Introduction
The introduction of different species in non-native places around the world has occurred mainly due to human intervention in search of populating new territories and maintaining trade routes. This has resulted in the existence of a diversity of synanthropic species that can generate negative impacts on invaded ecosystems (Correoso, 2005). Squamate reptiles have been one of the most frequently introduced groups to different regions of the world due to their morphological characteristics that allow resistance to extreme handling and transport conditions (Castaño-Mora, 2002; Arroyave, 2015). Such is the case for the family Gekkonidae, which is an almost cosmopolitan group, as some species are now distributed around the globe due to both natural and human-mediated interoceanic dispersal (Vences et al., 2004; Detwiler & Criscione, 2014; Hoogmoed & Avila-Pires, 2015). The accidental introduction of species of geckos to different parts of the world has been facilitated by some morphological characteristics, such as small body sizes, high fecundity, rapid maturation, calcareous eggs resistant to saltwater, and high population densities (Brown & Duffy, 1992; Case et al., 1994; Kolbe et al., 2016; Somaweera et al., 2020). These characteristics allow geckos to disperse and adapt easily to urban environments, sometimes including environments with a more reduced anthropic impact (Case et al., 1994; Short & Petren, 2008; Zozaya et al., 2015; Cyriac & Umesh, 2021).
Lepidodactylus (Fitzinger, 1843) is one of the genera of geckos with the widest distribution, which currently groups 41 species naturally distributed in southeastern Asia and some Pacific islands (Palacio et al., 2012; Nania et al., 2020; Uetz et al., 2021). Within the genus, Lepidodactylus lugubris is the species with the most extensive geographic range, as it has been introduced around the world through human-mediated transoceanic transport (Vitt & Caldwell, 2009; Jiménez & Abarca, 2015; Hoogmoed & Avila-Pires, 2015). Lepidodactylus lugubris, commonly known as the mourning gecko, has an extensive capacity for colonization and adaptation in anthropic ecosystems favored by its parthenogenetic reproduction and thermal range of activity between 25 and 35°C (Palacio et al., 2012). Additionally, the species has considerable genetic diversity exhibiting both diploid and triploid populations, each comprising several genetically distinct clones (Ineich, 1999; Yamashiro et al., 2000). Most of these clones exhibit unique patterns of dorsal coloration that permit their identification in the field (Ota, 1994; Ineich, 1999; García et al., 2006; Nania et al, 2020). In general, both diploid and triploid populations coexist throughout their native and introduced ranges (Ineich, 1999; Yamashiro et al., 2000). Most of the populations currently distributed in the Americas have a diploid genetic load (corresponding to clone A sensu Ineich (1999) (Daza et al., 2012). However, triploids (corresponding to clone C) have been reported in Costa Rica and Ecuador. Furthermore, in Colombia, triploid clones C and E have recently been identified for the localities of San Cipriano (Valle del Cauca department) and Barranquilla (Atlántico department), respectively (Palacio-Sierra et al., 2012; Hoogmoed & Ávila-Pires, 2015; Ineich, 2015).
In the Neotropics, L. lugubris has been introduced in México, Nicaragua, Costa Rica, Panamá, Colombia, Venezuela, Ecuador, Perú, Brazil, Suriname, Chile, Cuba, and Guadeloupe (French territory) (Krysko et al., 2011; Daza et al., 2012; Montes et al., 2012; Hoogmoed & Avila-Pires, 2015; Bosch & Páez, 2017; Nania et al., 2020; Urra et al., 2020; Uetz et al., 2021). In Colombia, the species was recorded for the first time in 1941 in the southwestern Pacific lowlands (Daza et al., 2012). Later, its distribution was extended to other parts of the Pacific and Caribbean lowlands (including the islands of San Andrés and Providencia) and Cordilleras Occidental and Central, with an isolated record in the Cordillera Oriental. The current distribution in Colombia comprises 12 departments: Antioquia, Atlántico, Bolívar, Boyacá, Cauca, Chocó, Córdoba, Nariño, Quindío, San Andrés y Providencia, Sucre, and Valle del Cauca (Ayala, 1986; Castro-Herrera & Vargas-Salinas, 2008; Montes et al., 2012; Daza et al., 2012; Vanegas-Guerrero et al., 2016; Mendoza et al., 2018).
