Introduction
Piper marginatum is a medicinal plant from the Piperaceae family widely distributed in the Amazon rainforest and popularly known as capeba, malvarisco, pepper-do-mato, nhandi, nhandú, or pepper-of-the-Indians (Andrade et al., 2008). It is commonly used in infusions against gastrointestinal and liver diseases and snake and insect bites (Hurtado et al., 2016; Chaves & Santos, 2002). Chemical studies of P marginatum have revealed that the plant tissues accumulate amides, prenylated benzoic acids, flavonoids, phenylpropanoids, and aristolactams (Reigada et al., 2007). In previous studies with essential oils from P marginatum leaves, terpenes and phenylpropanoids have been detected and shown to have antioxidant, leishmanicidal, larvicidal, and antifungal activities (Brú & Guzman, 2016; Moraes et al., 2014). Essential oils are usually obtained by hydrodistillation by crushing the plant in water and heating it in a Clevenger apparatus (Lima et al., 2021; Melo-Guerrero et al., 2020). The hydrodistillation of essential oils can cause the loss and degradation of certain volatile compounds due to the long extraction times by thermal or hydrolytic effect (Elyemni et al., 2019). There are many advanced and innovative methods for obtaining essential oils with optimized extraction times and temperatures besides reduced artifacts and the loss of polar molecules, but hydrodistillation remains the simplest and oldest method used to obtain essential oils (El Asbahani et al., 2015). In our study, we aimed at obtaining essential oils from P. marginatum leaves by hydrodistillation and pH control of the decoction water to evaluate possible variations in the chemical composition and antimicrobial activity.
Materials and methods
Botanical material
Leaves of P. marginatum specimens were collected on March 2019 from a fragment of forest located in the city of Recife, state of Pernambuco, Brazil. The species was identified by Dr. Margareth F. de Sales of the Department of Biology of the Federal Rural University of Pernambuco and compared with a voucher specimen previously deposited in the Vasconcelos Sobrinho Herbarium of UFRPE with the number 48210 (Ramos et al., 2022a).
Obtention of essential oils
For each oil sample obtained, 500 g of fresh leaves of P. marginatum were crushed and submitted to hydrodistillation in a modified Clevenger apparatus for 2 h. To obtain the oil in a basic medium, the decoction water was adjusted to pH 10.0 using a 0.1 molL-1 sodium hydroxide solution. To obtain the oil in an acidic medium, the decoction water was adjusted to pH 4.0 using a 0.1 molL-1 sulfuric acid solution. To obtain the oil in a neutral medium, distilled water was used (pH 7.01). The oils were treated with anhydrous sodium sulfate and stored at ±5 °C for further analysis.
Essential oils analysis
Oil samples were analyzed using a Hewlett-Packard 5890 Series II GC chromatograph equipped with a flame ionization detector and a J & W Scientific DB-5 fused silica capillary column (30 m x 0.25 mm i.d.) with a programmed temperature of 60 to 246 °C at 3 °C/min. The temperatures of the injector and the detector were 260 and 280 °C, respectively. Hydrogen was used as carrier gas at a flow rate of 1.0 mL/min; injection was in split mode (1:30) and the injection volume was 1.0 µL of a solution containing 10 mg/mL of oil in hexane. The amount of each compound was calculated from GC peak areas in the order of DB-5 column elution and expressed as a relative percentage of the total area of the chromatograms.
