Introduction
Currently, intensified agro-industrial processes have increased the generation of waste from raw materials in the cultivation stage to product commercialization. Colombia, a country with an extensive agro-industrial activity, generates around 6.1 million tons of fruit with the subsequent impact, as well as the added value of byproducts that can improve the benefits per year in this field (Martínez & Quintero, 2017). Agro-industrial waste, mostly the lignocellulosic biomass (Patiño, 2014), has a negative impact on the environment due to its high concentration of organic matter and inadequate final disposal (Díaz, 2011). Therefore, the use of this type of waste represents a challenge in the way of mitigating environmental consequences (Tafur, et al., 2006). Today, agro-industrial waste is used mainly for power generation and the production of animal concentrates (Cardona, et al., 2012).
Lignocellulosic biomass is composed of cellulose, hemicellulose, lignin, ash, and extractives (Abril & Navarro, 2012). The bioactive compounds, i.e., the secondary metabolites of plants, are present in the extractives and are of great interest to the food and pharmaceutical industries for their health benefits (Helkar, et al, 2016). Phenolic compounds represent an important group of bioactives with the ability to inhibit or delay the oxidation of other molecules due to the presence of the hydroxyl group in its structure (Chen, et al., 2013). The antioxidant activity of phenolic compounds protects the organism from reactive oxygen species and free radicals associated with chronic and degenerative diseases such as cancer, diabetes, cardiovascular disorders, liver damage, aging processes, and Alzheimer's (Chen, et al., 2013).
Phenolic compounds can be extracted using different solvents such as water, hexane, ethyl-acetate, ethanol, or methanol, the latter being one of the most frequently used for the subsequent quantification of total phenolic compounds (Kumar, et al., 2017). The quantification of these compounds, as well as the determination of their antioxidant capacity, is done by spectrophotometric or colorimetric methods (Grupta, 2015). The antioxidant capacity is measured by the antioxidant molecules present in a certain organic material. Most of the tests used to determine it measure the ability of antioxidant compounds to react with a given free radical, or the potential they have to reduce complexes formed by ions and a reagent (Valenzuela, 2015).
The methods for measuring the antioxidant capacity differ in the oxidizing agent (capture of electrons or hydrogens), the substrate used, the evaluation time, the sensitivity, the selectivity, and the interactions of the sample with the reaction medium. Some of the most common methods include flavonoids quantification, ferric reducing antioxidant power (FRAP) and total peroxyl radical trapping antioxidant parameter (TRAP) determination, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and the 2,2'-azinobis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assays, as well as the reducing power of antioxidant activity (RPAA) and the oxygen radical absorption capacity (ORAC) determination, among others (Grupta, 2015; Schaich, et al, 2015).
Colombia is an agricultural country with high consumption of fruits both by the general population and agro-industries and, therefore, the generation of fruit byproducts is also high. Some of the most frequently consumed fruits in the country are soursop, lulo, mango, tree tomato, tangerine, orange, lemon, grape, pineapple, arazá, peach, papaya, peach palm, banana, passion fruit, and sapote. Previous studies have shown that fruit byproducts have structural compounds with a high antioxidant capacity. According to Ghosh, et al. (2019), orange and mango peels have FRAP values in a range between 17.48 ± 0.38 to 568.92 ± 4.03 μM Fe (III) / mg, and their flavonoid content can vary between 1.59 ± 0.02 and 6.83 ± 0.09 mg of quercetin per gram of dry sample depending on the solvent used for extraction. Grape residues, characterized by having a high antioxidant capacity, have a flavonoid content between 3.26 and 6.71 mg of quercetin per gram of dry sample (Zeinab, et aL, 2019) while their ORAC value is between 347 and 660 μM trolox per gram of dry sample (Ferreyra, et al., 2019).
Extracts from citrus fruit byproducts have been found to be an important source of compounds like ferulic acid, 5-hydroxyvaleric acid, vanillic acid, 2-oxybenzoic acid, hesperidin, and naringin. These extracts have applications in the pharmaceutical, cosmetic, and food industries and they have been incorporated into products such as vitamin C tablets, antiseptic mouthwash, ointments, shampoo, perfumes, sunscreen, juices, sauces, and jams, among others (Mahato, et al., 2019). On the other hand, grape seed has been used to obtain over-the-counter dietary extracts and supplements rich in gallic acid, hydroxybenzoic, and cinnamic acid derivatives, quercetin, kaempferol, (+)-catechin, (-)-epicatechin, gallocatechin, procyanidins, vitamin E, carotenoids, and phytosterols (Lucarini, et al., 2018).
