Introduction
The Amazonian medicinal plant sucuba [Himatanthus sucuuba (Spruce ex Müll. Arg.) Woodson] is widely used in ethnopharmacology with high pharmacological potential and isolated substances with proven biological activity (García-Ortiz, et al., 2017; Da Silva, et al., 2021). Among its traditional uses, its leaves, stem, and latex serve in infusion and decoction as antitumor and antifungal substances for the treatment of gastritis, ulcers, and arthritis (Larrosa & Duarte, 2005), of cough, flu, diarrhea, hemorrhoids, warts, dislocations, anemia, as well as for blood clearance, aphrodisiac and analgesic (Rodrigues, et al., 2010; Linhares, et al., 2011). Studies with H. sucuuba have shown its anti-inflammatory (Fakhrudin, et al., 2014; Calero-Armijos, et al., 2020), analgesic (Miranda, et al., 2000), leishmanicidal (Soares, et al., 2010), acaricide (Sprenger, et al., 2016), and antibacterial action (Silva, et al., 2010).
There are several studies about H. sucuuba, but none about its endophytic microorganisms. However, several promising endophytic microorganisms and their secondary metabolites have been discovered in medicinal plants (Gos, et al., 2017; Abdalla & McGaw, 2018; Duhan, et al., 2020). Furthermore, the antimicrobial activity of medicinal plants may be associated with the proportion of endophytic antagonists to pathogens (Egamberdieva, et al., 2017). We should remember that endophytes are fungi and bacteria that live inside plants, in the roots, stems, and leaves, without causing damage to the host (Khare, et al., 2018).
Although endophytic fungi have been described for over a century, it was in the late 1970s that studies focused on their taxonomy and ecology, and their ability to protect plants against predators and pathogenic insects, other fungi, and bacteria (Mantzoukas & Eliopoulos, 2020; Peters, et al., 2020). Currently, these microorganisms are considered a second microbiological layer for plant defense (Dini-Andreote, 2020). Endophytic fungi are known to be an important resource of different secondary metabolites (Aboobaker, et al., 2019; Bibi, et al., 2020). They can provide more resistance to their hosts against water stress, alter the physiological properties of plants, and produce plant hormones, enzymes and other compounds of biotechnological importance (Kusari, et al., 2014), which has increased the interest in exploring their potential.
The main factor driving the search for new active molecules is microbial resistance to conventional antibiotics. Current projections indicate that by 2050 there will be more deaths from bacterial infections than from current leading causes of death such as cancer. As indicated by the World Health Organization (WHO), some strains are a priority in the fight against Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (López-Jácome, et al., 2019).
In this sense, our study aimed to make the first description of mutualistic endophytic fungi for the Amazonian medicinal plant sucuba (Himatanthus sucuuba) and to evaluate the antibacterial activity of their secondary metabolites.
Materials and methods
Collection of botanical material and herborization
Three plants were randomly collected at different sites of the Zoobotanical Park (9°57'8 "S - 67°52'25" W) located at the Universidade Federal do Acre (UFAC) in Rio Branco, State of Acre, Brazil, between January and April 2015 (Figure 1). The leaves and stems were collected in the early morning hours and the plants were selected during the flowering period for identification in the UFAC herbarium using the manual by Lorenzi (1998); the three plants collected were identified as belonging to Himatanthus sucuuba (Spruce ex Müll. Arg.) Woodson and deposited in the UFAC herbarium collection under number 22001 in January 2016 (Figure 2). The region where the sampling point for H. sucuuba seedlings is located has a hot and humid climate, a temperature variation of 1 to 2°C with average values between 24 and 26°C, heavy rains between November and March, droughts between May and September, and an average annual rainfall of 2,300 mm/year (Fisch, et al., 1998).
