Introduction
It is estimated that there are close to 20000 lichenized fungal species globally, with approximately 7000 occurring in the Neotropics (Sipman and Aptroot, 2001; Lücking et al., 2009, 2017). The great majority of these are found epiphytically on bark. Colombia has 1634 species reported in the most recent Catalogue (Lücking et al., 2021), while 3300 species have been predicted (Lücking et al., 2009), which suggests a large gap in the knowledge of the Colombian lichen biota. In addition, if we consider that the vast majority of studies and collections have been restricted to a few regions of the country, particularly the Andean region with departments such as Cundinamarca, Boyacá, Caldas, Cauca and Valle del Cauca, it is expected that there are many more taxa to be recorded or described (Soto et al., 2021).
Dry forests are a life zone characterized by annual rainfall between 1000-2000 mm and mean temperatures of 25 °C. These ecosystems are characterized by often having thorny woody plants with various adaptations to desiccation. The dry forest in Colombia is restricted to the inter-Andean valleys (Cauca and Magdalena), the Caribbean region, and some small high mountain areas. Currently, dry forests are one of the most threatened ecosystems in Colombia, with only 5% of their original area remaining (Ramos and Silverstone, 2018). Despite their small area, they represent the least known ecosystem in terms of their the diversity of lichenized fungi, with many new discoveries to be expected (Lücking et al., 2019). The objective of this study is to show new records of genera and species of lichens for Colombia
Materials and methods
The material studied is deposited in the CUVC Herbarium of the Universidad del Valle (Colombia). Specimens were determined by examining characteristics of the thallus and ascomas such as ascospore type and size. The material was reviewed using a stereoscope and an Olympus SX-21 microscope. The ascospores were measured with a micrometric ruler. Photographs of all specimens were taken with a digital camera Canon PowerShot SX16. Chemical tests were also carried out with reagents K (10% potassium hydroxide), C (10% sodium hypochlorite) and lugol's iodine (IKI) for hymenium and ascospores.
Results and discussion
Dirina paradoxa (Fée) Tehler
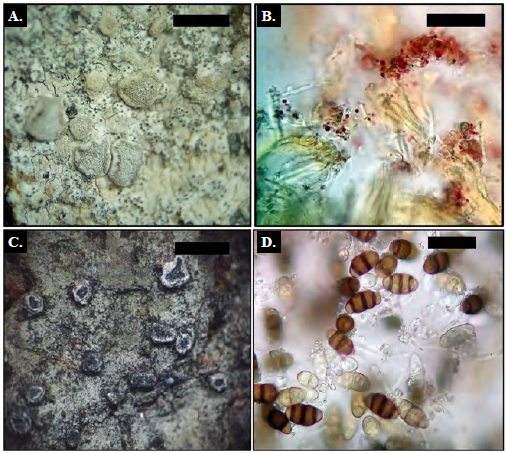
Description. Thallus corticolous, crustose, verrucose to squamulose, whitish gray, surface with pruina. Photobiont green. Apothecia sessile, disc gray with white pruine; white thalline margin, entire to wavy, 0.5-1.7 mm diam. Ascospores 8 per ascus, hyaline, 3-septate, 1620 x 5 |xm. Pycnidia not observed. CHEMISTRY: Thallus UV-, K-, C+ red; medulla C-, K-, KC-. Disc pruina C- or faintly C+ red, I+ red (lecanoric acid, erythrin). (Fig. 1 A-B)
Habitat and distribution. This species grows on acid rock or on bark in the Antilles and Venezuela (Tehler et al., 2013). Most species of the genus occur in Europe or Africa, and in South America it has been registered in Brazil, Cuba, Ecuador, Mexico, Perú and Venezuela (Tehler et al., 2013). In Colombia, it is reported for first time as a corticolous in dry forests of the Chicamocha Canyon. It is a typical element of dry forests.
Specimens examined. COLOMBIA: Santander: Municipality Los Santos, lower andeans orobiome; 06°47'32''N, 73°5'55''O, 1560-1700 m; open grasslands; 22 December 2018, Jiménez and Romero 785 (CUVC).