In the present study, we evaluated the bioactive potential of thirty fruit byproducts (peels, seeds, stem, and stalk) by determining the total flavonoids present in them and analyzing their antioxidant capacity using the FRAP, RPAA, and ORAC methods to define their potential use for obtaining compounds with added value.
Materials and methods
Obtention and pretreatment of byproducts
We selected the byproducts from the fruits with the highest production in Caldas and Valle del Cauca departments. We obtained them from supermarkets and fruit processing industries in these two departments. We selected 30 byproducts from peels (16), seeds (12), stems (1), and stalks (1). In table 1, we present the selected fruits along with their scientific name, the byproducts used, and the nomenclature for each of them. Once obtained and separated, the byproducts were stored at a temperature of -20 °C until use. Subsequently, the byproducts were thawed at room temperature for one hour and cut manually to reduce their size. Finally, they were dried at 45 °C for 60 hours in a Terrigeno D8 muffle and grounded to a particle size of 1 mm or less using a disk crusher.
Table 1 Selected waste for the study
| Fruit | Scientific name | Waste | Nomenclature |
|---|---|---|---|
| Soursop | Annona muricata | Peel | Soursop-P |
| Seed | Soursop-S | ||
| Passion fruit | Passiflora edulis | Peel | Passion fruit-P |
| Lulo | Solanum quitoense | Peel | Lulo-P |
| Sugar mango | Mangifera indica | Peel | Mango-P |
| Tree tomato | Solanum betaceum | Peel | Tree tomato-P |
| Seed | Tree tomato-S | ||
| Stem | Tree tomato-St | ||
| Arrayana tangerine | Citrus reticulata | Peel | Tangerine-P |
| Seed | Tangerine-S | ||
| Valencia orange | Citrus X sinensis | Peel | Orange-P |
| Seed | Orange-S | ||
| Tahití lemon | Citrus x latifolia | Peel | Lemon-P |
| Seed | Lemon-S | ||
| Isabella grape | Vitis labrusca | Peel | Grape-P |
| Seed | Grape-S | ||
| Stalk | Grape-St | ||
| Honey gold pineapple | Ananas comosus | Peel | Pipeapple-P |
| Arazá | Eugenia stipitata | Peel | Arazá-P |
| Seed | Arazá-S | ||
| Peach | Prunus persica | Peel | Peach-P |
| Seed | Peach-S | ||
| Melona papaya | Carica papaya | Peel | Papaya-P |
| Seed | Papaya-S | ||
| peach palm | Bactris gasipaes | Peel | peach palm-P |
| Seed | peach palm-S | ||
| Banana passion fruit | Passiflora tarminiana | Peel | Banana passion fruit-P |
| Seed | Banana passion fruit-S | ||
| Zapote | Quararibea cordata | Peel | Zapote-P |
| Seed | Zapote-S |
Extraction of phenolic compounds
For the extraction of the bioactive compounds, we weighed 250 mg of the dry byproduct in a centrifuge tube and added 1 ml of 60% ethanol EMSURE®. The tube with the sample was vortexed for one minute and centrifuged at 10,000 rpm for 15 minutes in an Eppendorf centrifuge 5424C, and then we obtained the supernatant and the precipitate. The supernatant was recovered in a 2 mL centrifuge microtube and we added 500 uL of ethanol 60 % to the precipitate, stirred the mix in the vortex and centrifuged under the same conditions mentioned above. The supernatants were bound, and we added ethanol 60 % until we completed a volume of 2 mL (Ruales, et al., 2015). The ethanolic extracts we obtained were used in flavonoids for the determination with FRAP, RPAA, and ORAC.
Flavonoids determination
We determined flavonoids following the methodology described by Kim, et al. (2003): We took 20 μL of the sample, 115 μL of water, and 7.5 μL of NaNO2 5% (EMSURE®), we homogenized them, and then we allowed their reaction for 5 minutes. After, we added 30 μL of 2.5% AlCl3 (Sigma-Aldrich), homogenized, and allowed the reaction for 6 minutes. Finally, we added 50 μL of 1 M NaOH (EMSURE®) and 50 μL of water, homogenized, and measured the absorbance at 500 nm 5 minutes later. We used a reference substance of quercetin (Sigma-Aldrich) and distilled water as a photometric target: For this analysis, we used a Multiskan GO UV/Vis microplate spectrophotometer (Thermo Scientific). The result of the test was expressed in equivalent micrograms of quercetin per gram of dry-based sample (μg QE/g Sdb).