Our current knowledge of the distribution of L. lugubris in Colombia is exclusively based on specimens deposited in biological collections and reported in the literature (Daza et al., 2012; Vanegas-Guerrero et al., 2016). Thus, it is possible that the current distribution is underestimated, and other sources of data could provide additional localities not yet reported in the literature. Among those alternate data sources, smartphone applications devoted to the documentation of biodiversity now represent an unprecedented amount of information about the distribution of taxa, including introduced species such as L. lugubris. For example, the application iNaturalist (iNaturalist, 2021) allows the photographic documentation of species, including amphibians and reptiles, by experts and non-experts while providing strategies to ensure data quality (Jacobs, 2016). Despite this, the potential use of these applications in documenting the geographic distribution of many taxa remains unexplored, and L. lugubris is no exception. In this context, our aim was to update and extend the distribution of L. lugubris in Colombia based on both previously unreported specimens deposited in herpetological collections and photographic reports compiled from the digital platform iNaturalist.
Materials and methods
To generate an updated map of the distribution of L. lugubris in Colombia, we compiled all records of this species available in the literature. Most of these previously published records were compiled and reported by Daza et al. (2012), with a few additional reports published since then (Rubio-Rocha et al., 2012; Mendoza et al., 2018). As the geographic coordinates of many of these records were not reported in the literature, we estimated them using Google Earth. To complement these published records, we also examined both previously reported and unreported specimens of L. lugubris deposited in the following Colombian herpetological collections: Museo de Historia Natural C.J. Marinkelle at Universidad de Los Andes (ANDES-R; Bogota), Centro de Colecciones Científicas at Universidad del Magdalena (CBUMAG; Santa Marta), the reptile collection of the Instituto de Ciencias Naturales, Universidad Nacional de Colombia (ICN-R and JDL [John D. Lynch field number]; Bogotá), Colección Zoológica de Referencia Científica del Instituto para la Investigación y la Preservación del Patrimonio Cultural y Natural del Valle del Cauca-INCIVA (IMCN; Cali); Colección de Reptiles, Museo de Historia Natural at Universidad de Caldas (MHN-UCa-R; Manizales), Colección de Reptiles, Instituto Amazónico de Investigaciones Científicas-SINCHI (SINCHI-R; Leticia), the herpetological collection at Universidad Industrial de Santander (UIS-R; Bucaramanga).
We also reviewed the records of the species available on the virtual database iNaturalist (iNaturalist, 2021) up to June 13, 2021, where we selected those with photographs allowing species-level identification and with a precise locality. After, we also filtered out localities at more than 5 km away in a straight line from the nearest report of the species (or 1 km for localities on San Andres and Providencia Islands). We followed this course of action to prevent selecting redundant observations in nearby localities. Further, we only selected observations labeled as "Research Grade", which indicates that two-thirds of the identifiers agree on the same identification for a given observation (Liebgold et al., 2019). Finally, we complemented all the above records with our own photographs of individuals and those taken by colleagues. These specimens came both from localities where the species has been already reported and from those with no previous reports. For some of these specimens and additional, uncollected individuals from the same localities, we took anecdotal observations of their behavior and habitat use, which we discuss below. For all specimens, we noted the year they were found to determine the date of new records and verify if there are more recent records in the previously known range of L. lugubris, which would suggest that such populations have become established.