Isolation of endophytic fungi
Healthy leaves and stems were collected to prepare the culture medium with their extract and isolate their endophytic fungi using potato-dextrose-agar (PDA) and Sabouraud-dextrose-agar (SDA) culture media with or without 10% plant extract. The extract was prepared with 100 g of fresh plant tissue (leaf or stem) grounded with 500 mL of distilled water and filtered on filter paper. For the preparation of the PDA+extract medium, 500 mL of 200 g of potato infusion were added to 500 mL of plant extract while for preparing the SDA+extract medium, 500 mL of water and the reagents used in the preparation of each culture medium were solubilized. Chloramphenicol (100 mg/mL) was added to all media to inhibit bacterial growth (Azevedo, et al., 2000). The media supplemented with H. sucuuba extracts were used to provide the plant substances used for the growth of the endophytic fungi present in its tissues.
Leaves and stems were disinfected by immersion in 70% ethanol (1 min), 2.5% sodium hypochlorite (3 min), 70% ethanol (30 sec), and sterile distilled water (1 min) twice. Fragments with 5 mm in diameter were placed in the culture medium and incubated at 18°C or 28°C for 30 days. Endophytic fungi were purified and preserved using the mineral oil and distilled water methods (Azevedo, et al., 2000).
For taxonomic identification, fungi isolated from H. sucuuba were grouped into morphospecies based on macromorphological characteristics (mycelium shape, mycelium texture, fungus color, agar color, and pigment diffusion in the medium). The macro and micromorphological characteristics of the morphospecies were observed and compared with those in the genera identification literature (Barnett & Hunter, 1998).
Bioassay of antibacterial activity
Endophytic fungi were cultivated in the PDA medium at 28°C for 14 days. Ten fungal discs were transferred to an Erlenmeyer flask containing 20 mL of potato-dextrose broth (PD) and incubated at 28°C, for 14 days. Two mL of metabolite medium were extracted by a liquid-liquid partition with ethyl acetate and solubilized in 300 |iL of dimethylsulfoxide (DMSO) (Vitolo & Pessoa JR, 2015).
Extracts from endophytic fungi were evaluated to verify their activity against pathogenic bacteria using the disc infusion method (NCCLS, 2003). S. aureus (ATCC 12598), S. pneumoniae (ATCC 11733), E. coli (ATCC 10536), and K. pneumoniae (ATCC 700603) were grown at 37°C for 4-6 h and turbidity adjusted to a scale of 0.5 McFarland. Bacteria were inoculated in Petri dishes containing Muller-Hinton medium and the paper discs were placed in the medium and on 20 μL of extract. Samples were then incubated at 37°C for 24 h. Endophyte metabolic extracts inhibiting bacterial growth around the disc were considered as having antibacterial activity and the halo of inhibition was measured in millimeters. DMSO was the negative control and chloramphenicol 30 mg was the positive control. The inhibition zones were considered from 10 mm but we observed some up to 36 mm.
Molecular characterization
The endophytic fungi showing activity against the four bacteria tested and the highest inhibition halos were chosen for molecular characterization. Genomic DNA extraction from these fungi was performed using the Quick-DNA Fungal/Bacterial miniprep kit (Zymo Research) following the manufacturer's instructions. The amplification of rDNA from the ITS region was done in a 50 μL reaction mix, which included 2 μL of DNA template (1-20 ng), 0.4 μM ITS1 and ITS4 primers (White, et al., 1990), 1.5 mM MgCl2, 0.2 μM dNTPs, 5 μL Taq buffer, and 1.25 U Taq DNA polymerase (Qiagen). PCR amplification was performed on a PCR cycler (Bio-Rad) with an initial denaturation at 95 °C for 2 min followed by 35 cycles of amplification (95 °C for 30 sec, 55 °C for 30 sec, 72 °C for 1 min) and an extension step (72 °C for 7 min). PCR products at around 600 to 700 bps were purified using the QIAquick PCR Purification Kit (Qiagen) and quantified on a 2% agarose gel. The purified products were sent to LACTAD (Campinas, Brazil) for sequencing.
The sequences obtained were analyzed and edited in Bioedit 7.0.9.1, aligned to the region sequences (ITS1-5.8S-ITS2) using the Clustal W program, deposited in the GenBank databases while the species similarity index was calculated with the BLASTn tool. After identifying the species of interest, a phylogenetic tree was constructed using the neighborhood method and the MEGA 7.0 software (Tamura, et al., 2007).