Ferric reducing antioxidant potential (FRAP) determination
The ferric reducing antioxidant potential was determined following the methodology described by Benzie & Devaki (2018). To measure the ferric reducing potential, we prepared the FRAP reagent, which consisted of a mixture of 300 mM acetate buffer, 10 mM TPTZ (Sigma-Aldrich), and 20 mM FeCl3 (Sigma-Aldrich) in a 10:1:1 ratio. First, we took 150 μL of the FRAP reagent and incubated it for one minute at 37 °C, then we homogenized it and measured the absorbance at 600 nm. Subsequently, we added 20 μL of the sample, homogenized it, and allowed it to react for 8 minutes. Finally, we measured the absorbance at 600 nm and used trolox (Sigma-Aldrich) as the standard substance and distilled water as a photometric target. For this test, we used a microplate spectrophotometer Multiskan GO UV/Vis (Thermo Scientific). Our results were expressed as the equivalent micrograms of trolox per gram of dry-based sample (μg TE1/g Sdb).
Determination of the reducing potential of antioxidant activity (RPAA)
We determined the reducing potential of antioxidant activity following the methodology described by Ballester (2016). For the RPAA test, we took 100 μL of the sample, 100 μL of 0.2 M phosphate buffer, and 100 μL of 1% potassium ferricyanide (Sigma-Aldrich).
The mixture was placed in a 50°C water bath (Memmert® WNB14) for 20 minutes, then we added 100 μL of 10% trichloroacetic acid (PanReac) and centrifuged in an Eppendorf centrifuge 5414D for 10 minutes at 6500 rpm. Finally, we took 100 μL of the mixture, and we added 100 μL of water and 20 μL of 0.1% iron chloride (III) (Sigma-Aldrich), homogenized, allowed to stand for 10 minutes, and then measured the absorbance at 700 nm. We used gallic acid (Sigma-Aldrich) as the reference substance and distilled water as a photometric target. For this test, we used a microplate spectrophotometer Multiskan GO UV/Vis (Thermo Scientific! Our results are expressed as an equivalent in micrograms of gallic acid per gram of dry-based sample (μg GAE/g Sdb).
Determining oxygen radical absorption capacity (ORAC)
We determined the oxygen radical absorption capacity following the methodology described by Ou, et al. (2001). To determine the oxygen radical absorption capacity, we took 20 μL of the sample and we added 120 μL of 120 nM fluorescein (Sigma-Aldrich), and then we incubated for 15 minutes at 37°C in the absence of light. Subsequently, we added 60 μL of 40 mM AAPH (Sigma-Aldrich) and the fluorescein intensity was read every minute for 2 hours using 538 and 485 nm emission and excitation filters. For this analysis, we used trolox (Sigma-Aldrich) as the reference substance. We used the phosphate buffer as a photometric target and positive control of the test in a microplate fluorimeter (Fluoroskan™ Ascent, Thermo Scientific). The result was expressed as the equivalent micrograms of trolox per gram of dry-based sample (μg TE2/g Sdb).
Statistical analysis
We performed all the analyses in triplicate; the values reported are presented as average values along with their standard deviations. For the statistical analysis, we made correlations between the analyzed variables based on Pearson's correlation coefficient (R) and the probability (p<0.05) to detect relationships between them. Statistical analyses were performed using Microsoft Excel ®.
