of diet, time of activity, and vertical position in these cloud forest Pristimantis assemblages revealed significant overlap and no guild structure for resources partitioning in the community (Arroyo, et al., 2008; Gutiérrez-Lamus, 2003). In our study, we compared some characteristics and the time of reproductive activities in the three most abundant coexisting Pristimantis species from one of the assemblages to determine whether the reproductive phenology and/or the distribution of age-sex classes might segregate the species temporally.
Materials and methods
Study area
The study site was located in La Sierra forest reserve at Santuario de Fauna y Flora Guanentá - Alto Río Fonce (national reserve) on the west flank of the Cordillera Oriental in the Colombian Andes, southern Santander department adjacent to the department of Boyacá (73° 15' 7'' W, 6° 03' 7'' N, 2000 to 2400 m of altitude). The area has a bimodal regime of rains with two peaks (April and October), an average annual rainfall of 1891.3 mm, a mean temperature of 12.1°C, and two types of forests: native and planted oak forests (Querchus humboldtii), abundant arborescent ferns, epiphytes, and Cecropia sp. trees (Galindo-Tarazona, et al., 2003).
Frog sampling
The frogs we analyzed were the same used in a previous study to determine their microhabitat, diet, time of activity, and assemblage structure (Gutiérrez-Lamus, 2003; Gutiérrez-Lamus, et al., 2004). The specimens were collected during eight months between July 2001 and November 2002 by visual detection of individuals during the day, afternoon, and night. Each month, three different people sampled three trails in the forest from 9:00 to 11:00, 15:00 to 17:00, and 20:00 to 22:00 h. Transects were sampled once a day to avoid disturbances. Each person looked for frogs in the vegetation and hand-collected them removing litter from the ground.
Additionally, in individuals collected in December 2000 sex ratio, sexual dimorphism, and size-fecundity relationships analyses were done. There was an involuntary gap in the samples during the rainy months of April and May. This sampling bias was partially corrected with the collection of the same species for a later study (2004) in a neighboring area with the same type of vegetation and altitude (Cachalú Biological Reserve, 73° 07' 109 09'' W, 6° 05' 3' 9'' N, 2000-2400 m altitude) (Cortés, et al., 2008).
All collected specimens were euthanized by submersion in 10% ethanol, fixed in 10% formalin, preserved in 70% ethanol, and placed in the herpetological collection of the Museo de Historia Natural, Escuela de Biología, Universidad Industrial de Santander (UIS-A-).
Laboratory methods
We measured the snout to vent length (SVL), the head length, the height and width, mouth width, body height and width, and length of the foreleg and hind limbs with a Vernier caliper (± 0.02 mm). We made a mid-ventral incision and recorded the following morphometric data for each sex: In males, we calculated the length of the abdominal fat bodies and the width (a) and length (b) of the left testes to determine their volume using the ovoid spheroid formula: V = (πa2b)/6 (Wiederhecker, et al., 2002. Then they were fixed in Bouin's solution, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin. The stage of spermatogenesis was determined in each individual by analyzing the histological slides and the stages were classified following Saidapur (1983): Stage I, primary and secondary spermatocytes; stage II, early spermatids; stage III, late spermatids; stage IV, presence of sperm bundles at the luminal border of the seminiferous tubule, and stage V, mature sperm in the seminiferous tubule lumen. In females, we recorded the number and diameter of the largest ovarian follicles and the width and convolution of the oviducts. We classified the reproductive condition of adult females by direct observation as previtellogenic (ovaries without yolked follicles) and vitellogenic (ovaries containing vitellogenic follicles and convoluted oviducts).
Data analyses
We used the monthly summaries of data describing SVL, sex, and reproductive condition for each species to characterize juvenile, adult female, and adult-male age and sex classes. We used age classes to determine sex ratios. We applied G tests to detect significant differences in the composition of the population over time. To establish significant differences in frogs' body-size distribution over time, we conducted a variance analysis (ANOVA) followed by post-hoc tests (Tukey test).