Data analysis
The frequency of fungal colonization (FC) was calculated using the formula where NCOL is the number of colonized fragments and TN corresponds to the total number of fragments. The relative frequency (RF) of the isolation was calculated as the number of isolates of a species divided by the total number of isolates and expressed as a percentage.
To calculate the diversity, we used the Simpson's formula: 2 while the Shannon-Wiener's formula was used to calculate the colonization frequency of the species in a sample: The evenness of species was calculated using the formula: where S is the number of species in the sample. As for the antibacterial analysis, the halos formed were measured in millimeters (mm) which enabled the calculation of means and standard deviation on the Excel program.
Results
Endophytic fungi
A total of 581 fungi were recovered from leaves and stems of H. sucuuba with a 97.9% colonization frequency (CF). There was a greater number of fungi isolated from the leaf (338 fungi - 58.2%) compared to the isolation of endophytic fungi from the stem (243 fungi - 41.8%) (Table 1).
Table 1 Number and relative frequency percentages of fungal endophytes isolated from Himatanthus sucuuba according to the plant tissue, culture medium, and temperature
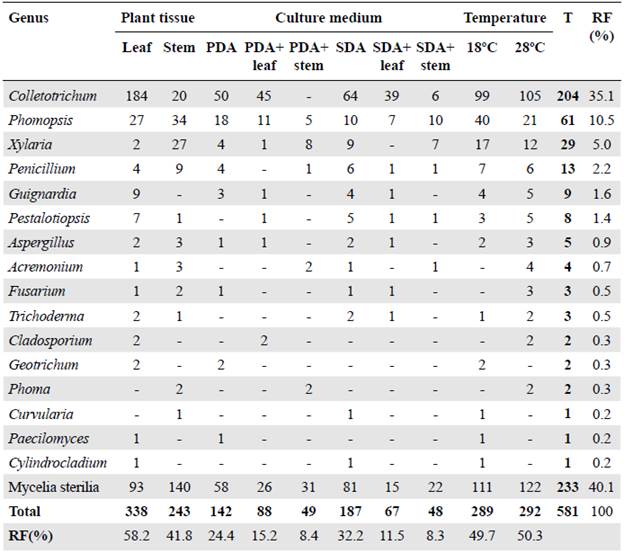
T: Total identified in the sample; RF: Relative frequency
The culture media without plant extract registered the highest number of fungi while the SDA medium had the highest isolation frequency with 187 fungi (32.2%) (Table 1). The second culture medium with the highest frequency of isolation was PDA with 142 fungi (24.4%). The media with extracts used for isolation influenced the number of fungi recovered, as 155 fungi (26.7%) were isolated in the culture medium with leaf extract and 97 in the medium with stem extract (16.7%) (Table 1). On the contrary, the temperatures used in the isolation had little effect on the number of fungi isolated with 292 fungi (50.3%) isolated at 28°C and 289 fungi (49.7%) at 18°C (Table 1).
Endophytic fungi were classified into 259 morphospecies and 135 (52.1%) were identified distributed in 16 genera (Table 1). The isolates that formed reproductive structures were identified at the genus level after analyzing the macro and micromorphological aspects (Figure S1, https://www.racceiyn.co/index.php/racceiyn/article/view/1525/3199). However, 124 (47.9%) morphospecies were not identified using morphological analysis given that they do not have reproductive structures.
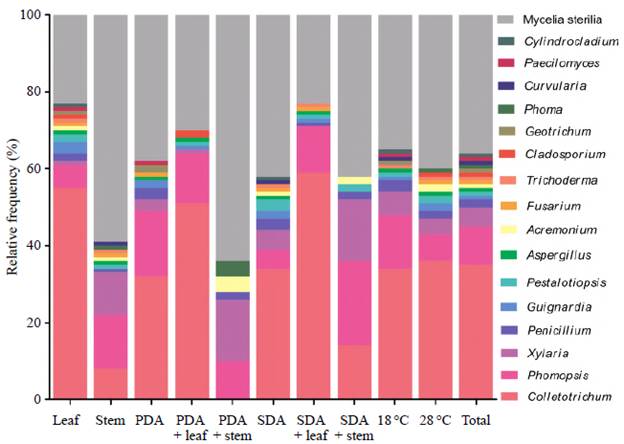
Colletotrichum, Phomopsis, and Xylaria were the most dominant genera and some showed specificity. Guignardia, Cladosporium, Geotrichum, Paecilomyces, and Cylindrocladium were isolated only from the leaf while Phoma and Curvularia were isolated only from the stem (Figure 3A). The effect of temperature on fungus isolation evidenced that Curvularia, Geotrichum, Paecilomyces, and Cylindrocladium were isolated only at 18°C, and Acremonium, Cladosporium, Fusarium, and Phoma only at 28°C (Figure 3B). Among the identified genera, only Phomopsis was isolated from all tissues and culture conditions used (Figure 3C).
