Introduction
Covid-19 caused by SARS-CoV-2 emerged in China in 2019 (Zhu, et al., 2020). Due to the pandemic by the dissemination of the virus, several control and restricted community-based measures have been adopted worldwide (Ebrahim, et al., 2020). Coronavirus is transmitted directly from person to person or by contamination with fluids from infected individuals; wearing facemasks and hand washing are the most commonly used personal protective measures to avoid infection spread (Morawska & Cao., 2020; Roshan, et al., 2020).
The recommended test during acute infection is the nucleic acid amplification test (NAAT) using reverse transcriptase polymerase chain reaction (RT-qPCR) for the detection of viral RNA in secretions from the respiratory tract, mainly nasopharyngeal and oropharyngeal swabs (World Health Organization-WHO, 2020). Serological tests are used to determine previous exposure to microbial agents; they are usually faster, cheaper, and some can be used as point-of-care (POC) tests. Serological assays to measure SARS-CoV-2 specific antibodies are useful for surveillance studies and to determine the rate of exposure (Lisboa-Bastos, et al., 2020). There are different methods available including lateral flow immuno-chromatography (LFI), ELISA, and chemiluminescence (CLIA) with a wide brand offer (González, et al., 2020). Among other variables, serological tests can differ from antigens used for antibody detection and performance characteristics such as sensibility and specificity (Lisboa-Bastos, et al., 2020; Müller, et al., 2021). A recent study has shown that the humoral immune response against SARS-CoV-2 is driven primarily by the spike (S) and nucleocapsid (N) viral proteins (Shrock, et al., 2020).
IgA and IgM-specific antibodies can appear 5 days post-infection (pi) on average. IgG can be detected at day 14 pi (Guo, et al., 2020) and most people have already seroconverted between day 15 to 21 pi (Guo, et al., 2020; Zhao, et al., 2020). IgG-specific antibodies against N (nucleocapsid) protein or S (spike) protein can be detected as far as eight months after acute infection (Dan, et al., 2021).
Most epidemiological and serological-specific population studies have been conducted in high-risk communities such as health workers and institutions (Houlihan, et al., 2020; Lumley, et al., 2021). Reopening university campuses represents a challenge due to several factors including population commuting from different places, people socialization and gatherings, and indoor activities such as lectures, laboratories, and workshops which help the virus to spread. In this context, our goal was to assess the SARS-CoV-2 seroprevalence in individuals attending the Universidad de Los Andes campus in Bogotá, Colombia, before its reopening in 2021 and after the first peak of SARS-CoV-2 transmission in the city during 2020.
Type of study and population
We conducted a cross-sectional study at the Universidad de Los Andes located in Bogota, Colombia, which has a population of 15,581 students, 1,808 teaching staff, and 2,333 employees (Universidad de Los Andes, 2021). The participants were working at the campus during city lockdown while teaching was mostly remote. Individuals working in place were categorized and characterized according to their age and the presence of comorbidities; neither they nor the members of their households had a risk of complications due to SARS-CoV-2 (age over 65 years, risk of contagion, and COVID-19-related risk comorbidities). Risk characterization of individuals was based on the regulations of the Colombian health authority (resolution 666 and communication # 30, 2020).
Blood sampling
Sampling was carried out between the last week of November and the first week of December 2020. Blood samples were drawn from the antecubital vein using a vacutainer without anticoagulants (BD, Franklin Lakes, NJ, USA). We centrifuged blood at 2,500 rpm for 5 min, separated the sera, and stored them at 4°C until their use for antibody detection the following day.
IgG anti-SARS-CoV-2 antibody detection
We used the Abbott IgG Architect SARS-CoV-2 chemiluminescence assay (Abbott, Abbott Park IL, USA) following the manufacturer's instructions. For antibody detection, we used the nucleocapsid protein (N) from SARS-CoV-2 as the antigen. Serology results were determined following the manufacturer's instructions: index S/C units (sample/cutoff point). We considered as positive an index value of >1.40.