We identified the specimens deposited in the collections and photographic records based on the diagnostic characteristics including large digital pads, first digits without claw, and basal membranes on digits (Savage, 2002; Hoogmoed & Avila-Pires, 2015). Additionally, whenever possible, we compared the reported specimens with available descriptions of the various distinctive coloration patterns of the clones of the species. These clonal morphotypes differ from each other in the arrangement and shape of the dark markings on the dorsum that they exhibit (Ineich, 1988; Yamashiro et al., 2000).
In general, L. lugubris differs from members of the genus Hemidactylus because it lacks the claw of the first digit of the hands, in addition to having calcium deposits on the neck that are not observed in the species of this genus (Savage, 2002). Compared to most Hemidactylus species reported so far for the Colombian territory (specifically from H. angulatus, H. mabouia, H. frenatus, and H. palaichthus), these present an arrangement of dorsolateral tubercles that can be round and flattened, conical and heterogeneous, or conical and trihedral interspersed between the dorsal scales (Avila-Pires, 1995; Cole et al., 2013; Dueñas et al., 2018; Gómez-Martínez et al., 2020; Kluge, 1969; Rosler & Glaw, 2010; Vásquez-Restrepo & Lapwong 2018) while the dorsum of L. lugubris lacks tubercles and is uniformly granular. On the other hand, H. garnotii differs from L. lugubris in that the second pair of geneial scales of the former is not in contact with the infralabial scales (Morales et al., 2017; Vásquez-Restrepo & Lapwong, 2018). As for the genera Thecadactylus and Phyllodactylus lizards, they also present claws on their five digits, unlike members of the genus Lepidodactylus. Additionally, both species of Thecadactylus (i.e., T. rapicauda and T. solimoensis) have extensive webbing on the hands and feet, which is not observed in L. lugubris (Bergmann & Russell, 2007), while Phyllodactylus differs in having a tuberculate dorsal texture (Ayala & Castro, 1985; Savage, 2002).
Results
Our compilation of historical and previously unreported localities of L. lugubris resulted in a total of 89 records, of which 32 were based on collected specimens previously reported in the literature, while 57 represent new records. Of the localities newly reported herein, ten correspond to previously unreported specimens in biological collections and 47 to photographic records, five of which were obtained by us and 42 from the iNaturalist platform, from which we had originally compiled a total of 306 records. However, we had to remove 255 records as they did not meet the cutoff of minimum distance to a previously published locality for the species we had established. Of the remaining 51 records, nine were removed because the lack of an exact locality or the quality of the photographs available prevented us from confirming if they were L. lugubris. In general, the new records we compiled allowed us to report for the first time the species for five departments of the country (Caquetá: specimens collected in 2015 and 2021; Cundinamarca: based on iNaturalist observations from 2020 and 2021; Meta: based on iNaturalist observations from 2020; Risaralda: based on iNaturalist observations from 2017, 2020, and 2021, and Santander: photograph taken in 2009 and several specimens found in collections since then) (Figure 1). The new records also indicate that L. lugubris is distributed in Colombia between 0 and 2000 m (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307).
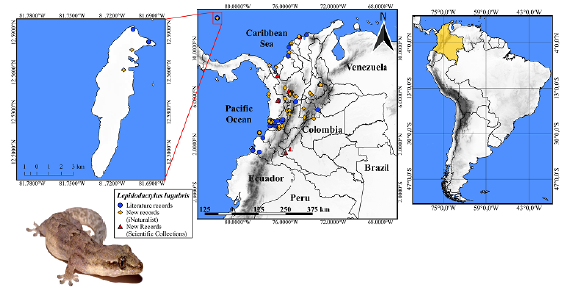
Figure 1 Records of Lepidodactylus lugubris in Colombia (blue circles: literature records; yellow circles: new records from iNaturalist; red triangles: new records from scientific collections. Photographs: A. Montes-Correa. The inset map at the left shows San Andres Island in the Eastern Caribbean Sea.