To test for sexual dimorphism in size, we used the following data: SVL, head length, width and height, mouth width, body height and width, the average length of the segments of the hind limbs (femur, tibia-fibula, and tarsal and metatarsal-phalange), and forelegs (humerus, radio-ulna, and carpal-phalange). We used t-tests to analyze the differences between male and female SVLs. For the other variables, we used covariance analyses (ANCOVA) of data transformed to logarithms (base 10). We used the body size (SVL) as a covariate to adjust the data of all other variables. Given the low sample size of adult females, we took a random subsample of 10 to 20 adult males to balance sample sizes between sexes during statistical testing.
The reproductive stage of each specimen served to determine the overall reproductive activity for males and females per month and the whole sampling time, as well as the minimum size of sexual maturity for each population determined by the body size (SVL) of the smallest vitellogenic female and the smallest male with sperm in testis (stages IV-V). To detect intra- and inter-sex variation per month (synchrony) and over time (seasonality) we used G tests.
We calculated linear regressions between testicular volume and abdominal fat bodies with the SVL from every adult male in each species. We obtained the residuals of these regressions to eliminate the effect of body size on the reproductive variables. We conducted a variance analysis (ANOVA) or a Kruskal-Wallis test on the residuals (as appropriate) followed by post hoc tests (Tukey or Nemenyi tests, respectively) to determine significant differences in these variables and discriminate which months were different (Zar, 1996).
We used linear regressions to test for significant relationships among testicular volume, accumulation of abdominal fat bodies, and rainfall. We did not find significant differences between the historical values for the mean precipitation and temperature during the sampling years [precipitation (2001: t 0.05,11 = -0.76, p = 0.46 and 2002: t 0.05,4 = 0.36; p = 0.74) and temperature (2001: t 0.05,11 = -0.31; p = 0.76 and 2002: t 0.05,4 = 1.47; p = 0.22)], therefore, we used the historical rainfall data corresponding to monthly precipitation and temperature averages over the last 20 years recorded at the climatological station La Sierra (IDEAM) in municipality of Duitama (Boyacá) located 73°10'W, 5°58'Nm at 2700 masl. The low numbers of captured adult females prevented us from using them in the statistical analyses.
We calculated the clutch size as the number of yolked follicles observed in each female. We evaluated the relationship between female and clutch sizes using linear regressions.
Results
We collected 292 adult males, 26 adult females, and 249 juveniles (130 immature females, 38 immature males, and 81 juveniles of undetermined sex) from the three species. Adult females were few in all three species and most of them were reproductive; however, we observed some differences in P. miyatai regarding the occurrence of adult females during the sampling (Figure 1). There were significant differences in the number of males from the three species and of P. merostictus and P. uisae juveniles; in June, one of the driest months of the year, we collected very few individuals of any of the species (Table 1,Figure 1).