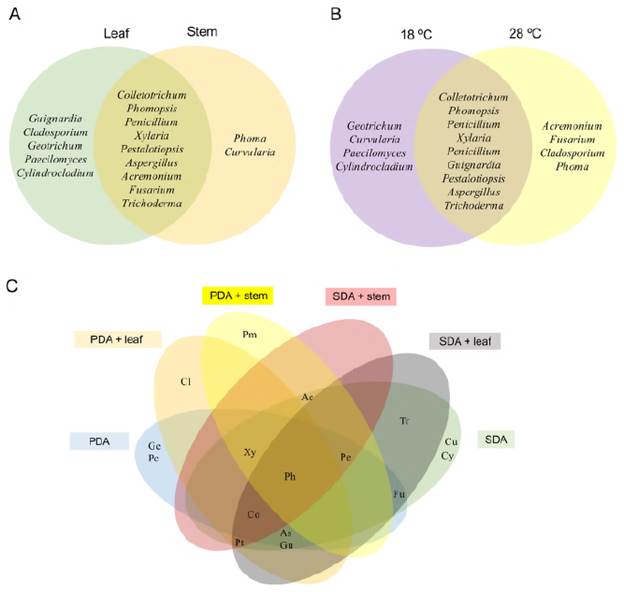
Figure 3 Venn diagram showing the effect of experimental conditions on the isolation of endophytic fungi isolated from H. sucuuba. A) Genera isolated according to plant tissue. B) Genera isolated according to temperature. C) Isolated genera according to the culture media used (Co: Colletotrichum; Ph: Phomopsis; Xy: Xylaria; Pe: Penicillium; Gu: Guignardia: Pt: Pestalotiopsis As: Aspergillus; Ac: Acremonium; Fu: Fusarium; Tr: Trichoderma; Cl: Cladosporium; Ge: Geotrichum; Pm: Phoma; Cu: Curvularia; Pc: Paecilomyces; Cy: Cylindrocladium)
Furthermore, the culture media used allowed the isolation of different H. sucuuba endophytic fungi. The Bi-plot and the load correlation graph from the principal component analysis (PCA) (Figure 4A and B) and the dendrogram showed that endophytic fungi such as Colletotrichum, Aspergillus, Guignardia, and Fusarium were more abundant in SDA and SDA+leaf extract (cluster 1) (Figure 4C). Trichoderma and Pestalotiopsis were more abundant in the SDA medium while Curvularia and Cylindrocladium were exclusive to this medium (cluster 2) (Figure 4C). Similarly, the Phomopsis and Penicillium genera were more frequent in the PDA medium and Paecilomyces was exclusive to this medium (cluster 3) (Figure 4C). Figure 5 shows the distribution frequency of the genera, as well as the sterile mycelium, with the treatments under study.