Chemical identification
Oil samples were analyzed using a Varian GC/MS (GC: Varian 431/GC-MS: Varian 220-MS) system operating in the EI mode at 70 eV equipped with a J & W Scientific DB-5 fused silica capillary column (30 m x 0.25 mm i.d.) and a programmed temperature of 60 to 246 °C by 3° C/min. The temperatures of the injector and the detector were 260 and 280° C, respectively. The carrier gas was helium, the flow rate was 1 mL/min, and we used the split mode (1:30) with an injected volume of 1.0 µL of a solution containing 3 mg/mL of oil in hexane. The initial identification of the separated components of the essential oil was done by comparing with previously reported values of retention indices obtained by co-injection of oil samples and C11-C24 linear hydrocarbons and calculated using the Van den Dool & Kratz equation (Van den Dool & Kratz, 1963). Subsequently, the MS acquired for each component was matched with those stored in the Wiley/NBS mass spectral library of the GC-MS system and with other published mass spectral data (Adams, 2007). Terpenes (β-pinene, y-terpinene, E-nerolidol, caryophyllene a-humulene, and a-pinene,) and phenylpropanoids (Z-asarone and E-asarone) purchased from Sigma-Aldrich, Brazil, were used to identify the volatile components. Dillapiole was previously obtained from Piper aduncum leaves essential oil (91.0 %) (Ramos et al., 2022b). All analyses were carried out in triplicate.
Antimicrobial activity
We analyzed this material to evaluate the antimicrobial activity against Staphylococcus aureus (02), Escherichia coli (86), Bacillus subtilis (86) bacteria, and Candida utilis (1006) and Candida albicans (1009) yeasts. We used Saubouraud liquid culture media for yeasts and Mueller Hinton liquid medium for bacteria. The microplates were grown at 37 °C for 24 h for bacteria and at 30 °C for 72 h for yeasts. After the incubation period, the microplates were treated by adding 10 µL of a 0.01 % resazurin solution and incubated for 3 h. The MIC was defined as the lowest concentration of the samples that inhibited the growth of the microorganism, with the final concentration fluctuating between 2.5 and 2500 µg/mL (Ramos & Bezerra, 2021). Metronidazole was used as a positive control for bacteria and fluconazole for yeasts.
Results and discussion
The yield values of essential oils obtained from P. marginatum leaves in neutral (pH7-EO), acidic (pH4-EO), and basic (pH10-EO) mediums were 0.13, 0.06, and 0.12 %, respectively. Analysis by gas chromatography revealed qualitative and quantitative variations in the chemical profiles of the three samples (Figure 1). We identified a total of 37 compounds: 13 in the pH4-EO samples; 16 in the pH7-EO samples, and 17 compounds in the pH10-EO samples, which corresponded to 72.5 %, 93.0 %, and 69.9 % of the total chemical composition of each oil, respectively (Table 1). In the pH7-EO samples, we found monoterpenes, sesquiterpenes, and aromatic compounds, predominantly, phenylpropanoids such as Z-asarone (18.2 %), E-asarone (15.1%), dillapiole (13.0%) and croweacin (6.0%). The sesquiterpenes macrocarpene (10.8 %), viridiflorene (9.1 %), caryophyllene (7.7 %), and E-nerolidol (6.5 %) were the major compounds identified in the pH10-EO samples.