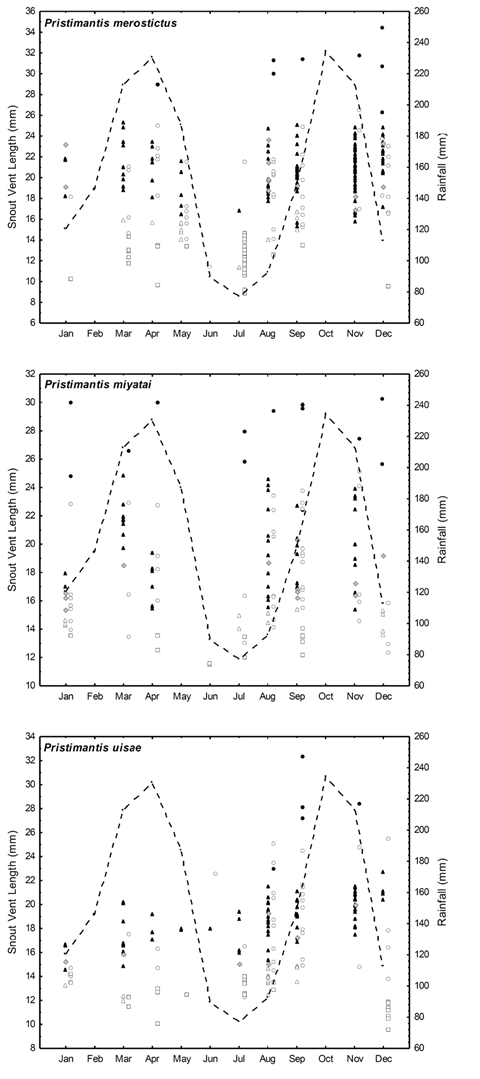
Figure 1 Distributions of age (SVL), sex, and age class of the individuals collected from the populations of Pristimantis uisae (a), P. merostictus (b), and P. miyatai (c). Reproductive adult males (filled triangles); non-reproductive adult males (grey rhombuses); immature males (open triangles); adult females (filled circles); immature females (open circles); and juveniles of undetermined sex (squares). Rainfall pattern (dotted line)
Table 1 Temporal changes in population structure and sexual dimorphism in Pristimantis merostictus, P. uisae, and P. miyatai
| Species | Population Structure | Sexual Dimorphism | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | N | G-Test | P | Variable | Sex | Mean±SD | N | R 2 | F-Test | Statistic Value | |
| P. merostictus | M | 144 | G0.05,9=112.2 | <0.001 | SVL | M | 19.75±2.16 | 10 | t0.05,16=9.2 | ||
| F | 8 | -- | -- | F | 30.28±2.74 | 8 | <0.001 | ||||
| J | 62 | G0.05,9=33.1 | <0.001 | ||||||||
| U-S | 45 | G0.05,9=89.0 | <0.001 | ||||||||
| P. uisae | M | 90 | G0.05,9=63.8 | <0.001 | SVL | M | 18.26±2.51 | 10 | t0.05,18=6.6 | ||
| F | 5 | -- | F | 26.18±2.83 | 10 | <0.001 | |||||
| J | 48 | G0.05,9=39.4 | <0.001 | ||||||||
| U-S | 23 | G0.05,9=8.9 | 0.5 | ||||||||
| P. miyatai | M | 58 | G0.05,9=17.2 | <0.05 | SVL | M | 19.05±2.92 | 20 | t0.05,38=7.16 | ||
| F | 13 | -- | -- | F | 25.66±2.92 | 20 | <0.001 | ||||
| J | 58 | G0.05,9=14.3 | 0.1 | Tibio- | M | 10.06±1.41 | 20 | 0.94 | F2,37=326.8 | F1,37=6.8 | |
| U-S | 13 | -- | -- | fibula | F | 13.79±1.42 | 20 | P <0.0001 | <0.010 | ||
| Humerus | M | 3.68±0.47 | 20 | 0.85 | F2,37=109 .8 | F1,37=6.5 | |||||
| F | 5.02±0.58 | 20 | P<0.0001 | 0.015 | |||||||
M: Adult males; F: Adult females; J: Juveniles; U-S: froglets with undetermined sex. Measurements given in mm. No significant variables in sexual dimorphism were omitted.
Juvenile recruitment
We recorded 63 Pristimantis froglets with very small body sizes (6 to 10 mm SVL) corresponding to 20% of all the juveniles in the sampling. We found significant differences in their occurrence (G0057 = 25.17; p < 0.001), and they were most abundant in the driest months (December and July). We did not include them in the analyses because it is not possible to differentiate among species when body sizes are so small (Lynch, pers. comm.).
We found somewhat bigger froglets, enough to identify their species, but we were not able to assign their sex given the minimal size of their gonads (juveniles of undetermined sex). We found P. merostictus smallest froglets during the two dry seasons (July and December), and P. miyatai's ones in June, July, and September. We registered P. uisae froglets' highest abundance in December after finding females with yolked follicles during the second rainy season of the year (Figures 1 and2).
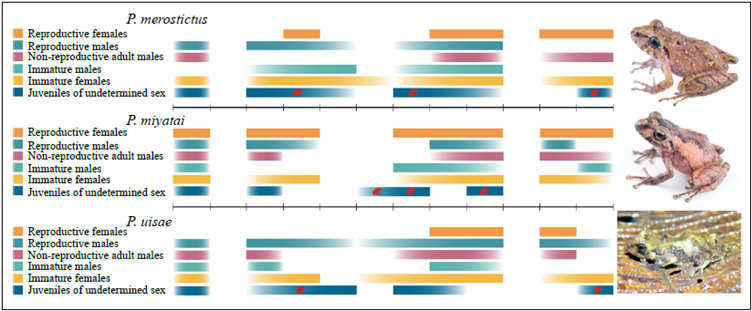
Figure 2 Reproductive events during the year for three species of Pristimantis. Colors in each bar are more intense when the abundance of this category is higher in the sampling months. The red froglets in the bar of juveniles of undetermined sex indicate the month in which the smallest froglets were found.