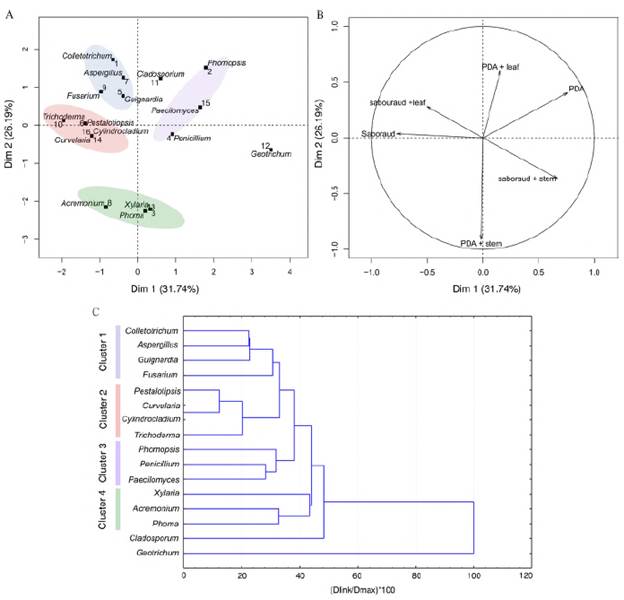
Figure 4 Principal component analysis (PCA) and dendrogram of fungal endophytic isolated on different culture media from H. sucuuba. A) Discriminations of endophytic fungi isolated on different media. B) Loading plot of media for the first two principal components, PC1, and PC2. C) Cluster analysis using Euclidean distance of endophytic fungi recovered from H. sucuuba based on different culture media
Diversity indices provide important information about uncommon or common species in a community (Table 2). Stem and leaf extracts exhibited a high Shannon-Wiener index, higher in the stem (4.87) than in the leaf (4.60). Simpson's index had the same value for stem and leaves (0.99). Species uniformity was greater in the stem (0.89) than in the leaf (0.74).
Table 2 Diversity indices of endophytic fungi from Himatanthus sucuuba according to the plant tissue, culture medium, and temperature
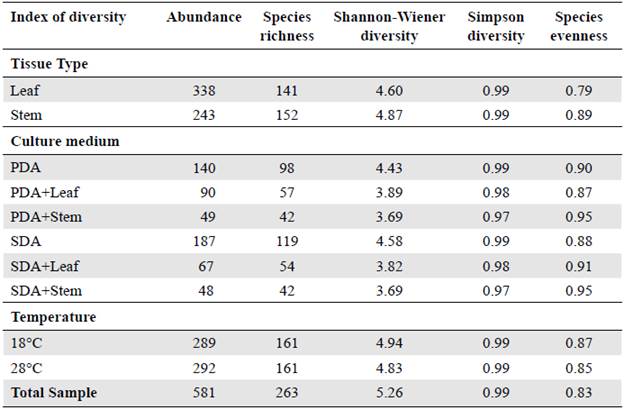
The Shannon-Wiener diversity index was higher for the SDA medium (4.58) followed by the PDA (4.43). The culture media with extracts showed higher rates with the leaf medium showing higher values than the stem medium. Simpson's diversity index was higher in the medium without extract, PDA, and SDA (0.99) followed by the medium with leaf (0.98) and stem (0.97) extracts. The uniformity of the observed species was greater for PDA+stem extract (0.95).
At 18°C (4.94) the highest values were registered with the Shannon-Wiener index followed by 28°C (4.83). Similar results were found for the evenness species at 18°C (0.87) and 28°C (0.85).
Antibacterial activity
A total of 235 endophytic fungal extracts were tested of which 28 (12%) had activity against E. coli, K. pneumoniae, S. pneumoniae, or S. aureus (Figure 6). Among the H. sucuuba identified endophytic fungi, the genus Xylaria presented more morphospecies with antibacterial activity. The Colletotrichum genus inhibited the growth of all tested bacteria, mainly S. pneumoniae and E. coli. Colletotrichum sp. 3 (code 2. 3659) morphospecies showed antibacterial activity against all bacteria tested. Based on the sequencing of the ITS rDNA gene, this fungus was identified as C. gloeosporioides under the GenBank accession number MN639701 (Figure 7).