Most records of L. lugubris in the country are concentrated in the Andean and Pacific regions, especially near cities and seaports such as Buenaventura, Barranquilla, different parts of the world, facilitating the colonization of L. lugubris in Colombia. Additional records of the species found far from Colombia's main seaports appear to be associated with major roads (such as those between the Atlantic Coast and Medellín, those connecting Tumaco or Buenaventura with Cali, and Medellín with Bogotá). This pattern suggests that the dispersal of L. lugubris within the country is potentially mediated by road transportation.
Interestingly, populations previously reported for the Pacific Region, the Caribbean coast of the country, and San Andrés Island appear to have subsisted for many years (since 1941, 1991, and 2012, respectively) (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307). Furthermore, most of the new records, except the new departmental records (Figure 1), fill gaps between the previous records of the species representing distribution extensions within the same city or between nearby municipalities. However, the new department records, except those from the department of Risaralda, represent significant distribution range extensions to the eastern part of the country (i.e., both the western and eastern slopes of the Cordillera Oriental). Also, the records from the southern Atlantic coast and the northern Pacific coast municipalities of Acandí, Necoclí, and Bahía Solano are almost 100 km from the nearest localities reported for the species; they are recent (first records for 2013, 2014, and 2018, respectively) (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307), they correspond to relatively understudied regions of the country.
All the specimens we revised from biological collections and photographs taken by us or obtained from the iNaturalist platform exhibit the dark markings on the dorsum considered characteristic of clone A of the species (Figure 2). Finally, anecdotal observations of the individuals we photographed and of others from the same localities suggest that L. lugubris tend to be more commonly found close to the sympatric introduced geckos Hemidactylus frenatus, H. angulatus, and/or H. mabouia. Sometimes, both were observed using the same light bulbs to forage and about 2 m from each other. However, it seems there are some slight differences in habitat use. Indeed, L. lugubris is less frequently found than Hemidactylus and seemingly in the shade and no higher than 1.5 m above the ground. In contrast, species of Hemidactylus (as adults) appear to be more commonly found in closer proximity to artificial light sources and at higher parts of walls (>2.5 m). Differences in habitat use seem to be maximized in the locality of Carepa (department of Antioquia), with L. lugubris being more commonly found in the external walls of buildings, on nearby boards, and in decaying leaf litter. In contrast, H. frenatus was more commonly found on the inner walls of buildings.
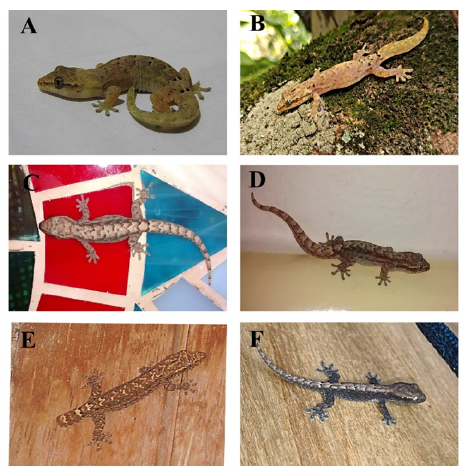
Figure 2 Variation in the color of the morphotype associated with the diploid clone A of Lepidodactylus lugubris in Colombia (A: Valle del Cauca - Cali; B: Caquetá - Florencia; C: Antioquia - San Jerónimo; D: Antioquia - San Jerónimo; E: Santander - Bucaramanga; F: Santander - Bucaramanga). Despite the variations, all specimens present the coloration pattern characterized by Ineich (1988) for the morphotype associated to clone A (=seven pairs of fine black dots on the dorsum located from the mid-dorsal region to the base of the tail). Photographic credits: A, Daniel Espitia; B, CC and ANM; C-E, JPR; F, Vladimir Quintero
Discussion
With the new distribution records for L. lugubris reported herein (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307, figure 1), the known distribution of the species in Colombia now comprises 17 of the 32 departments of the country. Our new records, along with other recently published range extensions (Rubio-Rocha et al., 2012; Palacio-Sierra et al., 2012; Montes et al., 2012; Mendoza et al., 2018), more than double the number of seven departments reported for the species in the latest summary of its distribution in the country (Daza et al., 2012). Considering that this compilation was published ten years ago, the fact that the known distribution range of the species has grown so much in such a short time span could indicate a very rapid increase in the range extension of L. lugubris in Colombia. Alternately, the species could have inhabited at least some localities much earlier than when they were first reported. For example, although L. lugubris has been seen in Bucaramanga (department of Santander) since 2009, only here is the species reported for that city. Thus, it is possible that the apparent rapid range expansion of L. lugubris in Colombia is an artifact and that the species has inhabited at least some of the newly reported localities for much longer than expected.