Heterocyphelium triseptatum Aptroot and M. Cáceres
Description. Thallus corticolous, crustose, smooth, grayish green to yellowish gray. Photobiont green. Perithecia solitary, becomes mazedia, laminar, ostiole margin white with black mass of ascospores, 0.3-0.6 mm diam. Ascus degrade quickly, leaving a mass of ascospores. Ascospores brown, 3-septate, 12-14 x 5-6 |rm. CHEMISTRY: Thallus UV-, K-; medulla C-, K-, KC- (no substances). (Fig. 1 C-D)
Habitat and distribution. This species grows on bark in Cerrado vegetation (Aptroot et al., 2017). Previously it has been reported from Brazil and Bolivia (Guzow-Krzemiñska et al., 2019). In Colombia, it is reported for the first time in forests of the middle Magdalena, in Río Claro (Antioquia).
Specimens examined. COLOMBIA: Antioquia: Municipality of Doradal, Magdalena temperate rainforest, Rio Claro, on the road between Río Claro an Doradal; 05°54'50'' N, -74°50'57'' W, 600 m; 4 July 2018, Soto s.n. (CUVC).
Julella sublactea (Nyl.) R.C. Harris
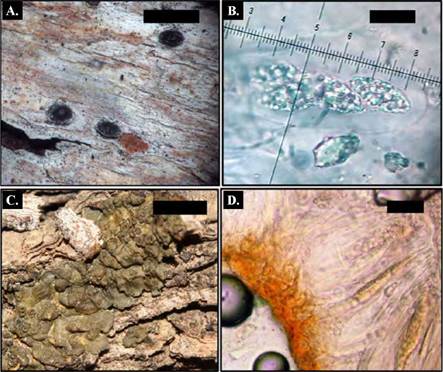
Description. Thallus corticolous, crustose, ecorticate, whitish gray. Photobiont green. Perithecia solitary, black, ostiole apical, 0.3-0.7 mm diam. Ascospores 8 per ascus, hyalines, muriforms, 30-34 x 10 μm CHEMISTRY: Thallus UV-, K-; medulla C-, K-, KC- (no substances). (Fig. 2 A-B)
Habitat and distribution. Julella sublactea is a corticolous species present in dry forests of North America and Mediterranean (Esslinger, 2019). In the Neotropics, this species has so far been registered for Mexico, the Caribbean, and recently Brazil (Aptroot and Spielmann, 2020; Harris, 1995, Roux et al., 2014), see GBIF.org (2019). In Colombia, it is reported for the first time as a corticolous in xerophytic dry forests of La Guajira
Specimens examined. COLOMBIA: Guajira: Municipality of Uribia, Ranchería Mapuain; 1°45'N, 88°08'W, 100 m; dry forest; 29 November 2018, Feria and Hoyos X53-103-NV35 (CUVC).
Opegrapha aurantiaca B. de Lesd.
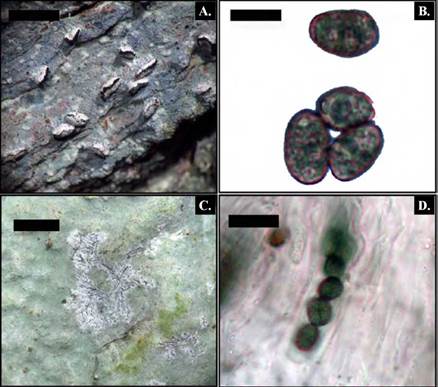
Description. Thallus corticolous, crustose, thin, smooth, green yellowish, with abundant yellow pruina, with indistinct prothallus. Photobiont green. Apothecia irregular with linear and branched shapes, only slightly raised above the thallus, 0.1-0.2 mm wide and 0.2-1.0 mm large; occasionally confluent, pale light grey throughout, with sparse yellow pruina, margins differentiated with abundant yellow pruina. Excipulum developed. Hymenium without gel, 65-72 ¡mi, IKI+ blue; paraphysoids anastomosing. Asci short ellipsoid to nearly globose, IKI-, dispersed in the hymenium, 30-50 x 18 ¡mi. Ascospores 8 per ascus, hyaline, clavate, 3-septate, 16-20 x 5 um, ends broadly rounded, wall and septum c. 1 mm thick. Pycnidia not observed. (Fig. 3 A-D)
Habitat and distribution. On smooth bark of trees in secondary rainforest. Only known from Florida, Cuba and Brazil (Ertz, 2009)
Specimens examined. COLOMBIA: Valle del Cauca, El Dovio, corregimiento of Bitaco, 4° 29.419'N, 76° 20968'W, 888 m alt., on bark of Caliandra sp., premontane cloud forest, 20 May 2019, E. Soto-Medina sn (CUVC 697373). 888 m
Peltula steppae (Kalb) Büdel, Kauff and Bachran
Description. Thallus corticolous, microfoliose, thin, smooth, lobes 1-2 mm large 0.5-1 mm wide, olive green to dark brown. Photobiont cyanobacteria. Apothecia sessile, laminar, cupuliform, thaline margin smooth, disc brown to dark brown, 0.4-0.9 mm diam. Ascus polisporus, hyaline, simple, subglobose, 3-5 x 3-4um (Fig. 1 D-F). CHEMISTRY: Thallus UV-, K-; medulla C-, K-, KC-. (Fig. 2 C-D)
Habitat and distribution. Peltula steppae grows on bark in very dry ecosystems of South America (Ferraro and Michlig, 2011; Kalb, 2001). Previously it has been reported in Argentina, Ecuador, Paraguay and Venezuela (Kalb, 2001, Ferraro and Michlig, 2011). In Colombia, it is reported for the first time in very dry shrublands typical of the Guajira, which are dominated by plants of the Cactaceae and Fabaceae families.