Pristimantis merostictus, P. miyatai, and P. uisae juveniles' body size showed a significant monthly variation (P. merostictus, F7,96 = 11.9, P < 0.001, P. miyatai, F7,61 = 2.44, P = 0.03, and P. uisae, F8,155 = 6.8, P < 0.001). Most of the froglets and juveniles were captured in the litter during mornings or afternoons.
Sexual dimorphism
In all three Pristimantis species, the mean SVL in adult females was greater than in males indicating a marked sexual dimorphism in body size (Table 1). We also observed sexual dimorphism in E. miyatai for tibia-fibula and humerus lengths, which was always longer in females regardless of their body size.
Sexual maturity and reproductive activity
Females from the three species reached reproductive maturity at larger body sizes than males, which may affect the population and operational sex ratios because immature females were more frequently found than adult females (Table 2).
Table 2 Sex ratio and minimum size at maturity (MSM) in the studied species. The operational sex ratio corresponds to the number of adult males per number of adult females. The population sex ratio includes adults and juveniles of each sex.
| Species | N | Sex ratio | MSM (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adult | Juveniles | Population | Operational | Males | Females | ||||
| Males | Females | Males | Females | Undetermined | ♂ : ♀ | ♂ : ♀ | |||
| P. merostictus | 144 | 8 | 12 | 50 | 45 | 3:1 | 18:1 | 15 | 26.28 |
| P. miyatai | 58 | 13 | 13 | 45 | 13 | 1:1 | 5:1 | 15.34 | 22.36 |
| P. uisae | 90 | 5 | 13 | 35 | 23 | 3:1 | 18:1 | 14.58 | 22.92 |
Males' reproductive activity seemed to be year-round in the three species. We did find few adult non-reproductive males (stages I-III), indicating that spermatogenesis may be discontinuous in these species (Figures 1 and2). The occurrence of reproductive males varied within the sampling months for the three species. P. merostictus (G005’9 = 70.7, < 0.001, N = 132) and P. miyatai (G005’9 = 34.2, < 0.001, N = 46) males were almost absent in June and July, the driest season of the year; conversely, P. uisae (G0.05’9 = 3 1, < 0.001, N = 82) males were found in all the sampling months, though more abundantly in the second semester of the year, during the transition from the driest season to the second rainy season and in its course when reproductive females are found. However, reproductive males from all three species are a permanent and available resource for females.
We found a positive relationship between body size and testis volume. Only P. merostictus showed significant variation in the testicular volume from month to month when we removed the effect of body size on testis volume (Table 3a). In all three species, testicular volume was significantly related to precipitation (Table 3a).
Table 3 Variation over time and precipitation in the adjusted testis volume (a) and the abdominal fat bodies (b) in adult males and their relationship with body size (SVL)
| (a) | N | SVL | Time | Precipitation | ||||
| Species | r2 | P | P | r2 | P | |||
| P. merostictus | 135 | 0.17 | < 0.001 | 0.001 | 0.07 | < 0.001 | ||
| P. miyatai | 60 | 0.68 | < 0.001 | 0.88 | 0.06 | 0.04 | ||
| P. uisae | 94 | 0.52 | < 0.001 | 0.26 | 0.03 | 0.05 | ||
| (b) | N | SVL | Time | Testis volume | Precipitation | |||
| Species | r2 | P | P | r2 | P | r2 | P | |
| P. merostictus | 136 | 0.024 | 0.04 | 0.32 | 0.095 | <0.001 | 0.002 | 0.3 |
| P. miyatai | 65 | 0.08 | 0.02 | 0.26 | 0.027 | 0.12 | 0.011 | 0.2 |
| P. uisae | 94 | 0.18 | 0.001 | 0.36 | 0.146 | <0.001 | 0.001 | 0.3 |
Interestingly, we found ovarian follicles dispersed in the interstitial tissue in the testes of some P. merostictus and P. uisae individuals in whose seminiferous tubules and ducts there was abundant free sperm indicating they were reproductive males; 3% of P. merostictus and 6% of P. uisae adult males had ovarian follicles in the testes.