Most of our new records concentrate on regions of the country where the species was already reported (the Caribbean, the Western and Central Andes, and the Pacific) (Daza et al., 2012; Hoogmoed & Avila-Pires, 2015). Furthermore, our new records from the Gulf of Urabá (municipalities of Carepa and Necoclí) and northern Chocó (municipality of Bahia Solano) support the idea expressed by Daza et al. (2012) that the distribution of this species is likely to be continuous between the Pacific and the Caribbean coasts. However, the new localities we report also expand the range of species to the central portion of the western slopes of the Cordillera Oriental and the northwestern edge of the Amazonia and Orinoquia regions of Colombia. Among these new departmental range extensions, two are of particular interest as they correspond to localities found in the transition between the Andean and the Amazon and Orinoquia regions of the country (Caquetá and Meta departments, respectively), areas where the species has not been reported yet. Such findings are worrisome considering that the proximity to these regions would suggest that the species could likely start colonizing them soon if it has not done so already.
Based on these new records, L. lugubris now exhibits an altitudinal range from 0 to 2000 m in Colombia (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307) representing an extension of ca. 500 m from the highest published report for the species (Medellín, Department of Antioquia) (Rubio-Rocha et al., 2013). In other South American countries where this species has been recorded, its altitudinal range reaches up to 900 m: between 7 and 729 m in Ecuador (Torres-Carvajal, 2020) and 875 m in Venezuela (Señaris et al., 2017). The elevational limits of L. lugubris may be related to the apparent inability of the species to tolerate climatic conditions in areas above ~2000 m. In general, reptiles have difficulties developing ecophysiological processes such as thermoregulation in more temperate or colder environments (Adolph & Porter, 1993), thus limiting their ability to feed or reproduce and, thus, their survival (Meiri et al., 2013). The original distribution of the species lies in tropical areas of Asia where the temperature rarely drops below 25°C (Savage, 2002; Lever, 2003). Ecophysiological studies, as well as additional sampling at altitudes above 2000 m, are needed to better understand the role of temperature and other environmental variables in determining the altitudinal range inhabited by this and other gekkonids in their introduced range and the effect of increasing temperatures and anthropogenic activities on determining the extent of its altitudinal distribution and geographic range in the future. In the future, we may also see situations like that observed for H. frenatus, which has maintained its population at 2600 m inside a brewery where the temperature is higher than the ambient temperature of nearby areas (Caicedo-Portilla & Dulcey-Cala, 2011).
Lepidodactylus lugubris has a high dispersal capacity, and it is known to have expanded its range to forested environments in parts of its distribution, for example, in Southeastern Asia (Case et al., 1994; McCoid, 1996). However, the dependence on urban environments appears to vary between clones, with clone A having lower densities than other clones in forested areas, at least in Hawaii (Short & Petren, 2008). In support of this idea, the establishment of L. lugubris in South America (where clone A is prevalent) appears to have been limited by the existence of man-made structures (Hoogmoed & Avila-Pires, 2015). For example, there are no records from the locality of Macanal (department of Boyacá) since 1981, despite being relatively well-sampled, which is probably a result of the abandonment of human structures in the area (D. Gómez, pers. comm. 2021). Interestingly, Macanal seems to be the only historical locality from which the species seems to have become extinct. Finding this to be the case would suggest that the other populations from previously known sites are likely to have become established and do not correspond to spurious or accidental records as has been reported for other lizards (e.g., the recent finding of Gymnophthalmus speciosus at 2000 meters above its normal altitudinal range) (Henao-Osorio et al., 2021). Further sampling is needed to confirm if this is the case and if the presence of human structures limits the distribution of L. lugubris on its introduced range or if it can disperse and get established in better-conserved regions.