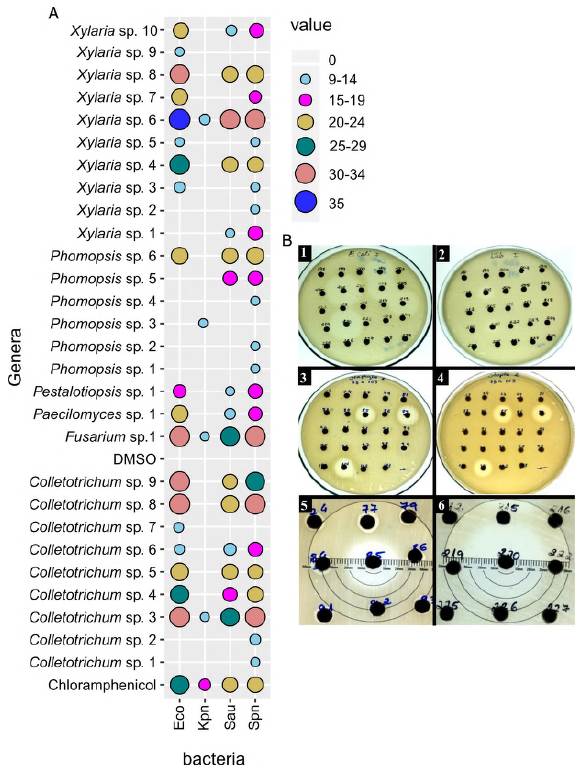
Figure 6 Antibacterial activity of endophytic fungi extracts isolated from H. sucuuba. A) Bubble chartshowing antibacterial activity of endophytic fungi. Eco = Escherichia coli; Kpn= Klebsiella pneumoniae; Sau = Staphylococcus aureus and Spn = Streptococcus pneumoniae. B) Examples of bioassay plates of endophytic fungi extracts showing growth inhibition zones for E. coli (1), K. pneumoniae (2), S. aureus (3), and S. pneumoniae (4). The numbers 5 and 6 show measurement of growth inhibition zones of tested bacteria
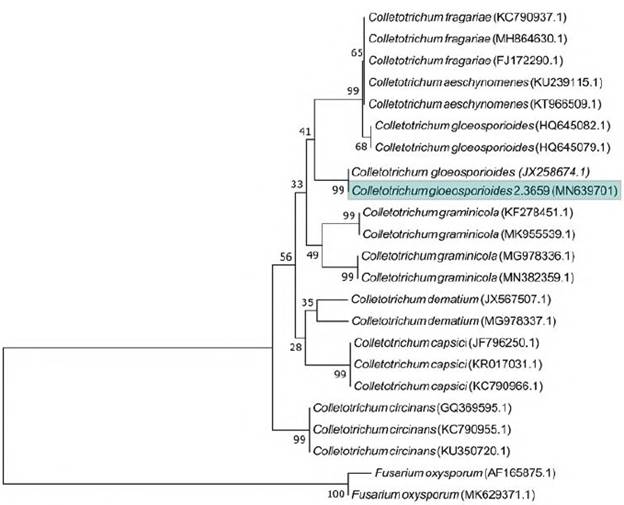
Figure 7 Neighbor-joining tree from ITS sequences showing the relationship between Colletotrichum species of the present study and other closely related Colletotrichum species retrieved from the GenBank. Bootstrap values (1000 replicates) are shown on the branches. Bar = 2 nucleotide substitutions per 100 nucleotides
Fungal extracts produced by Phomopsis showed inhibitory activity mainly against S. pneumoniae. The fungal extract of Phomopsis sp. 3 (code 2. 3008) showed specific activity against K. pneumoniae, which was the most resistant bacterium to all extracts.
The Fusarium genus presented activity against all tested bacteria. Although Fusarium isolates from H. sucuuba have not been effective as antibacterials, they can still be investigated for other biotechnological applications. Paecilomyces and Pestalotiopsis also had a single fungal extract showing activity against E. coli, S. pneumoniae, and S. aureus. The most sensitive bacteria to fungal extracts were S. pneumoniae and E. coli. K. pneumoniae was the most resistant when compared to the chloramphenicol control with (Figure 6).
Regarding the action of fungal extracts against pathogenic bacteria, only 10.7% of the extracts inhibited the four tested bacteria, but 39.2% of them inhibited E. coli, S. pneumoniae, and S. aureus, and 10.7% of the extracts specifically inhibited S. pneumonia (Figure S2, https://www.raccefyn.co/index.php/raccefyn/article/view/1525/3199).