Females' reproductive activity seemed to differ in the three species. In P. miyatai females, we observed vitellogenic ovaries during all sampling months indicating a continuous reproductive activity. P. merostictus reproductive females seemed to appear for few months during the rainy seasons while P. uisae ones only during the second rainy season.
During the first rainy season of the year, between April and May 2004, in a forest next to our study site, we sampled P. miyatai, and P. merostictus juveniles of different body sizes, abundant mature males, and some mature females, which suggests that they were reproductively active during this season. On the contrary, we only collected P. uisae juveniles and mature males (Figure 1).
This information, together with that of juvenile recruitment, allowed us to estimate the reproductive time of each species: in P. miyatai reproduction was continuous; P. merostictus, had two reproductive seasons at the beginning of each peak of rains as we collected small body juveniles during the following dry months, and P. uisae smallest juveniles appeared after finding vitellogenic females in the middle and at the end of the second rainy season suggesting that they have only one reproductive season per year. We found some P. uisae and P. miyatai non-reproductive adult females and, simultaneously, vitellogenic females suggesting reproductive intrasexual asynchrony between females of both species.
Clutch size
Size-fecundity relationships were difficult to establish due to the small sample of adult females. In P. miyatai (31 ± 14.07 eggs/clutch) and P. uisae (37.4 ± 4.62 eggs/clutch) the SVL was not related with fecundity (r2 = 0.026, F1,8 = 0.21, P = 0.66, and r2 = 0.65, F1,3 = 0.28, P = 0.21, respectively), whereas in P. merostictus (41.71 ± 8.93 eggs/clutch) there was a positive relationship between clutch size and body size (r2 = 0.65, F1,5 = 11.93, P = 0.018).
Abdominal fat bodies
We observed a positive relationship between the abdominal fat bodies of adult males and their body size (SVL) in the three species. We did not find any significant variation in fat body size during the sampling period in any of the species. There was a significant relationship between abdominal fat bodies and testicular volume in P. merostictus and P. uisae. The influence of rainfall on these reserves was not evident in any of the species (Table 3b).
Reproductive overlap
Pristimantis miyatai showed continuous reproductive activity as reproductive females and juveniles were collected during all study months with the greatest similarity between P. merostictus and P. uisae. In both species, female reproductive activity occurred during the rainy seasons and juvenile recruitment in the following dry months. However, P. uisae reproduction activity occurred mainly during the second rainy season of the year.
Discussion
Sex-ratio
We collected few adult females while reproductive males were more easily found because of their calling activity; this is particularly true for P. uisae males given that they call during the morning, afternoon, and night. However, males of the other Pristimantis species did not vocalize regularly nor in organized choruses, and all of them were collected without hearing them before. Additionally, possible differences in the use of the microhabitat by adults from both sexes could hamper the sampling of adult females. For example, Chinchilla-Lemus, et al. (2019) found significant differences between P. bacchus sexes in their use of the microhabitat with males preferring the stem of the bushes above 81 cm while adult females mostly used the leaf litter because of the substrate temperature. Therefore, the detectability of the males might be higher than that of females.
There may be other explanations for sex ratio differences in adult frogs. In the Pristimantis species under study, males reached their minimum-size sexual maturity at smaller body sizes than females, so the age and body size at which females and males reached sexual maturity differ with females requiring more time than males to mature. Thus, it is very common to collect small frogs of approximately the same body size composed by adult and reproductive males, as well as immature females.
On the other hand, some authors have suggested that species with prolonged breeding seasons have lower operational sex ratios and intersexual reproductive asynchrony (Wells, 1977; Arak, 1983; Howard & Kluge, 1985). The operational sex ratios found in the species of this assemblage were strongly male-biased as it was also observed for P. bacchus by Chinchilla-Lemus, et al. (2019). On average, males were three to 18 times more abundant than females in the sampling and mature males were reproductively active almost all the time, adult females were fewer and asynchronous in their reproductive activity. Population sex ratios, however, were higher than operational sex ratios suggesting that few females reached the body size at sexual maturity. This was also evident when we observed many more immature females than immature males in the three species, and in the overlap in body sizes of adult males and immature females. The fact that females must reach a larger size may answer to the fact that they have to live longer to reach the category of a mature adult. If the survival of the sexes is the same, there should be more mature males than mature females.