Results and discussion
Flavonoids determination
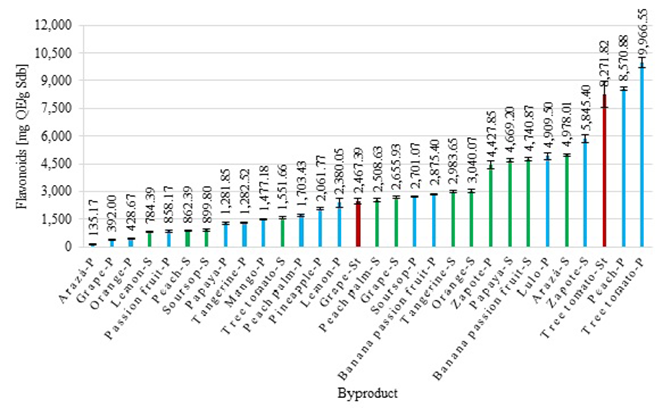
Figure 1 presents the results obtained from flavonoids for every byproduct. We found that the flavonoids contents were in a range of 135.17 ± 7.89 μg QE/g Sdb, in arazá peel, up to 9,966.55 ± 269.59 μg QE/g Sdb, in tree tomato peel. We established that tree tomato and peach peels, and the tree tomato stem, had high flavonoid contents. Considering the high flavonoid contents in these byproducts, we can assert that they could be used in the pharmaceutical, food, and cosmetic industries (Carullo, et al., 2018).
According to different reports, orange and mango peels have a flavonoids content of 3.22 ± 0.06 and 6.83 ± 0.09 mg of quercetin per gram of dry sample, respectively (Ghosh, et al., 2019). These data are higher compared to the results found in the present study (0.43 ± 0.02 mg QE/g Sdb for orange peel and 1.48 ± 0.03 mg QE/g Sdb for mango peel), which may be related to the fact that the cited authors used pure ethanol to carry out two extractions during four hours, which could influence the concentration of flavonoids obtained in the extracts. On the other hand, Suleria, et al. (2020) reported flavonoid values of 1.06 ± 0.07, 0.04 ± 0.01, 1.02 ± 0.08, and 1.47 ± 0.07 mg of quercetin per gram of dry sample for papaya, passion fruit, peach, and pineapple peels, respectively. These values are similar to those reported in the present study for papaya peel (1.28 ± 0.06 mg QE/g Sdb), however, they are lower than those found for passion fruit (0.86 ± 0.06 mg QE/g Sdb), peach (8.57 ± 0.12 mg QE/g Sdb), and pineapple peels (2.06 ± 0.08 mg QE/g Sdb), which suggests that, despite the short extraction time, it is possible to obtain extracts with a high content of flavonoids.
Taking into account that with a quercetin daily intake of approximately 50 mg, anti-inflammatory and antioxidant effects are obtained (Li, et al., 2016), byproducts extracts from tree tomato stems and peach and tree tomato peels should be analyzed to identify if quercetin is present in them and could contribute to the quercetin amount recommended for daily consumption as food additives or dietary supplements (Patel, et al., 2018).
Determining the ferric reducing antioxidant potential (FRAP)
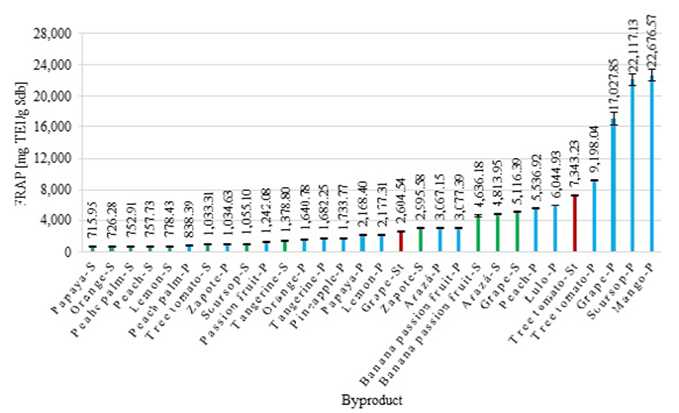
Figure 2 shows the results obtained from the ferric reducing antioxidant potential (FRAP) assay for every byproduct. FRAP values were between 715.95 ± 36.07 μg TE1/g Sdb for papaya seeds and 22,676.57 ± 759.71 μg TE1/g Sdb for mango peels. The Figure also shows that, in general, the peels have a higher FRAP value compared to the seeds, which may be related to the percentage of extractants present in them (Helkar, et al., 2016) and indicates that the extractable compounds in fruit peels have a high antioxidant capacity.
On the other hand, we observed that grape, soursop, and mango peels had greater FRAP values compared to fruits that stand out for having a high antioxidant capacity, such as raspberry and blackberry, with FRAP values of 9,828.89 and 9,999.09 μg TE1/g Sdb, respectively (Ruiz-Torralba, et al., 2018). This indicates that these byproducts have a high potential for obtaining valuable compounds that can be used as additives in the food and pharmaceutical industries (Ainswort & Gillespie, 2007).