Specimens examined. COLOMBIA: Guajira, Municipality of Uribia, dry forest, Ranchería Wourre, 1°45'N, 88°08'W, 100 m. 29-11-2018. Feria e Hoyos PH29-NV10 (CUVC).
Sarcographina cyclospora Müll. Arg.
Description. Thallus corticolous, crustose, mineral grey, thin, smooth. Photobiont trentepohlioid. Ascomata immersed in stromata; stromata white, immersed, irregularly rounded, 2-5 mm wide; lirelae thin, black, open, in irregular stellate clusters, 0.05-0.10 mm wide. Disc black to dark grey, white-pruinose. Hymenium 80-100 μm thick, not inspersed. Ascospores 8 per ascus, ellipsoidal to globose, dark brown and irregularly 2 x 2-locular, 10-13 x 6-8 μm, I-.
CHEMISTRY: Thallus UV-, K-; medulla C-, K-, KC- (no substances). (Fig. 4 A-B).
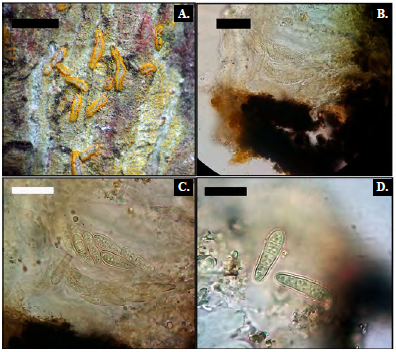
Figure 3 Opegrapha aurantiaca. A, habitus; B, section through apothecia; C, ascus with 8 ascospores; D, Ascospores. Bar scale: A = 1mm, B, C and D= 10 μm
Habitat and distribution. A very rare, corticolous species known thus far from northeastern Australia, Sri Lanka and Vietnam. In Colombia, it is reported for first time as corticolous in tropical forests of the Chocó Biogeographic region.
Specimens examined. COLOMBIA: Valle del Cauca: El Dovio, corregimiento of Bitaco; 17°14'11"S, 65°49'02"W, 1300 m; premontane cloud forest, on bark of Caliandra sp., 20 May 2019, E. Soto-Medina s.n. (CUVC).
Schistophoron tenue Stirt.
Description. Thallus corticolous, crustose, ecorticate, whitish. Photobiont trente-pohlioid. Ascomata sessile, elliptical to shortly lirelliform, 0.2-0.3 mm wide, 0.3-1.2 mm long and 0.1-0.2 mm high, ostiole a black slit. Ascospores 8 per ascus, grey to blackish brown, globose, submuriform, with reticulate ornamentation, 10-12 x 5-7 um. CHEMISTRY: Thallus UV-, K-; medulla C-, K-, KC- (no substances). (Fig. 4 C-D)
Habitat and distribution. Schistophoron tenue has been registered in Mexico, Guatemala, Costa Rica, Brazil, French Guiana, Ecuador, Peru, and Argentina (Torres et al., 2020).
Specimens examined. COLOMBIA: Valle del Cauca: El Dovio, locality of Vitaco; 17°14'11"S, 65°49'02"W, 1300 m; premontane cloud forest, on bark of Caliandra sp., 20 May 2019, E. Soto-Medina s.n. (CUVC).
These findings highlight the importance of exploring dry ecosystems in Colombia, which are perhaps the most threatened areas in the country. These species could be used as indicators of contamination or anthropic disturbance since they were collected in conserved sites. Sarcographina was only reported from Asia and Oceania, which shows the complexity of the distribution of lichenized fungi.