Discussion
Our results showed a high frequency of isolation of H. sucuuba endophytic fungi (CF = 97.9%) and are similar to those observed for Piper glabratum Kunth (Oliveira, et al., 2015). However, this frequency is not always high in the isolation of endophytic fungi such as Euterpe precatoria Mart., with an IF of 33% (Batista, et al., 2018). This suggests that the frequency of isolates and the species of fungi isolated may vary depending on the plant investigated (Vaz, et al., 2014).
Besides the environmental conditions in which the collected plants are found in nature, plant tissue, the culture medium, and the temperature can influence the frequency of isolation. The greatest number of isolated fungi was observed in the leaves, which confirms that they are the main entrance for endophytic microorganisms occurring due to the presence of stomata and hydathodes, natural openings in the leaves. Although the leaf is considered the main entry of endophytes to the plant, they can migrate to other organs depending on the plant species, the seasonal conditions, and the nutritional variation of each plant according to age (Petrini, et al., 1993). A greater number of endophytic fungi isolated from the leaf has also been observed in Eugenia jambolana Lam. (Yadav, et al., 2015).
In the present study, the culture media with no extracts had the highest number of isolates while in the SDA medium we registered the greatest abundance followed by PDA. PDA is the most used culture medium in the isolation of endophytic fungi and here we used it for the isolation of Pinus radiata D. Don (Martínez-Álvarez, et al., 2016) and Polygonum hydropiper L. (Ye, et al., 2019).
Of the total of isolated fungi, 26.7% was recovered in the leaf extract medium and 16.7% in the stem extract medium (Table 1). The media with plant extract still showed good results, as they represented the isolation of 43.4% of endophytic fungi. Studies with plant extract to supplement culture media can be the object of more specific experiments to define concentrations and other tests that demonstrate that the nutritional conditions of the host plant are maintained.
Another important condition for the isolation of endophytic fungi is temperature. Most studies on endophytic isolation are done at a temperature between 28°C and 37°C (Farhat, et al., 2019; Ye, et al., 2019). However, isolation done at lower temperatures (for example, 18°C) allows the growth of less frequent or slower-growing fungi which offers a better representation of the endophytic community (Azevedo, et al., 2000).
Here, at 28°C we registered the highest number of H. sucuuba fungi despite the small difference as compared to the number isolated at 18°C. Another study also used 28°C as the standard temperature for isolation as it allows good conditions for the development of most endophytic fungi (Freire, et al., 2015). Different environmental and nutritional conditions are important for the expression of endophytic fungi diversity and some genera grow in culture media or environmental conditions similar to those of the host.
Of the 581 endophytic fungi isolated from H. sucuuba, 52.1% were identified and distributed into 16 genera. However, 143 (55.2%) morphospecies were not identified. The high rate of unidentified fungi is associated with the identification method used. The use of morphological analysis methods is not easy due to the lack of specialists in taxonomy and recent advances (Ding, et al., 2013). There is no ideal methodology for the characterization of microorganisms but the use of polyphasic taxonomy (micro and macromorphology, physiology, production of metabolites, and molecular data) is recommended given that using a set of techniques seems to help for better and more effective classification of fungi (Passarini, 2013).
Colletotrichum, Phomopsis, Xylaria, Penicillium, Guignardia, Pestalotiopsis, Aspergillus, Acremonium, Fusarium, Trichoderma, Cladosporium, Geotrichum, Phoma, Curvu-laria, Paecilomyces, and Cylindrocladium were identified as H. sucuuba endophytic fungi. Other studies in tropical regions also found these fungi to be endophytic. Acremonium, Aspergillus, Cladosporium, and Trichoderma were isolated from Opuntia ficusindica Mill. (Bezerra, et al., 2012), and Acremonium, Aspergillus, Colletotrichum, Fusarium, and Phoma from Theobroma cacao (Mill.) Bernoulli (Hanada, et al., 2010).
The Colletotrichum genus was the most frequent (Figure 5); its high frequency may be related to its facility to spread. Plants are subject to this genus of fungus at all stages of development, it can be disseminated from one plant to another by the wind, and the seeds can also infect seedlings (Osherov & May, 2001). They are commonly reported by authors of studies on endophytic fungi (Oliveira, et al., 2020; Santos, et al., 2020).