Viganó, et al. (2020) reported a FRAP value of approximately 18.00 mg TE1/g Sdb for an extract of passion fruit bagasse obtained by the pressurized liquid technique at 65 °C using 75% ethanol as solvent. In the present study, we obtained a FRAP value of 1.24 ± 0.06 mg TE1/g Sdb for passion fruit peels, which corresponds to approximately one-fourteenth of the value reported by these authors. Such low value demonstrates the importance of using unconventional extraction techniques to obtain extracts with higher quality and antioxidant capacity. Likewise, Diep, et al. (2020) reported a FRAP value of 21,199.56 ± 2,785.73 μg TE1/g Sdb for an extract of tree tomato peel obtained by using 50% methanol as solvent, i.e., 2.30 times higher compared to that obtained experimentally in this work (9,198.04 ± 103.64 μg TE1/g Sdb).
Table 2 shows Pearson's correlation coefficients (R) between the flavonoid content and the FRAP, RPAA, and ORAC tests. When comparing the FRAP test results with the data obtained for flavonoid content, it is clear that the relationship between them is very low, with an R of 0.0936 indicating that the byproducts antioxidant capacity can come from other antioxidant compounds different to flavonoids, such as terpenes, steroids, amino acids, or tannins (Alabri, et al., 2014; López, 2017). Mango peels, with the highest antioxidant capacity measured by the FRAP test, have a low flavonoid content, but it is expected to present other types of antioxidant compounds such as carotenoids, vitamins, and anthocyanins (Banerjee, et al., 2018; Singh, et al., 2013). On the other hand, tree tomato peels have a high content of flavonoids while its antioxidant capacity is low, so we can infer that flavonoids and other antioxidant compounds present in this byproduct do not have such a good antioxidant capacity compared with other byproducts such as those in mango, soursop, and grape peels. Therefore, it is necessary to perform an HPLC-MS analysis to identify the specific metabolites present in each of the extracts and their concentrations.
Determining the reducing potential of antioxidant activity (RPAA)
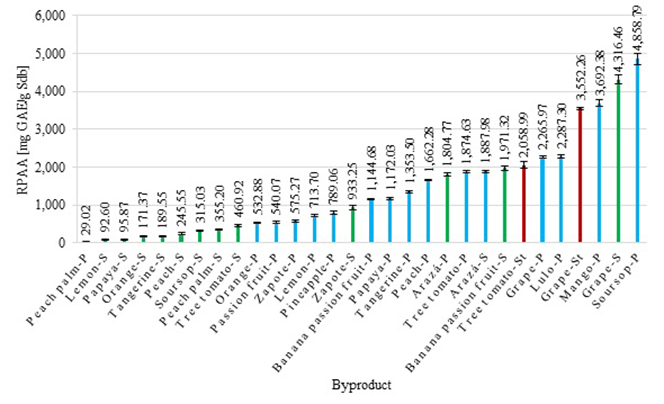
The results of the reducing potential of antioxidant activity test are presented in figure 3. We found that they were in a range of 29.02 ± 0.67 μg GAE/g Sdb for the peach palm peels and of up to 4,858.79 ± 156.71 μg GAE/g Sdb for the soursop peels. The figure shows that for most of the byproducts generated by the same fruit the values obtained by means of the RPAA test were higher in peels than in seeds. As mentioned earlier, in general, the analyzed peels have a greater percentage of extractants compared to the seeds, so that the compounds extracted from the peels have potential use in the food, pharmaceutical, and cosmetic industries (López, 2017). Byproducts such as soursop and mango peels and grape seeds and stalks have an antioxidant capacity with a high reducing power of free radicals and, therefore, they could be used to obtain compounds with beneficial effects on human health.
The results from the RPAA test had a very low directly proportional relationship with the flavonoid content in the byproducts with a correlation coefficient (R) of 0.1365. We can infer, then, that flavonoids were present in byproducts contributing to their antioxidant capacity and that the byproducts' composition had other secondary metabolites (Mosquera, et al., 2015). The soursop peel, the byproduct with the highest RPAA value, had low flavonoid contents given that there are other compounds in its structure such as vitamin C, as well as syringic and protocateic acids (Aguilar-Hernández, et al., 2019; Akomalafe & Ajayi, 2015), which are good electron donors and can inhibit the Fe3+/ ferricyanide complex (Ajila, et al., 2007). Likewise, we observed that byproducts such as mango peels, grape seeds, and grape stalks had a high antioxidant capacity as measured by the RPAA test and low flavonoid content. Furthermore, some byproducts such as peach palm peels and lemon seeds had low values for both flavonoid content and antioxidant capacity. However, it is important to identify and quantify the metabolites present in the extracts to corroborate the correlation between the flavonoid contents and the antioxidant capacity found by the RPAA test.