Lepidodactylus and Hemidactylus species can be easily confused (Bosch & Paez, 2017; Bandeira & Missassi, 2022) because they share similar morphological and ethological characteristics, such as the presence of nocturnal habits, inhabiting areas modified by man, and employing foraging strategies associated with artificial light (Case et al., 1994; Savage, 2002). Therefore, these similarities could explain the late reports of L. lugubris in some regions (Abarca, 2006). As mentioned above, despite L. lugubris inhabiting the city of Bucaramanga since at least 2009, the species has remained unreported there until the present study. Nevertheless, we are aware of specimens collected in this city and deposited in biological collections as early as 2015 that were confused with sympatric species of the genus Hemidactylus, which could be the case for other areas and museum collections.
Although our observations are anecdotal, L. lugubris seems to be commonly found in sympatry with the introduced gecko H. frenatus, a species with which they overlap in much of their distribution where they have been introduced (Rödder et al., 2008). Indeed, it has been observed that both species apparently are involved in resource competition, with H. frenatus potentially being able to outcompete L. lugubris (Case et al., 1994; Niewiarowski et al, 2012). Further studies on the interspecific interactions between L. lugubris and other sympatric geckos in their introduced ranges are needed, as our observations indicate that, at least in Colombia, H. frenatus is often more abundant than its counterpart, and it exhibits different substrate use.
The results of our revision of collected specimens and photographic records taken from the iNaturalist platform allow us to conclude that the existence of the morphotype associated with clone A in the Colombian territory is predominant. There is only one record for each of two triploid morphotypes (=C and E) (Hoogmoed & Ävila-Pires, 2015). Such a result supports the idea proposed by Ineich (2015) that clone A reached the American continent before World War II, while the other clones have started to colonize much more recently. However, it is necessary to clarify that identifying specimens of L. lugubris at the clone level based on color morphotypes is not ideal, especially when based only on preserved specimens or online photographs (I. Ineich, pers. comm. 2021). Indeed, this should be done by first depositing specimens under complete darkness for an hour, then illuminating them, and their dorsal pattern quickly photographed with the black marks being the only ones retained (I. Ineich, pers. comm. 2021). We recommend future researchers use this protocol to ensure consistency in the methods used to determine clonal morphotypes less ambiguously. In any case, future genetic studies are needed to corroborate the clonal identification of the specimens of L. lugubris found in the Colombian territory and in other countries where the species is introduced and to learn more about the geographical origin of the populations and their possible routes of dispersal.
Most of the specimens and new reports of L. lugubris recorded for Colombia have been provided by the iNaturalist platform (Table 1S,https://www.raccefyn.co/index.php/raccefyn/article/view/1742/3307). That is possible because, in our case, L. lugubris has a characteristic pattern of coloration on the dorsum and the absence of the claw on the first pedial digit sometimes can be observed facilitating, in most cases, its identification using good quality photographs. Thus, we highlight the importance of databases such as iNaturalist in providing more detailed and updated knowledge of the distributions of native and introduced species with the help of local communities (Wallace et al., 2019). Although the ideal is to collect specimens and deposit them in registered biological collections, we also recommend, with Auguste & Fifi (2020), to upload observations on various platforms such as iNaturalist with high-quality photographs and precise locality data (with coordinates). Likewise, as suggested by Liebgold et al. (2019), observations made on iNaturalist or other citizen science platforms that appear to correspond to distribution extensions should be verified by trained herpetologists.