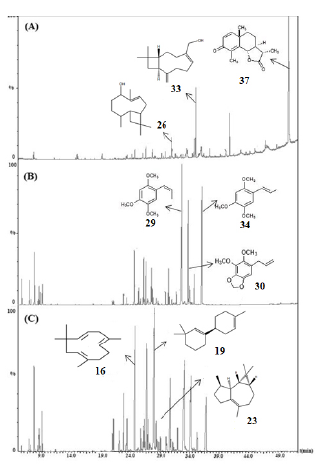
Figure 1 Chemical profiles by GC/MS of essential oils obtained from P. marginatum leaves in pH4 (A), pH7 (B), and pH10 (C)
Table 1 Chemical composition of essential oils obtained from P. marginatum leaves in neutral (pH7-EO), acidic (pH4-EO) and basic (pH10-EO) mediums
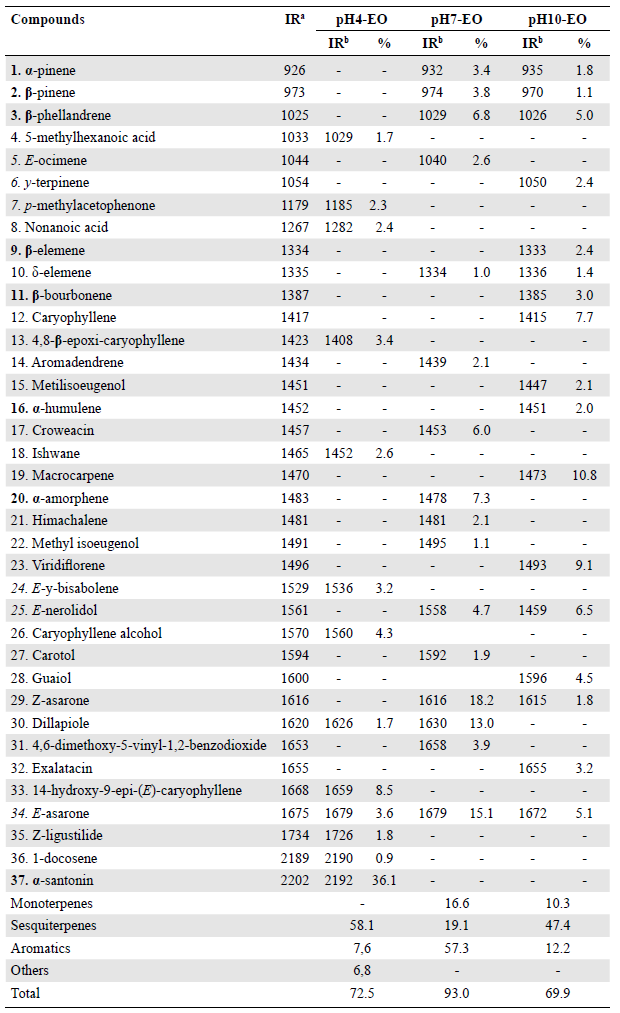
a Linear retention indices from the literature (Adams, 2007); b Retention indices calculated from retention times in relation to those of the n-alkanes series on a 30 m DB-5 capillary column
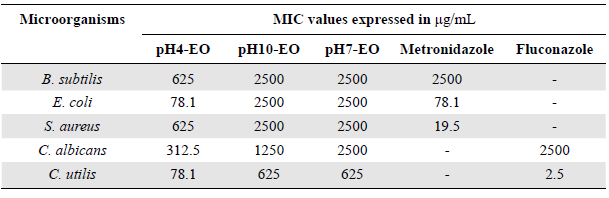
Table 2 MIC values for essential oils obtained from P. marginatum leaves in neutral (pH7-EO), acidic (pH4-EO), and basic (pH10-EO) mediums
The pH4-EO samples showed the greatest difference between the three oils analyzed; a-santonin sesquiterpene (36.1%) was the major compound and E-asarone was the only one present in the three oils. In an aqueous acidic medium, unsaturated terpenes undergo cyclization, hydration, and rearrangement reactions with the formation of carbocation intermediate resulting in mixtures of products similar to those obtained in mediums with neutral pH. Eleven compounds were found only in pH4-EO samples: six of them were identified as oxygenated terpenes and two as non-oxygenated. The six oxygenated terpenes were probably products of the hydration reaction that took place during the process to obtain the oil. In the pH4-EO samples, two carboxylic acids not found in pH7-EO and pH10-EO samples were also identified. The identification of carboxylic acids from essential oils obtained in an acidic medium was expected since the carboxyl groups are protonated while in a basic medium, carboxylates, which have an affinity for the aqueous phase, form.
A quantitative reduction of phenylpropanoids was observed for samples pH4-EO and pH10-EO when compared to sample pH7-EO. The reduction of phenylpropanoids probably occurs due to the oxidative degradation of Z-asarone, dillapiole, E-asarone, and exalatacin containing allylic and vinylic groups that are susceptible to oxidation in a basic or acidic medium overheating (Turek & Stintzing, 2013), and to the formation of phenylpropanoid dimers, which are non-volatile compounds.