Determining the oxygen radical absorbance capacity (ORAC)
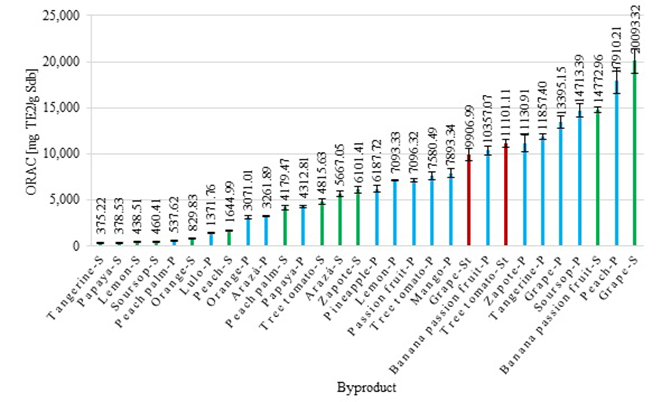
Figure 4 shows the results obtained from the oxygen radical absorbance capacity test for each one of the byproducts following the method described. We observed that the ORAC value varied from 375.22 ± 21.92 μg TE2/g Sdb for tangerine seeds to 20,093.32 ± 1,317.93 μg TE2/g Sdb for grape seeds. Byproducts such as grape seeds and peach peels showed a high antioxidant capacity measured by the ORAC test. In general, fruit peels had a greater ORAC value compared to seeds given the content of extractive compounds present in them. However, grape seeds had the highest ORAC value, which was expected taking into account previous results on their antioxidant capacity reported in the literature (Ferreyra, et al., 2019).
The ORAC test results are widely used to measure the antioxidant capacity of foods since it indicates the ability of a substance to inhibit the pyroxyl radical, which is the most frequent free radical in human biology (Prior, 2015). According to Prior (2015), it is advisable to consume foods that provide an ORAC value greater than 125,000 μg trolox. In this sense, extracts from grape, soursop and peach peels, and banana passion fruit and grape seeds could contribute 10% of the amount of antioxidants recommended for daily consumption. Considering these results, we can say that these byproducts are a potential source of antioxidant compounds capable of inhibiting lipid oxidation reactions and they can be used to obtain extracts with potential use in the food and pharmaceutical industries (Grupta, 2015).
Grape byproducts registered an ORAC value between 86,850.63 and 165,191.40 ug TE2/g Sdb (Ferreyra, et al., 2019), which differ from our results: 9,906.99 ± 0.06 for grape stalks, 13,395.15 ± 659.31 for grape peels, and 20,093.32 ± 1,317.93 for grape seeds. This difference is related to the fact that Ferreyra, et al. (2019) carried out the extractions using acetone as a solvent in an ultrasonic washer at 50 Hz and 60 °C for 60 min. Therefore, it is necessary to evaluate other operating conditions varying the extraction technique, the solvents, and the extraction time to optimize the process and obtain better quality extracts.
The flavonoid content and the values obtained for each of the byproducts in the ORAC test evidenced a low directly proportional relationship between them with an R=0.3179. As we already mentioned, this is due to the presence of other antioxidant compounds in fruit byproducts such as tannins, phenolic acids, and isoprenoids (Alabri, et al., 2014; López, 2017). We found that grape seeds were the byproduct with the greatest capacity to absorb oxygen radicals, however, its flavonoid content is not as high compared to byproducts such as tree tomato seeds. Several studies have reported the presence of antioxidant compounds such as gallic acid, catechin, epicatechin, and proanthocyanidins in grape seeds (Abhijit, et al., 2018; Yadav, et al., 2018), which suggests that these compounds are responsible for the absorption of oxygen radicals in this byproduct. Therefore, it is necessary to identify and quantify the metabolites present in the extracts to find the correlation between specific compounds and the antioxidant capacity registered in the ORAC test.