Sexual dimorphism in body size
Morphometric comparison of males and females in the three analyzed Pristimantis species showed that females attained greater SVL than males. We found similar results for other species in the genus (e.g., P. bacchus,Chinchilla-Lemus, et al., 2019). Sexual dimorphism in body size is common in frogs; according to Shine (1979), females are larger than males in 90% of the species of frogs and toads. Woolbright (1983) explained the general phenomenon of small male body size in anurans as the suite of constraints associated with reproductive behaviors in males, which require energy that would otherwise be used in growth.
This might also be due to differences in growth rates between sexes. In this sense, Arendt (1997) argued that the conditions that select for large size may actually select for slow growth rates and that if an increment in growth rate requires additional energy and materials, less energy would be available for reproduction. Moreover, studying Lithobates catesbeianusHoward (1981) suggested that the precocious maturity of males and stronger predation pressure on larger males explained this phenomenon. Thus, in females, fecundity may be favored by slow growth rates, and in males, rapid growth may be favored when predation is size-dependent. However, these hypotheses must be evaluated for Pristimantis species.
Relationship between body size and clutch size
Many studies have shown that larger females produce larger clutches or eggs and that this relationship is favored by natural selection (Salthe & Duellman, 1973; Crump, 1974; Howard & Kluge, 1985). Although a significant positive relationship between SVL and the number of yolked follicles was only observed in P. merostictus, it is very common in Terrarana species (Eleutherodactylus jonhstonei) (Bourne, 1997; Ortega, et al., 2005), in E. coqui (Townsend & Stewart, 1994), and Craugastor bransfordii (Donnelly, 1999; Wake, 1978). Thus, we can suppose that sexual dimorphism in body size in these species relates to the delayed maturity in females to favor the increase of the clutch size with larger body sizes, as well as to the time required for the allocation of energy for oocytes growth.
Juvenile recruitment
An important percentage of the collected frogs were small froglets unidentifiable at the species level that might correspond to neonates of our three species but also to other sympatric Pristimantis species such as P. bacchus, P. lynchi, or P. lutitus. Their body sizes ranged between 6 and 10 mm SVL and most of them were captured during the two dry seasons of the year. With an egg diameter of 3 to 4 mm, recently born froglets (less than 6 mm SVL) are usually absent in captures as their very small size makes them difficult to detect at sight and they can be hidden in the humid leaf litter where they were probably born. Post-hatching development and growth rates in froglets in these and other Pristimantis species are unknown; however, we can infer that their initial growth is fast and that the smaller specimens we collected had recently been born and they represented the recruitment of the Pristimantis populations in the area.
Our findings indicate that populations' age structure changed with time in P. merostictus, P. miyatai, and P. uisae, juveniles being more abundant than adults. Although, unlike the other two species, there were no differences in the occurrence of P. miyatai juveniles during the sampling months (Table 2), we inferred that froglets incorporated into the population during dry seasons grew until males reached sexual maturity, around six months later, when small reproductive adult males were more abundant (Figure 1).
Most of the Pristimantis adult males were initially considered as juveniles following the original description of the species where adult males have larger body sizes and very small testes and, in some species, there is a lack of vocalization. Accordingly, histological studies of the testes of these small-size species are necessary to avoid errors in the determination of the actual reproductive condition and minimal size at maturity.
Reproductive phenology
Males P. merostictus, P. miyatai, and P. uisae individuals were reproductive during all the sampling months. However, spermatogenesis appeared to be discontinuous since we observed that few adult males reinitiated their sperm production (stages I-III) when most of the males were reproductive?. Similarly, in some adult males from other Terrarana species a short testicular regression has been observed, although at population level there are reproductive males during the entire year (Geobatrachus walkeri,Pacheco-Flórez & Ramírez-Pinilla, 2014).