Previous studies with essential oil obtained from the leaves of allopatric species of P. marginatum have revealed great variability in the chemical composition with anethole (45.9%), isosafrole (37.3%), Z-asarone (32.6%), Z-asarone (30.4%), anisaldehyde (22.0%). and notosirnol (22.7%) presence, which points to the possibility of having more than seven chemotypes for P. marginatum leaves essential oil (Macédo et al., 2020; Ayres et al., 2021; Hurtado et al., 2016; Vogler et al., 2006). However, this is the first report of a-santonin sesquiterpene identification in the Piperaceae species. The proposed biosynthetic route for the a-santonin involves a sequence of cyclization reactions, rearrangements, and oxidation of germacrene from farnesyl diphosphate (De Kraker et al., 2001), reactions that can be favored in basic or acidic solutions. Forty compounds were identified in essential oils obtained from P. marginatum leaves, stems, and inflorescences including germacrene (Autran et al., 2009), a central intermediate in the biosynthesis of several sesquiterpenes (Xu & Dickschat, 2020). A new method developed to obtain and simultaneously fractionate essential oils by hydrodistillation allowed the obtention of qualitatively and quantitatively different chemical profiles of essential oils from P. marginatum (Ramos et al., 2022b). The variation in the chemical composition of the essential oil in a plant species is usually attributed to biotic and abiotic factors such as herbivory, climate, and soil composition, but the parameters of the method used to obtain the oil are usually neglected (Afshar et al., 2021; Vafadar et al., 2017; Silva et al., 2016; Paolini et al., 2010). Most studies available in the literature use hydrodistillation to obtain essential oils from plants but do not provide information on the control or specification of the water pH used to obtain the oil. The compounds commonly identified in essential oils obtained from plants are monoterpenes, sesquiterpenes, phenolics, phenylpropanoics, and heterocyclics, which contain various chemical groups of alcohols, ketones, aldehydes, carboxylic acids, esters, and acetates. These compounds in an aqueous base or acid medium under heating can undergo cyclization, hydrogenation, hydration, and dehydration reactions resulting in variations in the chemical composition of the essential oil (Turek & Stintzing, 2013).
Additionally, the antimicrobial potential of essential oils obtained at different pH values also exhibited variation in CMI values against bacteria and yeasts. Our results showed that the pH4-EO oil exhibited greater activity against the gram-negative bacteria E. coli, with a MIC of 78.1 µg/ml, while for the yeasts C. albicans and C. utilis the MIC was 312 µg/ml and 78.1 µg/ml, respectively (Table 2). The strong antimicrobial activity observed for the pH4-EO sample may be associated with the presence of the major compound a-santonin, a sesquiterpene that has been used as an effective drug against infectious diseases caused by worms, which was first isolated from the flower bud of Artemisia santonica (Wang et al., 2019). Activity for the pH4-EO sample can also be attributed to the presence of 5-methylhexanoic and nonanoic acids as oils rich in short-chain fatty acids exhibit strong antimicrobial activity (Holanda et al., 2020). The activities of the pH7-EO and pH10-OE samples were considered moderate to weak for all microorganisms tested, with a MIC that varied between 625 µg/ml and 2500 µg/ml for both oils.
Previously, the essential oil from P. marginatum leaves containing E-nerolidol, O-cymene, spathulenol, elemicin, and α-copaene as major compounds showed antimicrobial activity (dos Santos et al., 2021).
Conclusion
Our results showed the effect of varying the pH of the water used in the hydrodistillation on the composition and antimicrobial activity of essential oils from P. marginatum leaves. The compounds identified in the samples pH4-EO, pH7-EO, and pH10-OE evidenced intermediates and biosynthetic pathways in common, which indicates that the variations in the chemical profiles observed among these three samples were due to the variations in pH values. Our results also revealed the importance of water pH as a parameter that should be considered in future studies to obtain essential oils by the hydrodistillation method.