SARS-CoV-2 detection by molecular biology
Nasopharyngeal swabs were collected in a viral transport medium (containing Hanks balanced salt solution, heat-inactivated FBS-fetal bovine serum, gentamicin sulfate, and amphotericin B) (Gibco, NY, USA). The specimens were obtained from individuals attending the campus registered in the programs Comunidad Segura (epidemiological surveillance system for Covid-19 at the university) and COVIDA (free SARS-CoV-2 testing project). We conducted a retrospective analysis of molecular test results. Not all the individuals participating in the study had previous RT-qPCR. We used automated extractors (Genolution Nextractor® NX-48S, Seoul, Korea) and nucleic acid reagents (Hamilton MicroLab Starlet MagEx STARline, Washington DC, USA), as well as the Quick-DNA/RNA Viral MagBead extraction kit (Zymo Research, Orange, CA, USA) for RNA purification. The RT-qPCR test was performed to determine the presence of SARS-CoV-2 RNA using the U-TOP COVID-19 detection kit which targets two SARS-CoV-2 regions: ORF-1ab and N (SeaSun Biomaterial Inc., Daejeon, South Korea), using the RNAse P as the internal control gene. The assay was run following the manufacturer's instructions. A cycle threshold (Ct) below 38 was considered as a positive result.
Population's sociodemographic characteristics
We registered data on age and gender, commuting or residence boroughs, and type of work at the university. Individuals were asked for the presence of symptoms or signs associated with Covid-19 and contact with SARS-CoV-2 positive persons in the previous week. We used a questionnaire to evaluate the following symptoms: fever, cough, headache, expectoration, loss of smell, sneezing or running nose, shortness of breath, and muscular or joint pain.
Data presentation and analysis
Data were registered as percentages, mean or median, and their respective standard deviation (SD) or interquartile ranges (IQR). A normality test (Shapiro-Wilk) was used for quantitative variables while the U Mann Whitney test was used for non-parametric data and the t-Student test for parametric data. Statistical analyses were done using the PAST version 2.17c software (http://priede.bf.lu.lv/ftp/pub/TIS/datu_analiize/PAST/2.17c/download.html).
Results
A total of 237 individuals participated in the study; their age average was 36.14 years (± SD 9.66); 93 participants (30.44%) were females with an age average of 33.8 years ( SD 7.97) while males' age average was 38.06 years (± SD 9.83) (Table 1); no statistical differences were observed (p=0.17). Of the total 237 participants, 213 lived in 19 of the 20 boroughs in Bogotá with the exception of Sumapaz, the only rural locality of the city; the remaining 24 individuals came from other metropolitan municipalities (Figure 1) (Table 2). As regards their activities at the university, 96 (40.5%) worked in laboratories and workshops or were postgrad students, 81 (34.2%) had administrative jobs, 19 (8.0%) worked in general services, 24 (10.1%) in security, 11 (4.6%) belonged to the teaching staff, and six (2.5%) to the health services. The most commonly reported symptoms at the time of sampling were headache, 3.3%, muscle pain, 2.9%, and coughing and nasal congestion, 2.1%; no fever or dyspnea were reported. Seven individuals reported contact with someone showing Covid-19-related symptoms in the week before sampling. IgG-specific antibodies for SARS-CoV-2 N protein were detected in 32 individuals (13.5%): 10 women and 22 men (Table 1). The average index of seropositive individuals by CLIA was 4.7 (SD ± 2.58) and very few in this group had respiratory symptoms (2 with nasal congestion, 1 with coughing, and 1 with anosmia); no dyspnea was reported. Out of 32 individuals with positive antibodies, 13 worked in the security department (40.6%), 11 in the laboratories (34.4%), 6 in administrative services (18.1%), 1 in general services (3.1%), and 1 in health services (3.1%). According to the borough of residence, seropositive participants lived in 8 of the 20 in Bogotá, mostly from Rafael Uribe Uribe (6; 18%), Usaquén (5; 15.6%), San Cristóbal (4; 12.5%), and four (12.5%) lived outside Bogotá (Figure 1) (Table 2).