Testicular volume did not vary significantly through the months of study for P. miyatai and P. uisae while in P. merostictus there was a significant temporal variation, but histological analyses showed a continuous reproductive activity. This suggests that variation in testicular volume in this species is associated with the occurrence of mature females ready to mate, given that the month with the highest testicular volume was August when we captured vitellogenic females with large follicles. A similar observation was made for Geobatrachus walkeri by Pacheco-Flórez & Ramírez-Pinilla (2013).
Some of our Pristimantis male frogs had ovotestes which have been documented in other species such as Acris crepitans (Reeder, et al., 1998) and Telmatobius pisanoi (Montero & Pisanó, 1990) while some experimental studies with environmental contaminants have shown ovotestes and other gonadal abnormalities like hermaphroditism and multiple gonads (Hayes, et al., 2002). Our hypothesis is that the testicular anomaly we observed may respond to the use of agro-industrial chemicals near the study area but their potential effects on the sexuality of these species need to be examined in detail.
Abdominal fat bodies are special reserves for gonadal development (Brenner, 1969; Morton, 1981; Tsiora & Kyriakopoulou-Sklavounou, 2001, 2002) and their size reflects nutritional conditions (Jargensen, et al., 1986). The actual role of abdominal fat bodies in gametogenesis in tropical frogs has been discussed (Saidapur & Hoque, 1996; Huang, et al., 1997), and, as we observed, abdominal fat bodies in P. miyatai did not show any relationship with testicular size while in P. merostictus and P. uisae they were positively correlated with testes volume. These interspecific differences could be a species-dependent physiological variation. Otherwise, the lack of temporal variation and, therefore, of the influence of rainfall on this trait in males might respond to a high and permanent food availability in the area. In this sense, the study of Gutiérrez-Lamus (2003) showed that the frogs were active and well-fed all year-round. Besides, spermatogenesis is a process that does not require much energy, and, possibly, abdominal fat bodies in males are mainly required for calling, mate selection, mating, etc. (Girish & Saidapur, 2000) more than having a testicular function.
Chinchilla-Lemus, et al. (2019) found that a high proportion of non-breeding adult females are found in the forest litter but we found few adult females in this condition. In other Terrarana species, all adult females collected throughout the year were reproductive (Eleutherodactylus johnstonei,Ortega, et al., 2005; Geobatrachus walkeri, Pacheco-Flórez & Ramírez-Pinilla, 2013). The presence of adult females with ovaries but no yolked follicles may indicate that once a Pristimantis female inhabiting cloud forests oviposits, it takes a long time before it develops a new group of yolked follicles ready for ovulation and oviposition, especially when their clutch sizes are relatively high. Besides, some species such as P. bacchus exhibit maternal care of the egg clutch (Chinchilla-Lemus & Meneses-Pelayo, 2016). In contrast, female individuals of Andinobates virolinesis (Dendrobatidae) females, another terrestrial small frog found in a similar precipitation regime in a nearby locality, are reproductive year-round with a clutch size of only one egg per oviposition event but producing several continuous clutches with short inter-clutch intervals. Additionally, the males give parental care to clutch and tadpoles so females can optimize their egg production all the time (Valderrama-Vernasa, et al., 2010). These contrasting strategies in female reproductive activity in small terrestrial frogs are linked to their reproductive mode to face similar rainy regimes in these forests.
Although males seem to be permanently ready to mate, females define the reproductive activity in the population. Apparently, there is a relationship between reproduction and the seasonal distribution of rains for Pristimantis females. In our study, we proposed three seasonal patterns of breeding activity for the most abundant species in this anuran assemblage: Continuous reproductive activity (P. miyatai); reproductive activity during the two rainy seasons and recruitment in the following dry seasons (P. merostictus), and reproductive activity mainly during the second season of rains and juvenile recruitment in the following dry season (P. uisae). Although P. bacchus is also part of our assemblage (Gutiérrez-Lamus, et al., 2004), we found very few individuals. However, in an isolated population of this species living in a very small forest fragment further north, where it is the most common, with a rainfall regime similar to that of our site but a less drastic and prolonged dry season at midyear and a much more drastic dry season in December-February, Chinchilla-Lemus, et al. (2019) found a different pattern as males and females' reproductive activity was continuous for 10 months (January to October) and ceased during the two driest months with the maximum recruitment of juveniles in the transition from the driest season to the first rainy season (February to April). Thus, rainfall patterns seem to be an important factor in the breeding activity defining reproductive patterns. For some Terrarana species, rainfall also seems to be a key factor defining reproductive patterns. For example, in two populations of Eleutherodactylus johnstonei in Barbados (Ovaska, 1991), the reproduction peaked in the early wet season, and in Oreobates discoidalis in Argentina (Vaira, 2002), it was seasonal in response to the rainfall regime.