After Colletotrichum, Phomopsis, and Xylaria were the most frequent genera in this study. Phomopsis has more than 1,000 species, it is a plant parasite that grows on several plant species causing wilt, necrosis, cancer, rot, and dry stems and twigs, among other diseases (Udayanga, et al., 2011). However, several studies have also described this fungus as endophytic in Amazonian plants (Diniz, et al., 2020; Inácio, et al., 2021; Diniz, et al., 2021a; Diniz, et al., 2021b). Xylaria is described as a wood-damaging fungus isolated rather from tropical than subtropical plants (Photita, et al., 2001; Inácio, et al., 2021), which corroborates the findings in H. sucuuba.
Twelve percent of the tested fungal extracts showed antibacterial activity Xylaria being the genus with more fungi showing antibacterial activity. Several studies have reported the production of important substances by this genus, such as the compounds griseo-fulvin and dechlorhydriseofulphin with antibacterial and antifungal activity (Chapla, et al., 2018; McMullin, et al., 2020). In a study on the bioprospecting of Ficus pumila L. endophytic fungi, Xylaria sp. FPL-25 (M) showed a broad-spectrum antimicrobial activity against plant and human pathogens where the substance xylobovide-9-methyl ester was evidenced by bioautography. It is a promising hybrid product derived from natural polyketide as it is the basis for important antibiotics on the market (Rakshith, et al., 2020). Another endophytic strain of Xylaria sp., isolated from the stem of Isodon sculponeatus (Vaniot) Kudo, also produced six new xylariahgins A - F (1 - 6) compounds (Chen, et al., 2018). These studies support the idea that Xylaria strains are sources of potential drugs.
Colletotrichum can be found on many plant hosts as pathogens or endophytes, as well as saprobes (Ma, et al., 2018). In the present study, the endophyte inhibited the growth of all tested bacteria, mainly S. pneumoniae and E. coli. Colletotrichum sp. 3 (2,369) was identified by molecular characterization as C. gloeosporioides. In the literature, numerous bioactive substances from C. gloeosporioides endophytes such as coleoptic acid with activity against Bacillus subtilis, Sarcina lutea, Pseudomonas sp. and S. aureus have been reported (Hong Lu, et al., 2000; Zou, et al., 2000). The expression of the HupA gene by the C. gloeosporioides Cg01 strain has also been reported, which leads to the production of huperzine A, an important substance in Alzheimer's treatment (Kang, et al., 2019). The inhibitory activity of phosphoinositide 3-kinase (PI3Ka) is important for chemical therapy as it has shown antitumor activity; it was detected in the C. gloeosporioides (Uncaria rhynchophylla endophyte) strain due to the production of cycle L-leucil-L-leucyl and brevianamide F substances (Yang, et al., 2019) thus contributing to advances in pharmaceutical research.
Fungal extracts produced by Phomopsis exhibited inhibitory activity mainly against S. pneumoniae. Besides this inhibitory activity and the potential reported in several studies, the fungal extract produced by H. sucuuba Phomopsis sp. 3 (2.3008) showed specific activity against K. pneumoniae, the most resistant bacterium to all extracts, with great potential for the development of new drugs. It has been reported that Phomopsis strains have inhibitory activity against filamentous fungi, bacteria, and yeasts (Corrado & Rodrigues, 2004; Weber, et al., 2004). Furthermore, a recent study on the endophyte Phomopsis prunorum evicenced the presence of fomoterpenes and fomoisocoumarins with antibacterial activity (Qu, et al., 2020).
Considering our positive results on the antibacterial activity of H. sucuuba endophytic fungal extracts against Gram-positive and Gram-negative bacteria, this fungal community has promising research potential.
There is an endophytic diversity in the leaves and the stem of H. sucuuba. Besides, this species is a great host of endophytic fungi that deserve to be studied in other tissues such as roots and fruits. The extracts from these endophytic fungi have antibacterial activity, inhibiting Gram-positive and Gram-negative bacteria and, therefore, can be a source of broad-spectrum antibiotics. This is the first report on the endophytic fungal community of the Amazonian medicinal plant sucuba (H. sucuuba) and its antibacterial potential.