Table 1 Characteristics of the population and summary of results
| Age | N | Seropositives RT-qPCR+# | Seroprevalence % | Symptomatic* Number and percentage | ||
|---|---|---|---|---|---|---|
| Men | 38.0 | 144 | 22 | 9 | 10.8 | 7 (31.8) |
| Women | 33.8 | 93 | 10 | 4 | 15.3 | 3 (30.0) |
| Total | 36.1& | 237 | 32 | 13 | 13.5 | 10 (31.25) |
* Based on seropositive individuals
& No statistical difference, p: 017 t- student
# Positives by RT-qPCR according to the Ct
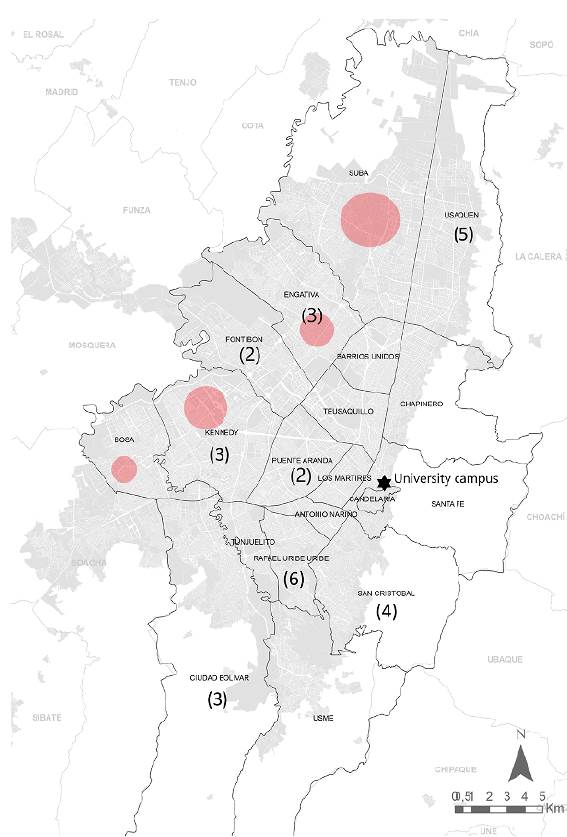
Figure 1 Map indicating the origin of the SARS-CoV-2 cases in Bogotá. The number in parenthesis indicate the seropositive cases among university employees and each borough where they live. Red dots show the boroughs with higher number of SARS-CoV-2 positive individuals in the city. Only seropositives from Bogota (28) are shown; four individuals came from outside Bogotá. (Generated in GIS on ArcGIS 10.8.1 software and gently provided by José David Pinzon, COVIDA program, Universidad de Los Andes)
Of the 32 individuals with reactive serology, 13 had a positive RT-qPCR for SARS-CoV-2 RNA (4 women and 9 men) and 10 out of 13 were symptomatic at the time of the molecular assay. The RT-qPCR test was done 91 days before the serological assay on average. One additional individual with positive RT-qPCR had no detectable specific antibodies by CLIA. Nineteen participants (6 women and 13 men) with previous negative RT-qPCR done routinely as part of the surveillance program were seropositive; none described Covid-19-associated symptoms and only one endorsed the previous contact with a SARS-CoV-2 positive person; 31.25% of the individuals with positive serology were symptomatic (Table 1). We determined clusters (defined as five or more epidemiologically-related cases) in the security staff and in engineering and sciences labs.
Table 2 Comparison of seropositive individuals at the university campus and SARS-CoV-2 cases by boroughs in Bogotá
| Borough* | Tested by serology | Positives by serology | Percentage | Number of cases & |
|---|---|---|---|---|
| Rafael Uribe | 11 | 6 | 18.8 | 24,176 |
| Usaquén | 27 | 5 | 15.6 | 34,279 |
| San Cristobal | 11 | 4 | 12.5 | 23,932 |
| Kennedy | 24 | 3 | 9.4 | 65,425 |
| Engativá | 16 | 3 | 9.4 | 55,013 |
| Ciudad Bolívar | 10 | 3 | 9.4 | 31,407 |
| Fontibón | 10 | 2 | 6.2 | 24,723 |
| Puente Aranda | 7 | 2 | 6.2 | 20,378 |
| Chapinero | 13 | 0 | 0.0 | 12,513 |
| Santa Fe | 16 | 0 | 0.0 | 8,480 |
| Usme | 6 | 0 | 0.0 | 18,226 |
| Tunjuelito | 1 | 0 | 0.0 | 12,343 |
| Bosa | 9 | 0 | 0.0 | 38,344 |
| Suba | 21 | 0 | 0.0 | 72,183 |
| Barrios Unidos | 5 | 0 | 0.0 | 10,443 |
| Teusaquillo | 16 | 0 | 0.0 | 11,044 |
| Los Mártires | 2 | 0 | 0.0 | 6,538 |
| Antonio Nariño | 2 | 0 | 0.0 | 6,987 |
| La Candelaria | 6 | 0 | 0.0 | 2,183 |
| Sumapaz | 0 | 0 | 0.0 | 13 |
| Outside Bogotá | 24 | 4 | 12.5 | NA |
| Total | 237 | 32 | 100,0 | 478,630 |
* Administrative division of Bogotá city
& Data until December 31, 2020 (Observatorio de Salud de Bogotá). Positive cases were determinated using RT-qPCR.