Rainfall may not directly affect reproduction in terrestrial frogs and other resources indirectly associated with reproduction, such as food, might also influence its timing. In C. bransfordii in Costa Rica, Donnelly (1999) found that reproduction was continuous, although Watling & Donnelly (2002) suggested that the abundance of juveniles during the dry season indicated an important period of juvenile recruitment for this species sustained by higher food availability. This could be related to the seasonal rhythm in the food supply that characterizes tropical zones due to seasonal changes in rainfall (Janzen & Schoener, 1968; Jetz, et al., 2003). In the Neotropical region, some studies have indicated that arthropod abundance in the leaf litter is high during dry seasons and the dry to wet season transition (Toft, 1980; Watling & Donnelly, 2002). Likewise, Watling & Donnelly (2002) suggested a link between food availability and the timing of reproduction in litter frogs in the tropics because many anurans time their reproduction in such a way that recruitment of juveniles occurs during the season of peak arthropod leaf litter abundance. Gutiérrez-Lamus (2003) found a high percentage of litter arthropods (isopods, collembolans, and diplopods) in the stomach contents of juveniles and most adults of our three Pristimantis species suggesting that they get some food from the ground. In this sense, it would be useful to study the distribution and abundance of arthropods and the effects of ontogeny on the consumption of these prey items in our study area.
Reproductive overlap
In Neotropical anuran communities, studies have shown that reproductive phenology is an important factor explaining the coexistence of sympatric species (Donnelly & Guyer, 1994; Bertoluci, 1998; Bertoluci & Rodrigues, 2002). When studying the phenology of calling activity and reproduction of a Neotropical anuran community, Gottsberger & Gruber (2004) found that temporal partition was apparent among different anuran groups according to their reproductive mode. For Pristimantis species they found that the calling activity was irregularly distributed over the study period and concluded that within the anuran groups (frogs with similar reproductive mode), the temporal patterns of this activity were very similar.
Assemblages of Pristimantis species in cloud forests in the northern part of the Cordillera Oriental vary in composition depending on geography and altitude, However, the third most abundant species are P. miyatai and P. merostictus while the rare and less common species differ among sites (Arroyo, et al., 2003; Suárez-Badillo & Ramírez-Pinilla, 2004; Gutiérrez-Lamus, et al., 2004). These two species are abundant in most of the cloud forests in the northern part of the Cordillera Oriental and they are considered generalist species as regards their microhabitat, diet, and habitat because they are abundant in different types of forests (native, non-native, and oak forests) (Lynch, 1994; Gutiérrez-Lamus, et al., 2004; Arroyo, et al., 2004, 2008; Cortés, et al., 2008). Our hypothesis is that this differential abundance of Pristimantis species among frog assemblages is related to and a consequence of the different patterns of their reproductive phenology.
We found that species overlap body size-wise during most part of their lives was similar to that in another study in the Colombian Cordillera Oriental (Arroyo, et al., 2003). Additionally, our reproduction analyses revealed that Pristimantis populations' breeding times coincide because the recruitment of most of the juveniles occurred during the driest months of the year. Lima & Moreira (1993) have found that relationships among species may depend more on the interaction among individuals of different sizes than on the relationships among heterospecific adults. In this sense, it would be important to evaluate the ontogenetic change in the use of resources such as the microhabitat, diet, and daily activity regimes because the ontogeny could be related to the segregation of these species.
Reproductive activity, age structure, and recruitment of juveniles revealed a significant temporal overlap among the three Pristimantis species. We concluded that reproductive phenology does not play an important role in the temporal segregation of these species. However, the different breeding activity patterns could represent a strategy to reduce multi-specific froglets overcrowding the site of growth and to facilitate the coexistence among potentially competing taxa.