Prospective use of fruit byproducts
The use of agro-industrial byproducts for the production of value-added compounds has become a challenge for industries that discard a large amount of byproducts such as peels and seeds every day since these have a negative impact on the environment due to their organic load besides the impact on the economy of the company given the costs associated with adequate storage, transport, and disposal (Vargas & Pérez, 2018). In Colombia, this type of byproducts is mainly used as a raw material for energy generation (Peñaranda, et al., 2017). However, agro-industrial byproducts can be used to produce valuable bioactive compounds (Bosco, et al., 2017).
In the specific case of the coffee-growing areas, in addition to their main agricultural product, the coffee, there are other fruit crops such as passion fruit, lulo, blackberry, banana, and tree tomato, among others (Cardona, et al., 2012), which produce a large number of byproducts from the moment of the crop through its distribution, processing, and consumption. The possibility of using them offers a great opportunity to implement the concept of biorefinery where each of the process's output streams generates other products of interest that can be marketed, like bioactive compounds and chemicals, as well as profits for the industry and benefits for the environment (Kiss, et al., 2016), which is advantageous since it would reduce the number of byproducts inadequately disposed of reducing the costs associated with their disposal (Vargas & Pérez, 2018). These byproducts can be used to obtain bioactive compounds that are highly valued in the market due to their great health benefits (López, 2017).
In the last years, the beneficial effects of flavonoids, such as the anticipated cardioprotective action and the antiviral, antimicrobial, anti-inflammatory, anticarcinogenic, and antiallergic potential have been extensively studied (Lesjak, et al., 2018; Patel, et al., 2018). Quercetin, one of the most common flavonoids, stands out for its protective function against diseases such as osteoporosis, some melanoma types, pulmonary and cardiac diseases, as well as against aging and inflammatory processes (Carullo, et al., 2018; Patel, et al., 2018). It has a catechol and an OH group in its structure, which confers it an optimal configuration to be the most potent eliminator of reactive oxygen species, inhibiting the action of substances such as peroxynitrite and the hydroxyl radicals, and avoiding the peroxidation of fats (Patel, et al., 2018).
It is necessary, then, to quantify the quercetin content of fruit byproducts such as tree tomato and peach peels and tree tomato stems to eventually use them in the pharmaceutic industry for the treatment of various diseases.
On the other hand, byproducts such as mango, soursop, grape, and peach peels and grape seeds and stalks have a high antioxidant capacity attributed to the presence of compounds different from flavonoids such as gallic, syringic, and protocateic acids (Aguilar-Hernández, et al., 2019), catechins, epicatechin, proanthocyanidins (Abhijit, et al., 2018), anthocyanins, carotenoids (Ajila, et al., 2007), terpenes (Aquilani, et al., 2018), steroids, amino acids, tannins (Singh, et al., 2013), and vitamins (Akomalafe & Ajayi, 2015). These fruit byproducts can be treated and processed to obtain antioxidant-rich extracts for their use in the production of functional foods (Rohm, et al., 2015), multivitamin supplements (Helkar, et al., 2016), nutraceuticals (Kumar, et al., 2017), and anti-inflammatory and anti-diabetes medications (Saleem, et al., 2019). However, it is vitally important to identify and quantify the metabolites present in the extracts to establish specific applications according to the benefits of each of the antioxidant compounds and evaluate the toxicity of the extracts to ensure their safety and measure the biological activity of the extracts through in vitro tests to guarantee their quality.
Conclusions
Based on our results, the byproducts with the highest flavonoid content were tree tomato and peach peels, and tree tomato stems, which can be used to obtain anti-inflammatory medications. Likewise, byproducts such as mango, soursop, grape, and peach peels, as well as grape seeds and stalk, had the highest antioxidant capacity, which makes them eligible as raw material for the food and pharmaceutical industries.
We also found that there was no direct relationship between the content of flavonoids and the antioxidant capacity of the byproducts based on Pearson's correlation coefficient. This must be corroborated by analyzing the composition of the extracts to identify other metabolites other than flavonoids such as phenolic acids, catechins, anthocyanins, terpenes, steroids, amino acids, tannins, carotenoids, and vitamins, which are good antioxidants.
Our results open the way for future studies to optimize the obtention of extracts by analyzing the influence of the technique, the operating conditions, and the solvent used. On the other hand, it is important to evaluate the composition of the extracts to identify different metabolites and their concentration and perform toxicity tests to guarantee their safety as raw materials for the food and pharmaceutical industries.