NA: not applicable
Discussion
The first imported case of SARS-CoV-2 infection in Colombia was reported in Bogotá on March 6th 2020 (Instituto Nacional de Salud-INS, 2020) and until December 31 2020 the city had reported 478,630 cases and a 2.1% lethality (SALUDATA, 2021)/ Bogotá started preventive lockdown on March 20th, the first peak of infection occurred during July and August, and then the city reopened. Then, employees and some low-risk postgrad students returned to the campus subject to biosecurity protocols after September 1st. The effective reproductive number R(t) in Bogotá then was 0.81 and by the time of our sampling it averaged 1.16 (SALUDATA, 2021)/ The most prevalent SARS-CoV-2 variant (near 50% of the sequences) in Colombia at the time was the B.1, a large European linage from the first outbreak (Laiton-Donato, et al., 2020). The reopening brought new challenges not only for the scholar community but also for the people and businesses around it. Preparedness for possible reopening for the next academic cycle during early 2021 included besides protocols and guidelines a seroprevalence assay for people attending the university campus. Only those under age 65 and no personal or family risk factors were allowed to attend. Most of the individuals performed activities in labs, workshops, and administrative offices.
Our study evaluated presence of the IgG anti-N protein of SARS-CoV-2 by CLIA (Piec, et al., 2021) in 237 individuals of the almost 300 people attending the campus in a working days. We selected the N protein as an antigen to continue the survillance with serology tests including vaccinated people while most platforms use the S protein. Interestingly, most of the cases did not come from the boroughs in Bogotá with the highest percentages of infection (Suba, Kennedy, Engativá, and Bosa) (SALUDATA, 2021)/Seroprevalence among individuals attending the Universidad de Los Andes campus in Bogotá was 13.5%, which differs from other studies done in Colombia. In the national seroprevalence survey done by the Colombian INS, crude and adjusted seroprevalence for Bogotá during October and November 2020 among 4,597 individuals was 26.3% and 30%, respectively (INS, 2021). A higher seroprevalence has been described for Colombian cities on the Caribbean reaching up to 55.3% (Mattar, et al., 2020). A study done in a university hospital in Bogotá during the first peak showed a baseline seroprevalence of 2.28% among health workers, which increased to 5.98% after 2-4 weeks of follow up with 38% of individuals categorized as pre-symptomatic or asymptomatic (Ariza, et al., 2020). Initial studies for SARS-CoV-2 IgG antibodies in Wuhan from March to April using a CLIA assay registered a seroprevalence between 3.2% and 3.8% reflecting the early spread and impact of the infection (Xu, et al., 2020); similar values were found in a systematic review worldwide until November 2020 showing a 3.2% (IQR 1.0-6.4%) seroprevalence in the general population (Bobrovitz, et al., 2020). These differences could be related to variables such as the detection method, the antigen used, the virus circulating variants, and the time of sampling. In our study, nearly 70% of seropositive individuals did not recall any symptoms before the serological assay. One study showed that during SARS-CoV-2 infection, the proportion of individuals without symptoms but positive RT-qPCR was 65.9% at the time of sampling and 41.2% when using serological tests (Oran & Topol, 2021).
During the reopening of colleges in Wisconsin (USA), SARS-CoV-2 transmission increased among students with infection clusters concentrated in three institutions; the virus sequencing showed a rapid dissemination to the community (Richmond, et al., 2020). The congregation of students on and off campus helped to spread infection in a university in North Carolina (Wilson, et al., 2020) as the close contact of young people at the campus regardless of the fact that they are less susceptible can also facilitate the spread of infection to the university staff and their households. Cluster outbreaks and rapid dissemination are the two ways of transmission described in colleges and universities (Wilson, et al., 2020; Walke, et al., 2020). Here we identified three clusters, the largest among security staff in line with a higher number of epidemiological contacts due to their activities. The other two clusters corresponded to laboratory staff and postgrad student gatherings.
Different measures have been adopted at the university to avoid spreading of the infection in the campus including characterization of individuals, online biosafety basic training, and global screening using RT-qPCR for both symptomatic and asymptomatic employees. Also, mandatory university guidelines including daily symptoms check through a cellphone App (SeneCare), handwashing, use of face masks, physical distance, and gathering limitations have been implemented.
Our study had some limitations such as the number of samples, no comparison by serology with other viral antigens and the retrospective analysis of RT-qPCR data which did not include all the individuals. Our data suggests that a very low percentage of the individuals has been infected despite the use of public transportation and daily commuting between the city boroughs with higher prevalence of infection. Although this is a descriptive study, we found a high percentage of asypmtomatic individuals and detected clusters that indirectly helped to take some control measurements. Seroprevalence studies could help better understand the dynamics of virus transmission in university populations, a subgroup of the broader general community similar to others such as commercial or industrial population subsets.















